Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
2023-09-05ZhangGuomeiLiHanLiuShanshanZhouXumingLuMingyangTangLiangSunLihua
Zhang Guomei, Li Han, Liu Shanshan, Zhou Xuming, Lu Mingyang, Tang Liang,SunLihua
Research Paper
Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
Zhang Guomei#, Li Han#, Liu Shanshan, Zhou Xuming, Lu Mingyang, Tang Liang,SunLihua
(; These authors contributed equally to this work)
Ustiloxins are vital cyclopeptide mycotoxins originally isolated from rice false smut balls that form in rice spikelets infected by the fungal pathogen. The toxicity of the water extract of rice false smut balls (RBWE) remains to be investigated. Studies have shown that RBWE may be toxic to animals, but toxicological evidence is still lacking. In this study, we found that the IC50values of RBWE to BNL CL.2 cells at 24 and 48 h were 40.02 and 30.11 μg/mL, respectively, with positive correlations with dose toxicity and time toxicity. After treatment with RBWE, the number of BNL CL.2 cells decreased significantly, and the morphology of BNL CL.2 cells showed atrophy and wall detachment. RBWE induced DNA presynthesis phase arrest of BNL CL.2 cells, increased the proportion of apoptotic cells and inhibited cell proliferation. RBWE up-regulated reactive oxygen species (ROS) levels and lowered mitochondrial membrane potentials. Additionally, Western blot and qRT-PCR results suggested that RBWE exerted the above effects by promoting the Nrf2/HO-1 and caspase-induced apoptosis pathwaysand. The contents of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bile acids in the serum of mice from Institute of Cancer were significantly up-regulated by RBWE. At the same time, RBWE can lead to increases in ROS and malondialdehyde contents, decreases incontents of oxidized glutathione, glutathione and reduced glutathione, as well as decrease in catalase and superoxide dismutase activities in mouse liver tissues, demonstrating that oxidative stress occurred in mice. Moreover, liver damage was further detected by haematoxylin-eosin staining and electron microscopy to verify the damage to the mice caused by RBWE. In general, RBWE may cause hepatotoxicityandvia the apoptosis pathway, which provides a reference for hepatotoxicity and its mechanism of action.
water extract; rice false smut ball; ustiloxin; liver injury; Nrf2/HO-1 pathway; apoptosis pathway
Rice false smut (RFS) is a disease caused by the fungus(anamorph:Takahashi) (Wu et al, 2020). Due to the greenhouse effect and the overuse of nitrogen, RFS has been reported as an emerging and devastating disease in most rice-producing countries, including China, India, Japan and the USA(Wang et al, 2016)..uses rice as the principal host, invades the stamen filaments of rice at the booting stage and converts kernels into smut balls by hijacking the host’s nutrient transport system (Yu et al, 2021). In addition, RFS transforms individual grains of the panicle into large velvety spore balls(Andargie et al, 2018), and the appearance of the spore balls is primarily orange to green-yellow, ultimately turning green-black during grain maturation (Kim and Park, 2007).
Ustiloxins are the major phytotoxins and mycotoxins produced by the fungus, and rice can be easily infected by this fungus. Moreover, ustiloxins are mainly produced on false smut balls that infect rice grain and contaminate raw agricultural commodities as well as processed foods and feeds consumed by humans and livestock (Qiu et al, 2019; Xiong et al, 2020). Unfortunately, the occurrence of ustiloxins in rice grain and brown rice from regions affected by RFS disease was confirmed to be significantly high (Fu et al, 2018; Sun et al, 2021). Meanwhile, traces of ustiloxins could also be detected in rice sold on the market (Cao et al, 2016). Therefore, for food safety considerations, it is urgent and vital to explore the potential health risks and influence of ustiloxin exposure.
Evidence from a number of studies has shown that both ustiloxins and their crude extract have antimitotic activity in plants by inhibiting tubulin assembly on phytotoxicity (Ludueńa et al, 1994; Li et al, 1995), inducing plant cell malformation and preventing the formation of the cellular skeleton (Miyazaki et al, 2009; Cheng et al, 2019). Many recent studies have focused on yield loss from disease and cytotoxicity, and little is understood about the toxicity of ustiloxins and the water extract of rice false smut balls (RBWE) to animals, except for a few reports. Multiple organ damage caused by ustiloxins in animals has been found by using mice and zebrafish as models. For example, the male-biased hepatotoxicity of ustiloxin A was confirmed in mice(Sun et al, 2022) and in developmental toxicity experiments (Hu et al, 2019). In addition, RBWE can cause erosions and ulceration of the forestomach and atrophy of the thymus (Nakamura et al, 1994), and apoptosis is induced in the liver, kidney and urinary bladder of mice by RBWE (Nakamura et al, 1993).
In our study, RBWE could induce oxidative stress by increasing the reactive oxygen species (ROS) level. Oxidative stress is an imbalanced pathological state between oxidation and antioxidation and is always accompanied by the massive generation of oxidative metabolites (Choudhari et al, 2014). Moreover, oxidative stress can lead to cell cycle arrest and cell apoptosis, resulting in organ damage. The liver is a vital organ in the human body due to its significant metabolic and detoxification functions. Because of this, the liver is easily affected by foreign chemicals. Nrf2/HO-1 signalling, as an important defence system, plays a vital role in fighting the oxidative stress response against liver injury (Zou et al, 2021). Considering the above background, our aim was to investigate the potential health risks of RBWE intake, and a subchronic intake experiment in mice was conducted to evaluate the relationship of RBWE ingestion with liver damage and to explore whether RBWE has hepatotoxicity and preliminarily speculate on its mechanism of action.
RESULTS
Effects of different concentrations of RBWE on cell proliferation in BNL CL.2 cells
Cell Counting Kit-8 (CCK8) assays were performed to assess the effects of RBWE on cell proliferation and viability. BNL CL.2 cells were treated with different concentrations of RBWE (0, 5, 10, 20, 30, 40, 60, 80, 100, 150 and 200 μg/mL) for 24 and 48 h. The IC50values of BNL CL.2 cells treated with RBWE for 24 and 48 h were 40.02 and 30.11 μg/mL, respectively. As shown in Fig. 1-A, RBWE inhibited BNL CL.2 cells in a dose- and time-dependent manner. The cells of the normal groups were uniform in size and shape and neatly arranged. However, in the treatment groups, some changes in cell morphology occurred with increasing drug concentrations. The apoptotic cells shrank, decreased, and separated from the surrounding cells (Fig. 1-B). While the chromatin in the nuclei was highly coiled, many vacuolizations appeared and were called cavitation structures.

Fig. 1. Effects of different concentrations of water extract of rice false smut balls (RBWE) on cell proliferation in BNL CL.2 cells.
