Suggestions for Promoting China’s Drug Regulatory Agency to Join Pharmaceutical Inspection Co-operation Scheme -PIC/S
2023-09-26HuShigaoWuZhiang
Hu Shigao,Wu Zhiang
(1. School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China;2. Anhui Center for Drug Evaluation and Inspection, Hefei 230051, China;3. Yeehong Business School, Shenyang Pharmaceutical University, Beijing 10055, China)
Abstract Objective To identify and reduce the gap between China’s drug GMP inspection and pharmaceutical inspection co-operation scheme (PIC/S) audit checklist,find out the key improvement items,and revise them pertinently,which will promote the process of China joining PIC/S.Methods The general situation of PIC/S organization and audit checklist were introduced first,and then the accession of several countries that joined the organization was analyzed.Meanwhile,the process of China’s participation in PIC/S was sorted out.After referring to the contents of PIC/S audit checklist,the problems of GMP inspection system in China were studied.Results and Conclusion There are still many problems in GMP inspection in China.Some suggestions are put forward for improvement and change,which can provide reference for the development of drug inspection agencies at all levels in China.
Keywords: GMP inspection;pharmaceutical inspection co-operation scheme (PIC/S);audit checklist;drug regulatory agency
On September 24,2021,China’s National Medical Products Administration (NMPA) formally sent a letter to the pharmaceutical inspection cooperation scheme (PIC/S) to apply for the initiation of the pre-entry procedure[1].With the start of the application process for NMPA to join PIC/S,it will take a long time to evaluate the whole GMP inspection system.It is expected that it will take 3 to 5 years for NMPA to join PIC/S in the future.
1 Introduction to pharmaceutical inspection co-operation scheme (PIC/S)
1.1 Process of joining PIC/S
PIC/S currently has 54 full member institutions,which were established in November 1995 and are composed of member national and regional drug inspection agencies[2].The process of joining PIC/S includes two stages.The initial stage is the “preaccession application” stage,and the longest period of the pre-accession process is 2 years.The applicant institution needs to submit questionnaire,relevant supporting materials,self-evaluation audit list and other documents[3].PIC/S needs to evaluate the gap between the drug inspection system of the applicant institution and PIC/S requirements.During this period,applicant members can have a detailed understanding of PIC/S standards and specifications.Upon completion of this assessment,the second stage,“formal application”,will start,during which applicant institutions should submit formal application to PIC/S[4].After entering the formal application stage,applicants need to pass the evaluation of PIC/S to become a full member.In theory,applicants can become a member of PIC/S within one and a half years at the earliest.However,PIC/S needs to conduct systematic and comprehensive evaluation of the applied institutions,which will often take a long time,up to 6 years[5].
1.2 Audit checklist of PIC/S
Audit checklist is the evaluation standard of GMP supervision system of pharmaceutical regulatory authorities.It adopts four evaluation methods:Documentation review (DR),on-site evaluation at inspectorate (OSEI),on-site evaluation at laboratory(OSEL) and observed inspection (OI).The whole inspection system is comprehensively evaluated by setting 11 modules,including requirements on laws and regulations,GMP standards,inspection procedures,etc.PIC/S is divided into three grades:Extremely important,very important and important according to their importance (Table 1).In addition,there are 38 sub-modules and 78 specific indicators[6].In order to simplify the evaluation process,13 subindicators are integrated with other similar subindicators,and 25 sub-indicators are actually evaluated[7].
