Gene Editing Offers New Hope for Treating Huntington’s Disease
2023-09-09ByYANFushengStaffReporter
By YAN Fusheng (Staff Reporter)
On February 16, a new study published in the journal ofNature Biomedical Engineeringdemonstrated the potential of CRISPR/Cas9 technology to combat Huntington’s disease (HD), a devastating neurodegenerative disorder.
A team, spearheaded by scientists from Jinan University and the CAS Guangzhou Institutes of Biomedicine and Health, successfully uses CRISPR/Cas9 gene editing in HD pigs to reduce levels of the toxic mutant protein that causes brain cell death.Their studies showed that a single injection of the gene editing molecules into the brains or bloodstream of the HD pigs improved their movement and lifespan.

Gene editing injection rescues neuron loss and movement deficits in Huntington’s disease pigs.(A) The CRISPR/Cas9 gene editing system was injected into the brain to replace mutant HTT exon 1 with normal human HTT (hHTT).(B) Footprint analysis shows impaired gait in an HD pig model (right,untreated), compared to improved strides after treatment with AAV-delivered CRISPR/Cas9 (left, treated).Brain injection of the gene editing system rescued motor dysfunction in the HD pigs.(Credit: YAN et al., Nature Biomedical Engineering)
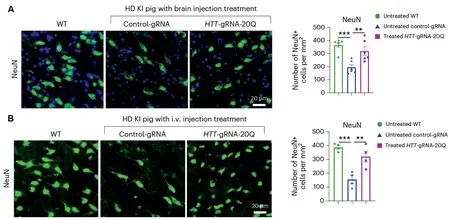
A single injection of the gene editing molecules into the brain (A) or bloodstream (B) mitigates the neuronal death occurring within the striatum—a brain region particularly affected by Huntington’s Disease (HD) – of genetically modified pigs that are engineered to mimic this disease.(Credit:YAN et al., Nature Biomedical Engineering)
HD is an inherited disorder caused by an abnormal expansion of DNA triplet repeats in the huntingtin gene (HTT), which leads to the production of mutant huntingtin protein that gradually impairs motor control and cognition.There are currently no effective treatments.
“We used AAV-mediated delivery of CRISPR/Cas9 to precisely cut out the expanded triplet repeats in the mutant huntingtin gene and replace them with a normal sequence,”explained Dr.LI Xiaojiang, one of the senior authors of the study.
In detail, the researchers first wrapped up the CRISPR/Cas9 system – essentially the “molecular scissors”that can cut out mutated DNA – into the adeno-associated virus that works as the Trojan horse.Then they injected them into HD knock-in pigs carrying the human mutant huntingtin gene.After cell entry,the CRISPR molecules were guided to the mutant DNA region and snipped it out.The cells’natural DNA repair system then used the injected normal DNA template to fix the cut, replacing the mutated gene sequence with a normal version.
Using genetically engineered pigs mimicking HD,they showed that delivering the CRISPR/Cas9 gene editing system through brain injection or intravenous infusion can both alleviate neurodegeneration in the striatum.Brain injection directly delivered the therapeutic molecules to the affected region, while intravenous infusion enabled the treatment to cross the blood-brain barrier and reach the striatum through circulation.
Strikingly, these one-time treatments dramatically reduced the toxic mutant protein levels in the pigs’brains and alleviated neurodegeneration.A single injection into the pigs’brains significantly improved their gait and motor coordination.Even more remarkably, a single intravenous (i.v.) injection in young pigs produced similar therapeutic benefits.
The researchers used adeno-associated virus serotype 9 (AAV9) to deliver the CRISPR/Cas9 components into the body and brain.As confirmed by the intravenous(i.v.) injection test, the AAV9 can cross the blood-brain barrier and spread through many tissue types, making it an ideal vehicle.The ability to rescue dying pig brain cells via intravenous infusion highlights the potential of harnessing gene therapy for neurodegenerative diseases.
The use of large animal models like pigs is an important step in validating new gene therapies for neurologic disease.Compared to non-human primate models, pigs are more distantly related to humans.However, pig models have the significant advantage of a faster breeding cycle (sexual maturation in 5~6 months) and large litter sizes (average 7~8 piglets per litter).Similar brain development and anatomy provide confidence that findings will translate to humans.
“In the future, monkey models could also be established using similar methods, but observing agerelated neurodegenerative symptoms through monkey models, and propagating them to observe symptoms in offspring, would involve a very long timeframe,”explained Dr.LAI Liangxue, one of the senior authors of the study.
While further research is still needed to ensure safety, using CRISPR-based strategies to lower mutant huntingtin expression holds great promise as a targeted therapy for this devastating disease.The use of a large animal model like pigs provides key insights into how this approach could translate to human patients.