A, Cell viability was measured by Cell Counting Kit-8 (CCK8) assay. Data are Mean ± SE (= 3). B, Cell morphology changes at 24 h after RBWE treatment. Scale bars, 100 μm. The red arrows indicate vascular degeneration in cells. C, Morphological changes in BNL CL.2 cells were observed under a scanning electron microscope. Scale bars, 10 μm.
BNL CL.2 cell morphology was observed by scanning electron microscopy (SEM)
To explore the apoptotic function of RBWE-treated cells, SEM was performed to observe the cell surfaces. As shown in Fig. 1-C, the cells in the normal group were neatly arranged with microvilli on the surface. In the RBWE treatment groups, SEM revealed that RBWE induced apoptotic changes in a significant number of BNL CL.2 cells, villi disappeared, cell membrane damaged and apoptotic bodies appeared.
Total transcriptomic analysis of BNL CL.2 cells induced by RBWE
The transcriptomic results showed that 1 099 genes were differentially expressed after induction by RBWE, of which 473 differentially expressed genes (DEGs) were up-regulated and 626 DEGs were down-regulated. In addition, Gene Ontology analysis revealed that these DEGs were mainly involved in DNA binding, cellular process, single-organism process and organelle (Fig. S1-A and -C). Kyoto Encyclopedia of Genes and Genomes analysis showed that these DEGs were enriched in DNA replication, cell cycle, IL-17 signalling pathway, and so on (Fig. S1-B and -D).
BNL CL.2 cell apoptosis was investigated by DNA ladder assay, cell cycle assay and mitochondrial membrane potential assay
As shown in Fig. S2, DNA ladder bands were observed on an agarose gel through a DNA ladder assay, which indicates internucleosomal cleavage of DNA and cell apoptosis. The histogram of the cell cycle distribution of the BNL CL.2 cells is shown in Fig. 2-A. Particularly in the cells treated with the highest concentration of RBWE, the signal of the DNA presynthesis (G0/G1) phase was highly intensive, while the signal intensity of DNA synthesis (S) and DNA late synthesis (G2) phases had decreased. Compared with the control group, RBWE markedly increased the quantity of cells in the G0/G1 phase, indicating that RBWE could induce cell apoptosis and G0/G1 phase arrest. The proportion of cells in apoptosis is also shown in Fig. 2-A. Compared with the normal group, the proportion of cells in apoptosis increased with increasing RBWE concentrations (Fig. 2-B). We then conducted a JC-1 assay to determine the mitochondrial membrane potential (Δψm) via flow cytometry. Our results indicated a decrease in Δψm compared with the control (Fig. S2-C).
Effects of different concentrations of RBWE on ROS content in BNL CL.2 cells
An ROS ELISA Kit was used to examine intracellular ROS levels. There were significant differences in ROS levels after treatment with different concentrations of RBWE for 24 h compared with the control (Fig. 3). Meanwhile, cells exposed to 5, 10 and 20 μg/mL RBWE had higher ROS levels than the control cells. The results suggested that RBWE could induce excess intracellular ROS and cause oxidative stress in BNL CL.2 cells in a dose-dependent manner.
Effects of RBWE on body weight, relative organ weight and liver injury of mice
After a three-month toxicity experiment, we observed the body weight of the mice from Institute of Cancer Research (ICR). Compared with the control group, the weights of the RBWE-treated ICR mice were significantly reduced (Fig. 4-A). The liver weights and the relative organ weights had similar trends, and the results showed significant decreases in 2.5 g/kg RBWE groups (Fig. 4-B and -C).

Fig. 2. BNL CL.2 cell apoptosis was investigated by cell cycle assay and mitochondrial membrane potential assay.
A, Histogram of cell cycle. G2, DNA late synthesis phase; S, DNA synthesis phase; G0/G1, DNA presynthesis phase. B, Histogram of cell apoptosis at 24 h after water extract of rice false smut balls (RBWE) treatment.
Data are Mean ± SE (= 3). **,< 0.01.

Fig. 3. Effects of water extract of rice false smut balls (RBWE) on reactive oxygen species (ROS) content in BNL CL.2 cells.
Data are Mean ± SE (= 3). *,< 0.05; **,< 0.01.
The effects of RBWE-induced liver histological abnormalities were examined by haematoxylin-eosin staining. The morphology of hepatocytes was normal, with intact structures and intact nuclear membranes (Fig. 4-D). In contrast, different concentrations of RBWE caused cytoplasmic vacuolation and cytoplasmic and nuclear blebbing. The RBWE treatment group showed obvious morphological changes, obvious swelling, loose cytoplasm, and occasional binuclear and diffuse light staining. Compared with the control group, nuclear pyknosis was clearly observed in the RBWE group, which further indicated that RBWE induced liver injury.
Effects of RBWE on alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bile acid (TBA) contents in mouse serum
Although the liver sections suggested liver injury, more evidence in clinical markers was necessary to further support this judgement. The intragastric administration of RBWE increased the serum levels of ALT, AST, ALP and TBA (Fig. 4-E and -F). Compared with the control group, the serum ALT and AST levels in 1.0 and 2.5 g/kg RBWE groups were significantly increased. However, only 2.5 g/kg RBWE group was significantly higher than the normal group for ALP and TBA contents, which indicated RBWE-induced liver injury.

Fig. 4. Water extract of rice false smut balls (RBWE)-induced changes in livers of mice.
A, Body weight curve. B, Liver weight. C, Liver weight to body weight ratio. D, Histopathological examination of liver by haematoxylin-eosin staining. Typical sections of the hepatic central venous region in mice. Scale bars, 10 μm.E, Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels. F, Total bile acid (TBA) concentration.
*,< 0.05; **,< 0.01. Values are Mean ± SE (= 12).
Effects of RBWE on indices of oxidative stress in mouse liver issues
The levels of ROS and malondialdehyde (MDA) and the activities of glutathione (GSH), oxidized glutathione (GSSG), reduced glutathione (GSH-Px), superoxide dismutase (SOD) and catalase (CAT) in the liver tissues are shown in Fig. 5. Consistent with the results of the cell experiments, the amounts of ROS in the liver tissues of the toxin treatment groups were increased in a dose-dependent manner compared with normal mice. Moreover, other factors of oxidative stress were evaluated in this experiment. Lipid peroxidation is a crucial parameter for measuring oxidative stress (Chowdhury et al, 2022). MDA is known as a byproduct of lipid peroxidation, which was increased significantly (< 0.01) in 2.5 g/kg RBWE group compared with the control. RBWE- treated groups showed decreased antioxidant enzyme activities in tissues. Compared with the control group, SOD and CAT activities were significantly decreased in the livers of the RBWE-treated groups (< 0.01). Moreover, GSH, GSSG and GSH-Px levels were also significantly decreased in the livers of RBWE-treated mice (< 0.01).