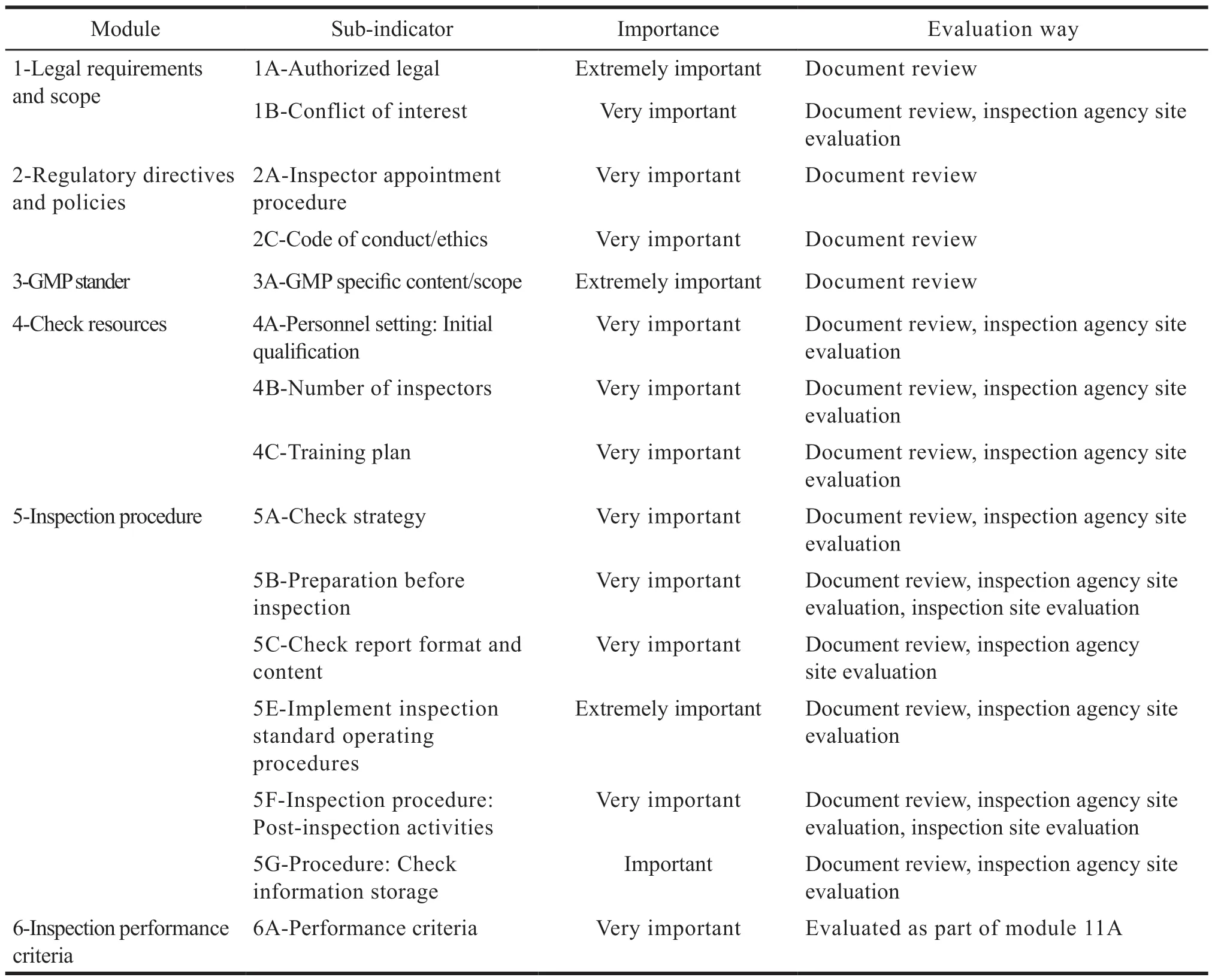
Table 1 Summary of audit checklist
1.3 Members of PIC/S
The members of PIC/S are GMP inspection bodies in different countries.In addition,there are several cooperative organizations,as of January 2022,there had been 54 members from 49 countries and regions and 4 cooperative organizations[2].
1.4 Significance of China’s accession to PIC/S
The safe use of drugs is related to the wellbeing of the public.Therefore,it is a general trend to comprehensively strengthen drug supervision and form a supervision chain of whole process and all varieties of drugs.At the same time,improving the national drug standard system and actively promoting the internationalization of standards is an important part of the “Healthy China 2030 Plan”[8].After being a member of ICH,joining PIC/S has also become one task to accelerate the internationalization process of China’s drug supervision.
PIC/S has now become an authoritative organization in the field of international good manufacturing practice (GMP),whose work is to build a unified drug GMP standard,operation mechanism,the inspector training system,and to achieve its goal of promoting the coordination of drug inspection standards and procedures among its members through the cooperation between the member inspection agencies.PIC/S can promote cooperation and networking among pharmaceutical authorities of member States,regional and international organizations,thereby enhancing their mutual trust.Therefore,in the process of internationalization of drug regulation in China,it is a key point to join PIC/S,which has great practical significance.
2 Review of China’s participation in PIC/S
China’s drug regulation has been actively in line with international standards.For example,it actively participated in many activities and forums of PIC/S,constantly improving and adjusting China’s GMP inspection standards.Fig.1 shows the process of GMP supervision in China actively integrating with international standards,indicating the scientific and forward-looking concept of drug supervision in China.

Fig.1 Timeline of China’s participation in PIC/S
3 Analysis of problems existing in GMP supervision in China
3.1 Imperfect system of laws and regulations related to GMP
In the process of implementing GMP in China,some laws and regulations still need to be continuously improved and issued.For example,the appointment of GMP inspectors also needs laws and regulations to be based on.Besides,once the conflict of interest occurs in the inspection process,there is no specific guidance for the declaration.