Fig. 5. Water extract of rice false smut balls (RBWE)-induced changes in oxidative stress indices in mouse livers.
ROS, Reactive oxygen species; MDA, Malondialdehyde; SOD, Superoxide dismutase; CAT, Catalase; GSH, Glutathione; GSSG, Oxidized glutathione; GSH-Px, Reduced glutathione.
*,< 0.05; **,< 0.01. Values are Mean ± SE (= 8).
Effects of RBWE on expression of Nrf2/HO-1 pathway-related proteins and genes
The protein expression and mRNA levels related to the oxidative stress pathway were then examined by Western blot and qRT-PCR, respectively. Nrf2 protein levels were obviously decreased when the RBWE concentrations were 10 and 20 μg/mL (Fig. 6-A and -B,< 0.01). As shown in Fig. 6-C, compared with the control group, the mRNA expression ofshowed a decreasing trend with RBWE content. The Haem oxygenase-1 (HO-1) protein level was significantly increased in 10 and 20 μg/mL groups (Fig. 6-B,< 0.01). Compared with the control group, qRT-PCR analysis showed that the mRNA expression ofin the RBWE group was markedly increased in a dose- dependent manner (Fig. 6-D,< 0.01). Meanwhile, in mouse livers, RBWE treatment promoted Nrf2 and HO-1 protein levels andandmRNA expression with increasing RBWE concentrations (Fig. 6-E to -H). To clarify the relationship between the Nrf2/HO-1 signalling pathway and hepatotoxicity caused by RBWE, Nrf2 protein expression levels in the nucleus were subsequently detected (Fig. 6-I to -L). Western blot experiments showed that the RBWE group had significantly increased intracellular protein expression of Nrf2 compared with the control group. Similar results were observed in liver tissue experiments, and the expression of Nrf2 was significantly higher in the liver tissue of mice in the RBWE groups than in the control group.
The protein levels of apoptotic marker genes were analyzed by Western blot (Fig. 7-A to -I). The data revealed that the expression levels of Bax, Caspase-9, cleaved Caspase-9, cleaved Caspase-3 and Caspase-3 were up-regulated significantly by RBWE in a dose- dependent manner, while the expression levels of Poly ADP-ribose polymerase (PARP) and Bcl-2 showed decreasing trends, leading to a decrease in the ratio of Bcl-2/Bax in a dose-dependent manner. To verify the results, we detected the mRNA expression levels of theandgenes by qRT-PCR. The results are presented in Fig. 7-J to -L. Compared with the control group, the mRNA expression levels of/were significantly decreased (< 0.01), consistent with the Western blot results.
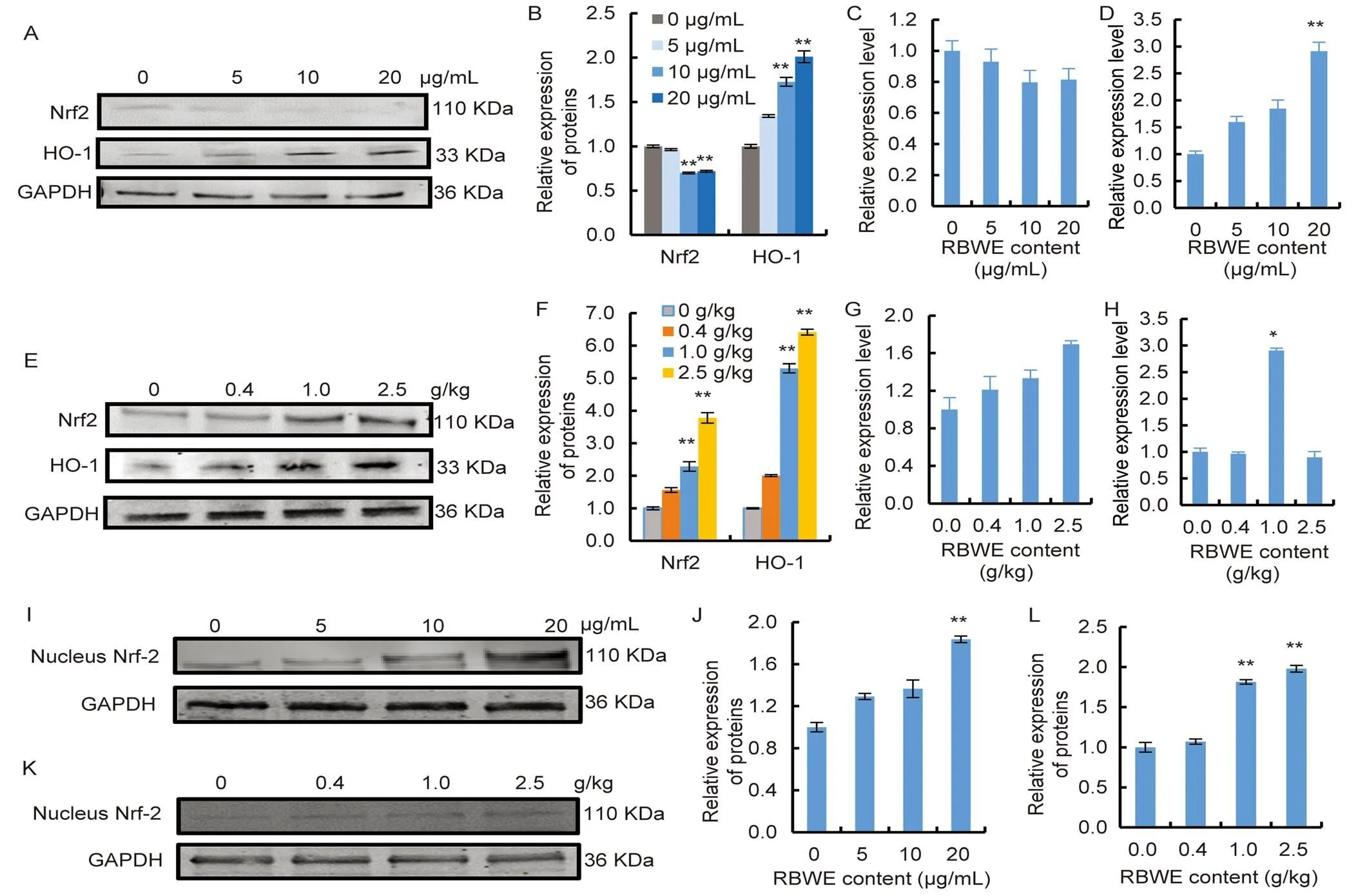
Fig. 6. Water extract of rice false smut balls (RBWE) affected oxidative stress-related proteins and genes.