3.2 China’s GMP inspection lack of timely update mechanism
Although the framework of China’s GMP has included the content of PIC/S audit checklist requirements,PIC/S requires the establishment of an update mechanism for GMP standards.However,China currently lacks an update mechanism for main documents.Although the appendix part has been updated,it is still necessary to establish an update mechanism that meets the requirements of PIC/S.
3.3 Lack of organization of inspectors
The lack of professional inspectors is an obvious phenomenon in China.Although China has issued documents on the team construction of inspectors,compared with the scale of the pharmaceutical industry,the problem of inadequate professional inspectors needs to be solved as soon as possible.According to the requirements of PIC/S specification,the specialization degree of inspectors is not high.Most drugs inspectors are part-time staff who come from the drug regulatory agency of different provinces,inspection institutions or the relevant business unit.Since part-time inspectors do not belong to the pharmaceutical inspection institutions,the drug inspection agencies at all levels cannot designate them to work directly.As a result,it affects the efficiency of inspection work.
In China,the training effect of drug inspectors cannot be evaluated,so the management method of part-time inspectors is inappropriate,which can lead to some problems in drug inspection.Because part-time inspectors may not be able to participate in some links of the inspection,problems such as insufficient preparation and inadequate review of rectification results exist.As part-time inspectors do not belong to drug inspection institutions at all levels,and their abilities are also different,it is hard to establish a complete and effective management assessment system.Therefore,the reward and punishment measures cannot be implemented,which is not conducive to the long-term development of the inspector team[9].The above problems will affect the construction and development of the inspector team.
3.4 Incomplete informatization of GMP inspection
The informatization of GMP inspection in China is not perfect,which is mainly reflected in the incomplete database.PIC/S requires its members to have procedures for checking data storage,so it is important to check the update of classification database for the current compliance status of enterprises.China’s drug GMP inspection includes overseas inspection,flight/tracking inspection,and the issuance of certification announcements[10].The audit and inspection center has a special database on the website of GMP certification announcement,but there are problems such as single function and insufficient efficiency in data storage system.Some information lacks relevant links,and it is not easy to find relevant certification and announcement content[11].In addition,there is no relevant information about overseas inspection results.Although some inspections have columns,they do not support fuzzy retrieval and only non-compliance information is publicized.No relevant database has been established for the data of GMP licenses suspended or revoked,and the database already in use still has the problem of unclear classification[12].
3.5 Lack of GMP inspection quality risk management system
China has established the evaluation index currently,but drug GMP inspection quality evaluation system has not been set up in the drug inspection institutions.There is a lack of evaluation index system of key elements such as the formulate plan,the situation of on-site inspection control,the ability and level of inspectors,the comprehensive evaluation based on risk and the application of inspection results.
At present,most of the GMP inspectors in China are administrative supervision or technical supervision staff of provincial and municipal bureaus,who are part-time inspectors with frequent job rotation and insufficient stability of the team.During the process of inspection,because different dosage forms or product risk points are not the same,as well as different enterprises,different varieties,combined with information such as the product sample,auditing case,adverse reactions monitoring,it is vital to formulate a personalized scheme based on risk.If the inspection scheme cannot highlight the risk points and key elements,the pertinence and accuracy of the inspection will decrease,which inevitably leads to poor quality inspection.Therefore,it is necessary to establish the quality risk management system for GMP inspection.If there is no scientific evaluation of the quality of inspection,there will be no corresponding performance appraisal system.All these are important factors that restrict the internationalization development of drug supervision in China.
4 Suggestions for promoting China’s smooth accession to PIC/S
4.1 Promoting the system of laws and regulations that needs to be improved
PIC/S requires that the institutions of member states should have the appointment process of inspectors and stipulates the ethical standards that they should abide by.These should be improved before the formal application.Therefore,China should issue some relevant laws and documents as soon as possible.At present,the documents such as the evaluation of inspectors’ ability are not publicly available,but only for internal public use,lacking transparency,which is not conducive to the public’s understanding of information.Therefore,according to the evaluation requirements of PIC/S,we should promote the work of improving relevant laws and regulations in China.
4.2 Establishing GMP standard updating mechanism and promoting dynamic inspection and supervision
Under the new situation of drug supervision,dynamic and whole process supervision has become the primary task of the supervision department to replace the previous static supervision.Besides,the measures of in-process and post-supervision will replace the pre-approval.This is a manifestation of the perfection of China’s drug supervision,and the effect and strength of supervision are unprecedented.