A and B, Nrf2 and Haem oxygenase-1 (HO-1) protein levels in BNL CL.2 cells. C,mRNA expression in BNL CL.2 cells. D,mRNA expression in BNL CL.2 cells. E and F, Nrf2 and HO-1 protein levels in mouse livers. G,mRNA expression in mouse livers. H,mRNA expression in mouse livers. I–L, Nuclear Nrf2 protein level. I and J, Nuclear Nrf2 protein level in BNL CL.2 cells. K and L, Nuclear Nrf2 protein level in mouse livers.
*,< 0.05; **,< 0.01. Values are Mean ± SE (= 3).

Fig. 7. Expression of apoptosis- and pathway-related proteins and genes in BNL CL.2 cells.
A, Expression levels of apoptotic factors in BNL CL.2 cells assessed by Western blot analysis. B, Content of Bcl-2. C, Content of Bax. D, Bcl-2/Bax relative intensity. E, Content of Caspase 3. F, Content of cleaved Caspase 3. G, Content of poly ADP-ribose polymerase (PARP). H, Content of Caspase 9. I, Content of cleaved Caspase 9. J–L, mRNA expression levels of(J),(K) and/(L) genes by qRT-PCR.
*,< 0.05; **,< 0.01. Values are Mean ± SE (= 3).
DISCUSSION
This study explored the hepatotoxicity of RBWEand. The hepatotoxicity of RBWE in BNL CL.2 cells was assessed by the CCK8 assays. The results showed that RBWE induced dose- and time-dependent cytotoxic effects in BNL CL.2 cells. We further observed the cell morphology by microscopy and SEM. The results showed that the cells had different degrees of morphological changes and cell membrane damages, and some apoptotic bodies appeared. After the antiproliferative activity of RBWE on BNL CL.2 cells was determined, further investigation into the potential mechanism was conducted. Total transcriptome analysis was performed on normal cells and RBWE-treated cells, suggesting that cell damage induced by RBWE was highly correlated with the cell cycle and DNA damage. Subsequently, a series of experiments were performed to further validate the transcriptome results.
The changes in the cell cycle were measured, and the results of flow cytometry showed significant differences compared with the control cells. The cell cycle of the treated BNL CL.2 cells was arrested, with a significant accumulation of cells at the G0/G1 phase and substantial declines in the cell population at the S and G2 phases. The disrupted cell cycle of the BNL CL.2 cells after treatment with RBWE strongly indicated that cell apoptosis may have occurred. Similar findings were observed by Tanaka et al (2003) and Wang et al (2017), who showed that RBWE has the same effect in plant cells, inhibiting their mitosis by influencing their cell cycle.
Following the test of cell cycle arrest, the mitochondrial membrane potential was measured to determine whether RBWE affected the mitochondrial function of the treated BNL CL.2 cells. Mitochondria play a necessary role in cell energy metabolism, and numerous studies have shown that mitochondria have become essential target sites for toxicity-induced hepatotoxicity (Ramachandran et al, 2018). The significant decrease in mitochondrial membrane potential is the result of the opening of the mitochondrial permeability transition pore and could subsequently lead to cell apoptosis. Therefore, the potential apoptosis of the treated BNL CL.2 cells was determined by JC-1, which is one of the most commonly used methods to detect cell apoptosis. After 24 h of treatment with RBWE, a large number of apoptotic cells were observed, even at a reduced treatment concentration. Subsequently, the ROS content in the cells was detected, which was significantly higher in RBWE-treated group than in the control group. ROS may be the intermediate medium in the process of apoptosis. ROS functions as redox messengers in intracellular signalling and regulation, wherein their levels are tightly regulated (Chen et al, 2018). Excessive ROS accumulation in cells may cause mitochondrial dysfunction and biomolecule oxidation, leading to changes in cytoskeletal organization and cell morphology and apoptosis (Zhang et al, 2021), and activating PARP and cysteine cascade. DNA fragmentation is one of the most specific markers of apoptosis. During cell apoptosis process, DNA is cleaved by DNA fragmentation factor (DFF) (Liu et al, 1997). One crucial factor in DFF activation is the caspase family. DFF cleaves nuclear DNA into internucleosomal fragments approximately 180 bp in size and multiples thereof (Majtnerová and Roušar, 2018). In our experiment, the DNA laddering pattern was observed.
Meanwhile, to investigate the hepatotoxicity of RBWE, ICR mice were given RBWE (0.4, 1.0 and 2.5 g/kg) by intragastric administration for 15 weeks. The liver is the most important metabolic organ in the body. Moreover, histopathological changes in the mouse livers were evident, including hepatocellular enlargement. Usually, AST, ALT, ALP and TBA are the most accepted biomarkers used in biochemical tests to predict liver damage. We found that the contents of AST, ALT, ALP and TBA in RBWE- treated ICR mice showed increasing trends, which indicated that RBWE exhibited a hepatotoxic effect on mice. Overall, RBWE can induce hepatotoxicity.
Based on certain cell experiments, this study hypothesized that ROS-induced oxidative stress and lipid peroxidation are significant contributors to RBWE-induced tissue damage in the liver. To further explore the injury effect of RBWE on mouse liver, ROS content and other related oxidative stress indices in mouse livers were detected. Oxidative stress is a condition in which the balance between ROS production and the antioxidant defence system is disrupted(Yang Q Y et al, 2022). The increased generation of ROS always activates the antioxidant systems (MDA, GSH, SOD, etc.) (Bhattacharjee et al, 2020), which facilitates the progression of lipid peroxidation and the rapid overproduction of MDA. At the same time, the activities of SOD and CAT (antioxidant system defence enzymes) and the concentrations of GSH and GSSG (antioxidants against cell damage), which are important indicators of the degree of oxidative stress, are also decreased significantly (Liu et al, 2022). GSH-Px is a critical enzyme that can protect cells from oxidative stress (Fan et al, 2021). In the present study, we found that RBWE induced oxidative stress in liver tissues by decreasing GSH, GSSG, GSH-Px, SOD and CAT activities with subsequent increases in ROS and MDA levels. The above data indicated that RBWE caused liver tissue damage through oxidative stress.
Nrf2/HO-1 plays an important role in oxidative stress injury, and haem degradation catalyzed by Nrf2/HO-1 generates numerous components that are critical to constitute an endogenous protective system against oxidative stress injury (Basu et al, 2016). Nrf2 is a transcription factor involved in regulating the balance of cellular redox in mammals (Gur et al, 2022). Nrf2 and its downstream genes play a critical role in the antioxidant system (Muhammad et al, 2018).is one of the genes regulated through, and the activity of its products has been shown to regulate key biological processes such as cell proliferation, inflammation and apoptosis (Hirao et al, 2020). Chen et al (2019) found that reduced Nrf-2 expression may increase ROS generation and oxidative stress damage to the liver. Similarly, in the cell experiments, the protein and mRNA expression levels of NRF2 were inhibited. However, we found that RBWE enhanced Nrf2 expression and increased the expression of HO-1 in the liver bystudy, which may be related to the protective response to oxidative stress damage. Nrf2 is sequestered in the cytoplasm under physiological unstressed conditions. Once oxidative stress occurs in cells, Nrf2 translocates into the nucleus, interacts with the antioxidant response element, and then activates the expression of antioxidant enzyme genes, including(Zhang et al, 2022). Therefore, the expression levels of Nrf2 in the nucleus are important. According to the protein expression levels of Nrf2 in the nucleusand, RBWE increased the expression of Nrf2 in the nucleus, and the nuclear translocation of Nrf2 was affected. In this study, abnormal expression of Nrf2 and HO-1 revealed that RBWE influenced antioxidative stress- related proteins or genes to promote oxidative damage. Hence, the Nrf2/HO-1 signalling pathway probably plays an important role in the occurrence of oxidative stress injury in mouse livers induced by chronic RBWE exposure.
Oxidative stress can lead to cell cycle arrest and cell apoptosis, resulting in organ damage. Cell apoptosis has been recognized as a kind of programmed cell death that plays an essential role in the genesis of a number of pathological processes (D’Arcy, 2019). Apoptosis is induced by various physical or chemical stimuli, such as ROS (Yang X et al, 2022). Specifically, Bcl-2 is an antiapoptotic protein, and Bax is a water-soluble related protein homologous to Bcl-2 and is an apoptosis-promoting gene in thegene family. Over-expression ofcan antagonize the protective effect of Bcl-2 and cause cell death. The combined activity of Bcl-2 and Bax coordinates apoptosis and activates the caspase family by stimulating mitochondrial membrane penetration. Different caspases have different specificities involving the recognition of neighbouring amino acids, and members of the caspase family that participate in apoptosis can be roughly categorized into initiator caspases (caspases 2, 8, 9 and 10) and executioner caspases (caspases 3, 6 and 7) (Cohen, 1997; Elmore, 2007). Normally, once caspases are initially activated in apoptotic cells, initiator caspases trigger executioner caspases (Peng et al, 2022), and there seems to be an irreversible commitment towards cell death. PARP is a multifunctional protein translation- modified enzyme that exists in most eukaryotes. It is activated by identifying DNA fragments of structural damage and is considered to be the receptor for DNA damage. At the same time, PARP is the cutting substrate of caspase, the core member of apoptosis, and caspase can also hydrolyse 113 kDa PARP into two fragments of 89 and 24 kDa. Moreover, Western blot analysis showed that Bax, cleaved Caspase-3, cleaved Caspase-9, Caspase-3 and Caspase-9 expression levels showed increasing trends, while the expression levels of Bcl-2 and PARP showed decreasing trends. Therefore, in theandexperiments, the promoter Caspase 9, the executor Caspase 3 and Bax were activated, and the Bcl-2 and PARP proteins were cleaved, indicating that apoptosis occurred.
In our study, RBWE caused increases in ROS and oxidative stress and then activated the Nrf2/HO-1 pathway, caspase family proteins and PARP to induce apoptosis and liver damage. In conclusion, the present study showed that RBWE can induce liver injuryand. Potential targets may include mitochondrial damage and cell apoptosis. However, the details of the molecular mechanism of RBWE damage remain unknown, attesting to the need for further exploration.
METHODS
Materials, antibodies and reagents
According to the cyclic peptide structure and water-soluble characteristics of the ustiloxins, we extracted and purified ustiloxins to obtain RBWE for the experiment. The specific methods were as follows: first, the rice false smut balls were fully ground in water, and the ratio of water to rice false smut balls was 5:1. This was followed by a 35 ºC water bath shock for 10 h. The liquid was centrifuged at 4 000 r/min for 15 min and then 12 000 r/min for 15 min. The supernatant was then collected, and the liquid was dried by a freeze-drying machine for 5 d to obtain the crude extract. Then, the crude extract was dissolved in water, and impurities were purified by fine resin filler filtration. The eluent was eluted with 20% methanol, and then freeze-dried again to obtain the RBWE for the experiment.
A CCK8 and a cell cycle assay kit were provided by Beyotime (Shanghai, China). A nuclear protein extraction kit was provided by Thermo Fisher (Massachusetts, USA). A mitochondrial membrane potential assay kit with JC-1 was provided by Absin (Shanghai, China). ROS, MDA, SOD, CAT, GSSG, GSH and GSH-PX ELISA kits were provided by MEIMIAN (Nanjing, China). ALT, AST, ALP and TBA assay kits were provided by PUREBIO (Ningbo, China). A bicinchoninic acid (BCA) protein assay kit, RIPA lysates, a GoldBand Plus 3-colour regular range protein marker, YeaRed Nucleic Acid Gel Stain and DNA ladder were provided by YEASEN (Shanghai, China). Antibodies against Nrf2, HO-1, PARP, Bcl-2, Bax, Caspase-3, Caspase-9, cleaved Caspase-3, cleaved Caspase-9, GAPDH and β-actin were provided by BOSTER (Wuhan, China).
Cell culture and animals
The BNL CL.2 cell line was maintained in dulbecco’s modified eagle medium with 10% foetal bovine serum and 1% penicillin-streptomycin (1000 U/mL and 10 mg/mL) at 37 ºC and 5% CO2in an incubator. Cells in the exponential growth phase were used for experiments.
ICR mice [total 48, specific pathogen free, male, weight (20 ± 2) g, 4-week-old] were purchased from the Animal Center, Zhejiang Academy of Medical Sciences, China. The mice were given free access to drinking water and kept in a polyethylene box. The laboratory conditions were a 12 h light/12 h dark cycle at room temperature (23 ºC–25 ºC) and a humidity of 40%–60%. After one week of acclimatization, the mice were randomly assigned to four groups (= 12 in each group): i) control group: the mice were given intragastric administration of deionized water; and ii) RBWE intervention groups: the mice were exposed to RBWE (0.4, 1.0 and 2.5 g/kg). The above process was performed daily for 15 weeks.