After more than 30 years of development and improvement,China’s GMP inspection has conformed to the basic framework of PIC/S regulations,but dynamic supervision requires an updating mechanism for standards.With the application of regulatory science,scientific supervision also needs to be supported by a mechanism that is updated from time to time.Therefore,it is necessary to establish an update mechanism for China’s GMP standards and implementation contents.It not only updates the main documents in a timely manner,the qualification and responsibility constraints of inspectors should also be updated and changed.In addition,dynamic GMP management reform and innovation should be carried out,which requires the inspection center should focus on talents instead of facilities.Lastly,the on-site management should be improved.These measures can reduce the risks and resistance that China’s pharmaceutical enterprises may encounter in the process of international declaration,which not only meets the requirements and evaluation contents of PIC/S,but also conforms to FDA,EU and other requirements for production site inspection.
4.3 Improving the organization and management system of inspectors
PIC/S has many requirements for inspector management,including selection procedures,training,and inspection performance standards.Firstly,we should strengthen the restraint and management of inspector ethics,which is an important link.Then we can increase the performance assessment of inspectors,conducting performance evaluation of their work,and carrying out rectification,rewards and punishments.
A database link platform for national and provincial inspectors should be established,which can keep track of the number of inspectors accurately.Then,the inspector information can be managed according to drug categories.On the one hand,the platform can reasonably allocate inspection tasks and improve their inspection quality of inspectors.On the other hand,it can dynamically adjust the workload of each inspector,scientifically and reasonably use a limited number of inspectors to achieve the optimal allocation of resources.
An inspector training system that meets the requirements of PIC/S should be established,which can grade inspectors through training and assessment.Besides,differentiated salary and welfare mechanisms must be set up.NMPA will lead the establishment of a diversified exchange platform on which provinciallevel training can be shared to strengthen information exchanges.
4.4 Strengthening the construction of information technology
According to the requirements of PIC/S,it is necessary to establish a system-wide sharing mechanism of GMP inspection information to improve the informatization level comprehensively.Firstly,we should improve the database construction,manage the information in the database uniformly and adjust the database structure of compliance information.Meanwhile,the data of provincial supervision departments should be consistent with the national audit and inspection center,which makes the information comprehensive and updated timely.The retrieval function should be improved and increase the instruction of database.Besides,a flexible and convenient retrieval method based on needs should be offered.Lastly,a special archive should be established to uniformly manage the previous GMP inspection information for inquiry.
The informatized database can use visual methods to classify inspection types and inspection results,and it can conduct corresponding screening to form a standardized report format,which can be retrieved and queried freely.Therefore,the publication of inspection results should form a unified format to meet the requirements of PIC/S,which will facilitate the unified entry and archiving of the database.It can also facilitate the later risk management and evaluation.A drug safety credit file management module should be established in the database.Then a blacklist of dishonest enterprises will be formed to improve the credibility of supervision.
4.5 Establishing quality risk management mechanism for GMP inspection
According to the specifications of PIC/S,it is necessary to strengthen risk management and design the scheme according to the risk initiation mechanism.The regulatory department should formulate an annual inspection plan and determine the inspection frequency according to the risk.It should adjust the inspection scheme in time and send some units to comprehensively evaluate the inspection and rectification according to the principle of risk assessment.Strengthening the awareness of risk prevention and control should go through the whole process of inspection management.Therefore,it is of great practical significance to establish an inspection quality risk assessment system,which can control the quality in the whole process.
5 Conclusion
The mission of PIC/S is to lead and coordinate members to form unified GMP standards.Its functions are to formulate general GMP standards,provide training for inspectors of all members,and coordinate global inspection procedures.Mutual trust and recognition of international drug GMP inspection results will become a trend.China’s drug supervision has already approached to international standards and is preparing to become a member of PIC/S.In the preparation stage,it is necessary to establish a sound legal and regulatory foundation and complete the construction of China’s GMP inspection system as soon as possible.Only when China builds a scientific quality risk management system,it can meet the requirements of PIC/S and become a full member in the near future.
杂志排行
亚洲社会药学杂志的其它文章
- Supervision of Public Opinion under the Background of Social Co-governance-Take the Changsheng Vaccine Incident as an Example
- Analysis and Enlightenment of Big Data Platform for Adverse Drug Reaction Supervision in China and the United States
- An Empirical Study on Organizational Efficiency of Drug Review lnstitutions
- The Impact of Population Aging on the Expenditure of Medical Insurance Fund for Urban Workers in China
- Exploration and Research on the Integrated Development of “Internet Plus Medical Treatment”
- A Systematic Review of Patient-Reported Outcome Measurement for Psoriasis in Chinese Population