Cell viability assay
Cell viability was quantitated by CCK8 assay. Cells were seeded in 96-well plates at a density of 5 × 103cells per well. Media containing different concentrations of RBWE (0–200 μg/mL) were added to the plate, with 6 replicate wells in each group. The cells were incubated at 37 ºC for 24 and 48 h. Then, 10 µL of CCK8 (Beyotime, Shanghai, China) was added to each sample. The plates were incubated at 37 ºC for 1 h, and the absorbance was then read at 450 nm. The viability of untreated cells was arbitrarily set to 100%, and cell viability was expressed as a percentage of CCK8 reduction. The IC50values were defined as the concentration of drugs that inhibited cell viability in 50% of the control cells. The half-inhibitory concentration was determined using GraphPad Prism 8 software.
Ultrastructural examination
The BNL CL.2 cells were incubated with RBWE (0 and 20 μg/mL) for 24 h. Then, the medium was discarded, the electron microscope fixation solution was quickly added without rinsing, and the cells were scraped off gently and collected in a centrifuge tube. The fixation solution was discarded, and a new electron microscope fixation solution was added to fix the cells at 4 ºC for storage and detection. After being dehydrated and sprayed with gold, the cells were observed by SEM.
Nanopore long-read RNA-Seq analysis of BNL CL.2 cells
RNA-Seq analysis method is described in File S1.
DNA ladder assay
The cells were transferred to a 6-well plate during the toxicity test for the apoptosis experiment. RBWE were set at 3 different concentrations (5, 10 and 20 μg/mL), and cultured in toxin-free medium as a control. After 24 h of culture, the cells (including the cells suspended in the medium without walls) were collected and washed with phosphate buffer saline (PBS), and DNA was extracted with an apoptosis DNA ladder extraction kit (centrifugal column) for DNA ladder detection. The extracted DNA was banded on a 1.0% agarose gel with l μg/mL YeaRed (100 V, 50 min) and on a gel imager.
Cell cycle assay
BNL CL.2 cells (2 × 105cells/well) were cultured in 6-well plates and treated with RBWE (0, 5, 10 and 20 μg/mL) for 24 h. After the treatment, the cells were collected and washed with cold PBS and mixed with cold 70% ethanol at 4 ºC overnight. The cells were washed with cold PBS three times and then stained with 500 μL propidium iodide for 20 min in the dark at room temperature. Finally, cell cycle analysis was performed using a flow cytometry.
Mitochondrial membrane potential assay
For each well of the 6-well plate, the culture medium was aspirated, and 1 mL of cell culture medium was added. The cell culture medium might have contained serum and phenolic red. JC-1 dye solution (1 mL) was added and mixed thoroughly. The cells were incubated for 20 min at 37 ºC. During incubation, an appropriate amount of JC-1 staining buffer (1×) was prepared at a ratio of 4 mL distilled water for every 1 mL JC-1 staining buffer (5×) and placed in an ice bath. After incubation at 37 ºC, the supernatant was aspirated and washed twice with JC-1 staining buffer (1×). Two millilitres of cell culture medium was added, which might have contained serum and phenolic red. Observation occurred under a fluorescence microscope or a laser confocal microscope.
ROS measurement of cells
A total of 1 × 106cells per well were inoculated in a 6-well plate. After treatment with different concentrations of drug media for 24 h, the extract was added and centrifuged to collect the supernatant. The assay was performed according to the kit instructions and incubated at 37 ºC for 10 min. The blank well was set to zero, the absorbance of each well was measured in sequence at 450 nm wavelength, and the reads were performed within 15 min after adding the stop solution.
Detection of oxidative stress index in livers
After the mice were sacrificed, liver tissue was quickly removed, flash-frozen in liquid nitrogen, and stored in a -80 ºC freezer. The activities of CAT and SOD and the contents of GSH, GSSG, GSH-PX, ROS and MDA were measured using kits according to the manufacturers’ instructions to evaluate the antioxidant capacity. The absorbance was measured at a wavelength of 450 nm using a microplate reader, and the concentrations of the above indicators in the samples were calculated from standard curves.
Western blot analysis
After BNL CL.2 cell and mouse liver interventions, total protein was extracted by Radio Immunoprecipitation Assay Lysis buffer. Nuclear proteins were extracted using a nuclear protein extraction kit (Thermo Fisher Scientific, Massachusetts, USA) according to the manufacturer’s instructions.
The protein concentrations were determined by the BCA method. All protein samples were diluted to the same concentration with cell lysis buffer. Finally, the protein samples were mixed with loading buffer (5×) at a ratio of 4:1 and boiled in a metal bath at 100 ºC for 8 min. After denaturation, the protein samples were centrifuged to separate the supernatant and stored at -20 ºC.
Total protein was separated by SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. The membranes were blocked with 2.5% nonfat milk and 2.5% Bovine Serum Albumin for 1 h. Then, every membrane was incubated with primary antibodies against PARP, Caspase-3, Caspase-9, Bax, β-actin, GAPDH and Bcl-2 overnight at 4 ºC. Next, the cells were washed with Tris- buffered Saline with Tween 20 for 10 min with three times and incubated with secondary antibody for 1 h at room temperature. The infrared scanning system Odyssey was used to detect the target protein bands. β-actin and GAPDH were used as the internal reference. The optical density integral value was used to analyze the target protein bands.
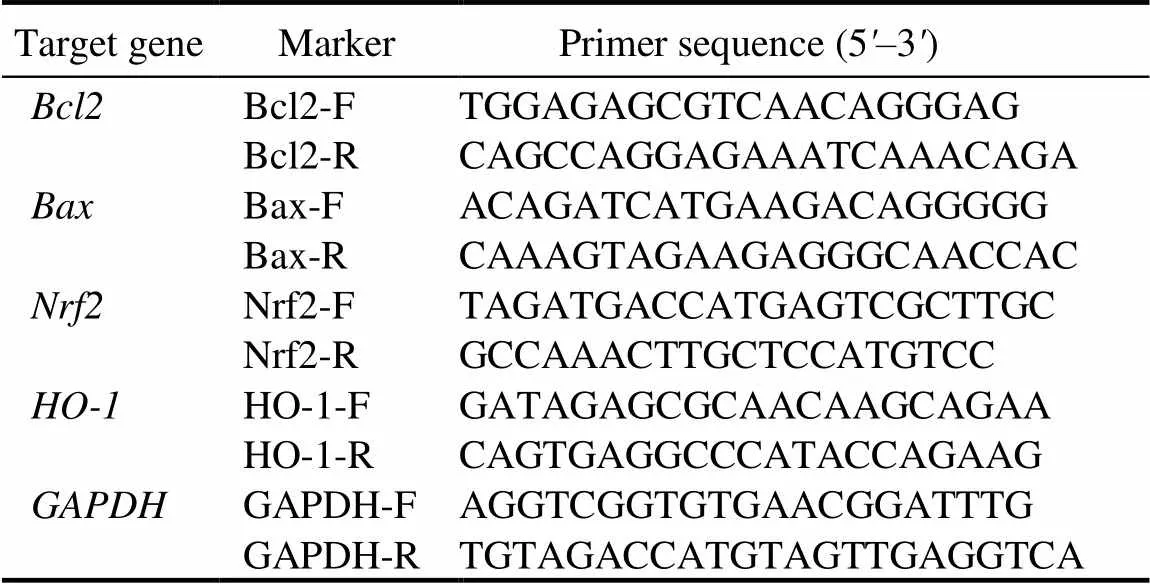
Table 1. Specific sequences of PCR primers.
qRT-PCR
Total RNA was isolated using TRIzol®reagent (TaKaRa, Kyoto, Japan). RNA was reverse transcribed into cDNA by a Prime Script RT Reagent Kit (TaKaRa, Kyoto, Japan) and analyzed by qRT-PCR. qRT-PCR was performed using a Bio-Rad CFX Connect Real-Time System (Bio-Rad Laboratories, California, USA). The conditions were 95 ºC for 10 min, 95 ºC for 15 s, and 40 cycles of 60 ºC for 60 s. The primers used for qRT-PCR were designed with online NCBI Primer Designing software.was used to normalize the qRT-PCR data through the 2-ΔΔCtmethod, and figures were shown as fold changes of target genes to the invariant control gene. The fold change of the gene expression of the treatment group was normalized to that of the control group. The specific sequences of the qRT-PCR primers are listed in Table 1.
Blood supernatant analysis
After having completed 15 weeks of treatment, mice were anaesthetized and sacrificed. Then, the blood samples were collected from the retrobulbar vein. The blood was placed in a 37 ºC water bath for 10 min. The serum was obtained via centrifugation at 3 500 ×for 15 min and stored at -80 ºC. For the detection of ALT, AST, ALP and TBA, kits were purchased from Ningbo Purebio Biotechnology, Ningbo, China.
Haematoxylin-eosin staining
The liver tissues were collected and fixed with buffered 4% formalin for 2 d. All the samples were sectioned at a thickness of 5 µm and stained with haematoxylin and eosin for histopathologic observation by a light microscopy (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data were presented as the mean ± standard deviation for at least three independent experiments. The data were analyzed by one-way analysis of variance (ANOVA) using GraphPad Prism software version 8.0 to compare the control group with RBWE-treated groups. Values of< 0.05 and< 0.01 were considered statistically significant.
ACKNOWLEDGEMENT
This study was funded by the Education Department of Zhejiang Province Foundation of China (Grant No. Y202249221).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
File S1. RNA-Seq analysis method.
Fig. S1. Bar charts and dot plots of the main Gene Ontology terms and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis.
Fig. S2. BNL CL.2 cell apoptosis was investigated by DNA ladder assay, cell cycle assay and mitochondrial membrane potential assay.
Andargie M, Li L Y, Feng A Q, Zhu X Y, Li J X. 2018. Mapping of the quantitative trait locus (QTL) conferring resistance to rice false smut disease., 15: 38–43.
Basu A, Singha Roy S, Bhattacharjee A, Bhuniya A, Baral R, Biswas J, Bhattacharya S. 2016. Vanadium(III)-l-cysteine protects cisplatin-induced nephropathy through activation of Nrf2/HO-1 pathway., 50(1): 39–55.
Bhattacharjee P, Borah A, Das S. 2020. Quercetin-induced amelioration of deltamethrin stress in freshwater teleost,: Multiple biomarker analysis., 227: 108626.
Cao Z Y, Sun L H, Mou R X, Lin X Y, Zhou R, Ma Y N, Chen M X. 2016. Analysis of ustiloxins in rice using polymer cation exchange cleanup followed by liquid chromatography-tandem mass spectrometry., 1476: 46–52.
Chen Y G, Liu K L, Shi Y F, Shao C S. 2018. The tango of ROS and p53 in tissue stem cells., 25(4): 639–641.
Chen Y Y, He L Y, Yang Y Y, Chen Y, Song Y R, Lu X, Liang Y M. 2019. The inhibition of Nrf2 accelerates renal lipid deposition through suppressing the ACSL1 expression in obesity-related nephropathy., 41(1): 821–831.
Cheng S Y, Liu H, Sun Q, Kong R, Letcher R J, Liu C S. 2019. Occurrence of the fungus mycotoxin, ustiloxin A, in surface waters of paddy fields in Enshi, Hubei, China, and toxicity in., 251: 901–909.
Choudhari S K, Chaudhary M, Gadbail A R, Sharma A, Tekade S. 2014. Oxidative and antioxidative mechanisms in oral cancer and precancer: A review., 50(1): 10–18.
Chowdhury F I, Yasmin T, Akter R, Islam M N, Hossain M M, Khan F, Aldhahrani A, Soliman M M, Subhan N, Haque M A, Alam M A. 2022. Resveratrol treatment modulates several antioxidantand anti-inflammatory genes expression and ameliorated oxidative stress mediated fibrosis in the kidneys of high-fat diet-fed rats., 30(10): 1454–1463.
Cohen G M. 1997. Caspases: The executioners of apoptosis., 326: 1–16.
D’Arcy M S. 2019. Cell death: A review of the major forms of apoptosis, necrosis and autophagy., 43(6): 582–592.
Elmore S. 2007. Apoptosis: A review of programmed cell death., 35(4): 495–516.
Fan R, Sui J D, Dong X P, Jing B, Gao Z Z. 2021. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis., 173: 29–40.
Fu X X, Wang W X, Li Y Y, Wang X H, Tan G Y, Lai D W, Wang M A, Zhou L G, Wang B M. 2018. Development of a monoclonal antibody with equal reactivity to ustiloxins A and B for quantification of main cyclopeptide mycotoxins in rice samples., 92: 201–207.
Gur C, Kandemir F M, Caglayan C, Satici E. 2022. Chemopreventive effects of hesperidin against paclitaxel-induced hepatotoxicity and nephrotoxicity via amendment of Nrf2/HO-1 and caspase-3/ Bax/Bcl-2 signaling pathways., 365: 110073.
Hirao H, Dery K J, Kageyama S, Nakamura K, Kupiec-Weglinski J W. 2020. Heme Oxygenase-1 in liver transplant ischemia- reperfusion injury: From bench-to-bedside., 157: 75–82.
Hu Z, Dang Y, Liu C S, Zhou L G, Liu H. 2019. Acute exposure to ustiloxin A affects growth and development of early life zebrafish,., 226: 851–857.
Kim K W, Park E W. 2007. Ultrastructure of spined conidia and hyphae of the rice false smut fungus., 38(6): 626–631.
Li Y, Koiso Y, Kobayashi H, Hashimoto Y, Iwasaki S. 1995. Ustiloxins, new antimitotic cyclic peptides: Interaction with porcine brain tubulin., 49(10): 1367–1372.
Liu L L, Yan X, Xue K Y, Wang X M, Li L Y, Chen H Y, Li R L, Li H, Lan J, Xin J J, Li X, Zhuo C L, Wu Z, Zhang D, Huang W J, Wang Y L, Li X Y, Jiang W, Zhang H Y. 2022. Prim-O- glucosycimifugin attenuates liver injury in septic mice by inhibiting NLRP3 inflammasome/caspase-1 signaling cascades in macrophages., 106: 154427.
Liu X S, Zou H, Slaughter C, Wang X D. 1997. DFF, a heterodimeric protein that functions downstream of Caspase-3 to trigger DNA fragmentation during apoptosis., 89(2): 175–184.
Ludueńa R F, Roach M C, Prasad V, Banerjee M, Koiso Y, Li Y, Iwasaki S. 1994. Interaction of ustiloxin A with bovine brain tubulin., 47(9): 1593–1599.
Majtnerová P, Roušar T. 2018. An overview of apoptosis assays detecting DNA fragmentation., 45: 1469–1478.
Miyazaki S, Matsumoto Y, Uchihara T, Morimoto K. 2009. High- performance liquid chromatographic determination of ustiloxin A in forage rice silage., 71(2): 239–241.
Muhammad I, Wang X H, Li S H, Li R, Zhang X Y. 2018. Curcumin confers hepatoprotection against AFB1-induced toxicityvia activating autophagy and ameliorating inflammation involving Nrf2/HO-1 signaling pathway., 45(6): 1775–1785.
Nakamura K, Izumiyama N, Ohtsubo K, Koiso Y, Iwasaki S. 1993. Apoptosis induced in the liver, kidney and urinary bladder of mice by the fungal toxin produced by., 38: 25–30.
Nakamura K I, Izumiyama N, Ohtsubo K I, Koiso Y, Iwasaki S, Sonoda R, Fujita Y, Yaegashi H, Sato Z. 1994. “Lupinosis”-like lesions in mice caused by ustiloxin, produced by: A morphological study., 2(1): 22–28.
Peng Y Y, Bishop K S, Ferguson L R, Quek S Y. 2022. Phenolic- rich feijoa extracts from flesh, peel and whole fruit activate apoptosis pathways in the LNCaP cell line., 383: 132285.
Qiu J H, Meng S, Deng Y Z, Huang S W, Kou Y J. 2019.: A fungus infects rice flower and threats world rice production., 26(4): 199–206.
Ramachandran A, Visschers R G J, Duan L Q, Akakpo J Y, Jaeschke H. 2018. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: Current understanding and future perspectives., 4(1): 75–100.
Sun Q, Liu H, Zhang Y K, Kong R, Yi X E, Liu C S. 2021. Detection of ustiloxin A in urine by ultra-high-performance liquid chromatography-tandem mass spectrometry coupled with two-step solid-phase extraction., 1181: 122916.
Sun Q, Liu H, Zhang Y K, Yi X E, Kong R, Cheng S Y, Man J G, Zheng L, Huang J B, Su G Y, Letcher R J, Giesy J P, Liu C S. 2022. Global distribution of ustiloxins in rice and their male-biased hepatotoxicity., 301: 118992.
Tanaka H, Sawayama A M, Wandless T J. 2003. Enantioselective total synthesis of ustiloxin D., 125(23): 6864–6865.
Wang X H, Wang J, Lai D W, Wang W X, Dai J G, Zhou L G, Liu Y. 2017. Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls., 9(2): 54.
Wang Y Q, Li G B, Gong Z Y, Li Y, Huang F, Fan J, Wang W M. 2016. Stachyose is a preferential carbon source utilized by the rice false smut pathogen,., 96: 69–76.
Wu N, Jiang H B, Bao Y D, Zhang C, Zhang J Z, Song W J, Zhao Y Y, Mi C X, He Y, Liu F. 2020. Practicability investigation of using near-infrared hyperspectral imaging to detect rice kernels infected with rice false smut in different conditions., 308: 127696.
Xiong M, Meng S, Qiu J H, Shi H B, Shen X L, Kou Y J. 2020. Putative phosphatase UvPsr1 is required for mycelial growth, conidiation, stress response and pathogenicity in., 27(6): 529–536.
Yang Q Y, Han B, Li S Y, Wang X Q, Wu P F, Liu Y, Li J Y, Han B Q, Deng N, Zhang Z G. 2022. The link between deacetylation and hepatotoxicity induced by exposure to hexavalent chromium., 35: 129–140.
Yang X, Fang Y, Hou J B, Wang X J, Li J Y, Li S Y, Zheng X Y, Liu Y, Zhang Z G. 2022. The heart as a target for deltamethrin toxicity: Inhibition of Nrf2/HO-1 pathway induces oxidative stress and results in inflammation and apoptosis., 300: 134479.
Yu J J, Yu M N, Song T Q, Cao H J, Yong M L, Pan X Y, Qi Z Q, Du Y, Zhang R S, Yin X L, Liang D, Liu Y F. 2021. UvSMEK1, a suppressor of MEK null, regulates pathogenicity, conidiation and conidial germination in rice false smut fungus., 28(5): 457–465.
Zhang Q, Wang L P, Chen G L, Wang M X, Hu T Z. 2021. Cylindrospermopsin impairs vascular smooth muscle cells by P53-mediated apoptosis due to ROS overproduction., 353: 83–92.
Zhang X X, Li M, Wu H, Fan W Y, Zhang J S, Su W W, Wang Y G, Li P B. 2022. Naringenin attenuates inflammation, apoptosis, and ferroptosis in silver nanoparticle-induced lung injury through a mechanism associated with Nrf2/HO-1 axis:andstudies., 311: 121127.
Zou J, Wang S P, Wang Y T, Wan J B. 2021. Regulation of the NLRP3 inflammasome with natural products against chemical- induced liver injury., 164: 105388.
Copyright © 2023, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2023.04.003
6 December 2022;
23 April 2023
Sun Lihua(sunlihua_002@163.com)
(Managing Editor: Li Guan)
杂志排行
Rice Science的其它文章
- Effect of GW8 Gene Editing on Appearance Quality of Erect-Panicle Type (dep1) Japonica Rice
- Transcriptome Analysis of oserf922 Mutants Reveals New Insights into Rice Blast Resistance
- ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice
- LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice
- Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa
- Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth
