Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth
2023-09-05MdDhinIslamAdamPricePaulHallett
Md. Dhin Islam, Adam H. Price, Paul D. Hallett
Research Paper
Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth
Md. Dhin Islam1, 2, Adam H. Price1, Paul D. Hallett1
(School of Biological Sciences, University of Aberdeen, St Machar Dr, Aberdeen AB24 3UU, the United Kingdom; Faculty of Agriculture, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur-1706, Bangladesh)
Rice is often grown as multiple seasons in one year, alternating between flooded and upland systems. A major constraint, introduced from the flooded system, is a plough pan that may decrease rooting depth and productivity of follow-on upland rice. Roots penetrating the plough pan under flooded rice system can leave a legacy of weaker root growth pathways. Deeper rooting rice cultivars could have a bigger impact, but no direct evidence is available. To explore whether a deep rather than a shallow rooting rice cultivar grown in a flooded cropping cycle benefited deeper root growth of follow-on rice in an upland, reduced tillage cropping cycle, a simulated flooded paddy in greenhouse was planted with deep (Black Gora) and shallow (IR64) rooting cultivars and a plant-free control. Artificial plough pans were made in between the topsoil and subsoil to form different treatments with no plough pan (0.35 MPa), soft plough pan (1.03 MPa) and hard plough pan (1.70 MPa). After harvest of this ‘first season’ rice, the soil was drained and undisturbed to simulate zero-tillage upland and planted rice cultivar BRRI Dhan 28. The overall root length density (RLD), root surface area, the numbers of root tips and branching of BRRI Dhan 28 did not vary between plough pan and no plough pan treatments. Compared with the shallow rooting rice genotype, the deep rooting rice genotype as ‘first season’ crop produced 19% greater RLD, 34% greater surface area and 29% more branching of BRRI Dhan 28 in the subsoil. In the topsoil, however, BRRI Dhan 28 had 28% greater RLD, 35% greater surface area and 43% more branching for the shallow rather than deep rooting genotype planted in the ‘first season’. The results suggested that rice cultivar selection for a paddy cycle affects root growth of a follow-on rice crop grown under no-till, with benefits to subsoil access from deep rooting cultivars and topsoil proliferation for shallow rooting cultivars.
plough pan; root growth; biopores; crop rotation;;preceding crop; zero-tillage
Intensive flooded rice cultivation with two or three rice crops per year in the same field is the main agricultural land use systems in the lowland tropics and subtropics of Asia (Yuan et al, 2022). Despite benefits of flooded rice such as weed control, easy transplanting, and good establishment of rice seedlings (Sanchez, 1973), it uses about 2 500 L water to produce one kilogram of rice, which is 2‒4 times greater than upland rice (Bouman et al, 2007). Furthermore, water scarcity (Steduto et al, 2012) and soil degradation from intensive puddling (Ladha et al, 2003) affect continuous flooded rice production systems. Puddling is widely used to prepare land for continuous flooded rice production, but the associated intensive tillage results in organic matter mineralization and structural destabilization that negatively impact soil (Chen et al, 2007). Moreover, farmers are affected by fuel costs and labour demands for the puddling operation (Ladha et al, 2015; Narang et al, 2020). The rotation of continuous flooded rice with upland crops (Timsina et al, 2010) or aerobic rice (Bouman et al, 2007) in a non-flooded condition has therefore become increasingly popular around the tropics and subtropics in the dry season.
Prolonged puddling in paddy systems or tillage in upland systems can create a compacted layer (plough pan) beneath the plough layer that restricts root access to deep water and nutrients (Li, 1992). Plough pans are commonplace in paddy rice production, with one study finding more than 50% of 18 studied sites in Bangladesh, Nepal and India to be affected (Singh et al, 2017). Under the water or nutrient limited upland rice production, yields decrease and resources may be captured by the plants less efficiently (Linh et al, 2015). When comparing yields between upland rice and a preceding crop of flooded rice, significant yield reductions can occur (Weller et al, 2016). Peng et al (2006) compared flooded rice with upland rice and found a 8%‒69% yield reduction, while Belder et al (2005) reported a 15%‒39% yield reduction that is mainly attributed to soil physical constraints. The greatest physical constraint is often at the plough pan below topsoil (Li, 1992), which is typically compacted with reduced granular structure, porosity and water stable aggregate (Huang and Ding, 1995; Zhou et al, 2014).
Typically upland rice production includes soil tillage to break up the previously puddled surface soil. A gradual transition to reduced tillage for upland rice production has been occurred in recent years. This is possible because rice seedlings, which are normally transplanted in the puddled soil, can also be transplanted in the soft un-puddled soil surface under zero-tillage (Kamboj et al, 2013; Haque et al, 2016). This un-puddled transplanted rice system reduces soil disturbance and tillage costs, saves water, and increases farm profit and energy use efficiency, potentially without any yield reductions (Gathala et al, 2015). Ambassa-Kiki et al (1996) found that rice yield does not vary between zero-tillage and conventional tillage with ploughing, but the cost of labour is 2.6 times greater for conventional tillage compared with zero-tillage. With a permanent change to zero-tillage without a puddling cycle when rice is flooded, Mishra and Saha (2008) found that initial decreases of rice yield under zero- tillage transitions to 13% increases in yield after 3 years due to decreasing penetration resistance over time. Therefore, zero-tillage has become increasingly attractive in many rice-growing regions for sustainable rice production (Huang et al, 2011).
The improved physical conditions for root growth that develop over time under zero-tillage require bioturbation from plant roots and soil organisms (Piron et al, 2012; Zhang et al, 2022). However, this needs to act against severe soil physical constraints that may remain, particularly plough pans and subsoil compaction. At the plough pan and subsoil, soil bulk density and soil penetration resistance increase markedly,resulting in decreased hydraulic conductivity and macroporosity (Aggarwal et al, 1995; Gathala et al, 2011). This compaction restricts rooting depth and root exploration (Rosolem et al, 2002), which lowers the uptake of water and nutrients (Lipiec and Hatano, 2003; Bingham et al, 2010).
However, against the mechanical impedance of a plough pan, bioturbation and decomposed plant roots have been shown to produce potential pathways for growing roots to reach subsoil (Cresswell and Kirkegaard, 1995; Islam et al, 2021). Under zero-tillage, such preferential growth pathways can extend from the soil surface through the plough pan, connecting topsoil with subsoil. Kautz and Köpke (2010) reported that biopores can affect the nutrient uptake by plant roots because they often grow along pre-existing biopores and re-enter into the bulk soil to access new potential nutrient sources. Studies on crops other than rice have reported that increasing the number of biopores in subsoil may be beneficial for subsequent crops by providing better penetration of air, water and roots (Elkins and Sickle, 1984; Bertollo et al, 2021). However, the abilities of plants to bioturbate soils and create biopores vary between species and cultivars due to their root system architectures (Cresswell and Kirkegaard, 1995; Islam et al, 2021). Deep rooting allows rice to utilize water in deeper soil that is inaccessible to cultivars with shallow root systems, so if the biopores produced by these cultivars could be accessed by the following crop, it would boost drought tolerance in upland rice after flooded rice (Fukai and Cooper, 1995).
Given the growing use of reduced tillage for upland rice production, this biological action of plant roots to improve soil structure is likely increasingly important to alleviate physical constraints to root growth. In previous research, a deep rooting rice cultivar (Black Gora) was found to be more effective than a shallow rooting rice cultivar (IR64) at penetrating compacted soil and accessing artificial macropores (Islam et al, 2021). The aim of the current study was to explore whether the legacy of root growth from these deep versus shallow rooting cultivars under a flooded puddled system affects root growth through a plough pan to subsoil of follow-on rice (BRRI Dhan 28) grown under simulated zero-tillage in an upland system. Conditions were highly controlled in a greenhouse to enable X-ray computed tomography (CT) scanning after both the flooded and upland systems, and to explore the impacts of plough pan strength. It is hypothesized that a previous deep rooting, but not shallow rooting rice cultivar would enhance deep root growth by a follow-on upland rice due to the legacy of bioturbation through the compacted plough pan. The research aimed to provide evidence to select for root traits that may improve rice production in flooded to upland rice rotations.
RESULTS
Aboveground responses
In the first season (flooded), IR64 was shorter and had less shoot mass than Black Gora, but it had more leaves and a similar number of tillers (Table 1). The compaction level of the plough pan only influenced shoot dry weight, which was 14% greater for Black Gora and 20% greater for IR64 for the no plough pan versus hard plough pan treatments. The only interaction between rice genotype and plough pan compaction was found for shoot fresh weight, whereas shoot fresh weight was not impacted by a plough pan in Black Gora, it was profoundly negatively affected by a plough pan in IR64 (Table 1). In the second season (upland), the only shoot property of BRRI Dhan 28 that was affected by the first season genotype or plough pan treatment was the shoot fresh weight, which was about 10% heavier if IR64 was the preceding crop (Table 2).
Data are Mean ± SE (= 4). For analysis of variance, values reported are the-values and asterisks indicate the level of significance. NS means non-significant. ***,< 0.001; **,< 0.01; *,< 0.05.
Table 2. Aboveground parameters for BRRI Dhan 28 grown under different treatments in the second season (upland).
Data are Mean ± SE (= 4). For analysis of variance, values reported are the-values and asterisks indicate the level of significance. NS means non-significant. **,< 0.01; *,< 0.05.
However, there was a significant effect of genotype and an interaction with plough pan strength on the total nitrogen (N) uptake from the soil by the plant (Table S1). Plant N content was not affected by a soft plough pan in both genotypes, but a hard plough pan positively influenced plant N content in Black Gora, whereas for IR64, hard plough pan decreased plant N content. The total N uptake by the plant was 24% greater for Black Gora than IR64 (Table S1). In the second season (upland), the preceding rice genotype or plough pan strength had no impact on plant carbon (C) and N contents or the uptake of total N by BRRI Dhan 28 (Table S2).
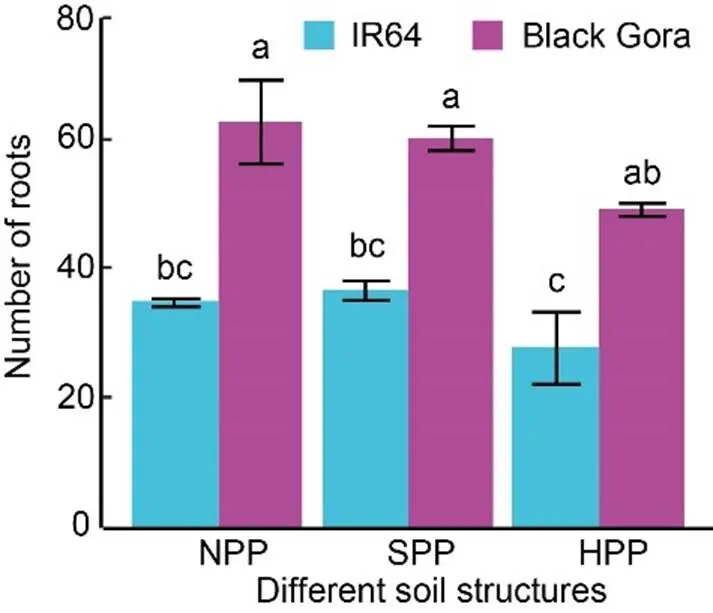
Fig. 1. Number of roots at the bottom surface of plough pan counted from X-ray computed tomography images for Black Gora and IR64 grown under different treatments in flooded season.
These roots were decomposed during the decomposition period which we assumed could provide root channels or biopores for the next season of upland rice. NPP, No plough pan; SPP, Soft plough pan; HPP, Hard plough pan.
Error bars are standard error of the mean (= 4). Different lowercase letters above the bars indicate significant difference at< 0.05.
Underground responses C and N contents in soil
Neither genotype nor plough pan strength affected C or N content in soil, and they were similar for both the first flooded and the second upland seasons (Tables S1 and S2).
Number of roots at the bottom surface of plough pan in the first season (flooded)
From the X-ray CT images, the deep rooting cultivar Black Gora was found to have 70% more roots at the bottom surface of the plough pan than the shallow rooting cultivar IR64 in the flooded season (Fig. 1). Although the soft plough pan and no plough treatments did not differ, there were about 20% fewer roots for the hard plough pan in the both genotypes (Fig. 1).
Root length density (RLD) of BRRI Dhan 28 in the second season (upland)
The overall RLD of BRRI Dhan 28 was not affected by the preceding rice genotypes (Black Gora and IR64), but the overall RLD was 22% greater if the cores were planted with rice in the first season rather than plant-free (Fig. 2-A and Table 3). The effects of soil structure and interaction were not significant for overall RLD (Table 3).
The flooded season treatments are no plant, Black Gora and IR64. For analysis of variance, values reported are the-values and asterisks indicate the level of significance. NS means non-significant. ***,< 0.001; **,< 0.01; *,< 0.05.
For topsoil, the RLD of BRRI Dhan 28 was 28% greater for IR64 than Black Gora grown as the preceding crop (Fig. 2-B and Table 3). There was no significant effect observed between planted and unplanted treatments. The plough pan did not affect RLD density in the topsoil (Table 3).
Fig. 2. Root length density (RLD) of BRRI Dhan 28 grown under different treatments in upland season.
A, Overall RLD for whole soil cores.
B‒D, RLD in topsoil (B), plough pan (C) and subsoil layer (D) of soil cores.
Genotype means preceding season treatments. WP, Without plant; NPP, No plough pan; SPP, Soft plough pan; HPP, Hard plough pan.
Error bars are standard error of the mean (= 4).
In the plough pan, although RLD was unaffected by the preceding rice genotype, planted cores produced 91% greater RLD than unplanted ones (Fig. 2-C and Table 3). The RLD was 49% lower for soft plough pantreated cores and 64% lower for hard plough pan treated ones compared with no plough pan treated ones.
For subsoil, RLD was 19% greater for Black Gora than IR64 grown as the preceding crop. However, about a 31% decrease in RLD was found for unplanted cores than planted ones, and plough pan strength and interaction effects were not significant (Fig. 2-D and Table 3).

Fig. 3. Root diameter of BRRI Dhan 28 grown under different treatments in upland season.
A, Average root diameter for whole soil cores.
B‒D, Root diameter in topsoil (B), plough pan (C) and subsoil layer (D) of soil cores.
Genotype means preceding season treatments. WP, Without plant; NPP, No plough pan; SPP, Soft plough pan; HPP, Hard plough pan.
Error bars are standard error of the mean (= 4).
Root diameter of BRRI Dhan 28
The average root diameter in the second season upland conditions was not affected by deep or shallow rooting rice grown in the preceding season flooded conditions, but the presence of preceding plants caused a 25% increase in root diameter (Fig. 3-A and Table 3). The average root diameter increased by 7%‒9% for plough pan than no plough pan treated cores, and there was an interaction between plough pan strength and the preceding rice genotype (Fig. 3-A and Table 3). These results suggested that difference between the preceding crop treatments was less obvious in the soft plough pan. With the no plough pan and soft plough pan, Black Gora as a preceding crop increased root diameter more than IR64, whereas in the hard plough pan, the preceding rice genotype had no impact.
For topsoil, the average root diameter of BRRI Dhan 28 in the second upland season was 21% less for cores that were not plant-free in the first season. The presence of a plough pan did not affect the root diameter of BRRI Dhan 28, but there were interaction effects between the preceding rice genotype and plough pan strength treatments (Fig. 3-B and Table 3). The roots were thicker when IR64 was grown as the preceding crop in the hard plough pan and no plough pan treated cores. With Black Gora as the preceding crop, BRRI Dhan 28 in the second season had thicker roots in the soft plough pan (Fig. 3-B).
In the plough pan, root diameter of BRRI Dhan 28 was affected by plough pan strength and there was an interaction with the preceding rice genotype, but not from the preceding genotype on its own (Table 3). The hard plough pan resulted in increased root diameters of 16% and 37% compared with the soft plough pan, and no plough pan, respectively (Fig. 3-C). Planted treatments from the first flooded season had 8%‒10% greater root diameter than the unplanted treatment in the second upland season (Fig. 3-C). The hard plough pan with IR64 as the preceding crop increased root diameter more than Black Gora did. In contrast, Black Gora treated cores had thicker roots in the soft plough pan and no plough pan than IR64 treated ones (Fig. 3-C).

Fig. 4. Average root tip density of BRRI Dhan 28 grown under different treatments in upland season.
A, Overall root tip density for soil cores.
B‒D, Root tip density in topsoil (B), plough pan (C) and subsoil layer (D) of soil cores.
Genotype means preceding season treatments. WP, Without plant; NPP, No plough pan; SPP, Soft plough pan; HPP, Hard plough pan.
Error bars are standard error of the mean (= 4).
For subsoil, the widest root diameter of BRRI Dhan 28 occurred when Black Gora was planted in the first flooded season. Moreover, having rice plants in the first upland season resulted in 20% thicker roots of BRRI Dhan 28 than the unplanted treatment in the second season upland condition. There was no influence of soil structure treatment on the root diameter for succeeding rice (Fig. 3-D and Table 3).
Root tip density of BRRI Dhan 28
The average root tip density did not differ significantly in BRRI Dhan 28 if either IR64 or Black Gora was the preceding rice genotype. On the other hand, there were 22% more root tips on average of BRRI Dhan 28 if either rice genotype was planted in the first season (Fig. 4-A and Table 3). Plough pan strength had no impact on the average root tip density.
For topsoil, the average root tip density of BRRI Dhan 28 was not affected by the preceding rice genotype or plough pan strength (Fig. 4-B and Table 3). However, there was about a 17% greater average root tip density of BRRI Dhan 28 if rice was planted in the first season (Fig. 4-B).
In the plough pan, there was about a 50% increase in average root tip density of BRRI Dhan 28 if rice was planted in the first season (Fig. 4-C and Table 3). Average root tip density in the plough pan was 58% less for a soft plough pan and 75% less for a hard plough pan compared with cores with no plough pan (Fig. 4-C).
For subsoil, average root tip density of BRRI Dhan 28 in the second upland season, having Black Gora as preceding crop in the first flooded season, resulted in a 20% increase over IR64, and a 50% increase over the no plant control (Fig. 4-D and Table 3). However, plough pan strength had no influence on subsoil average root tip density of BRRI Dhan 28 in the second upland season.
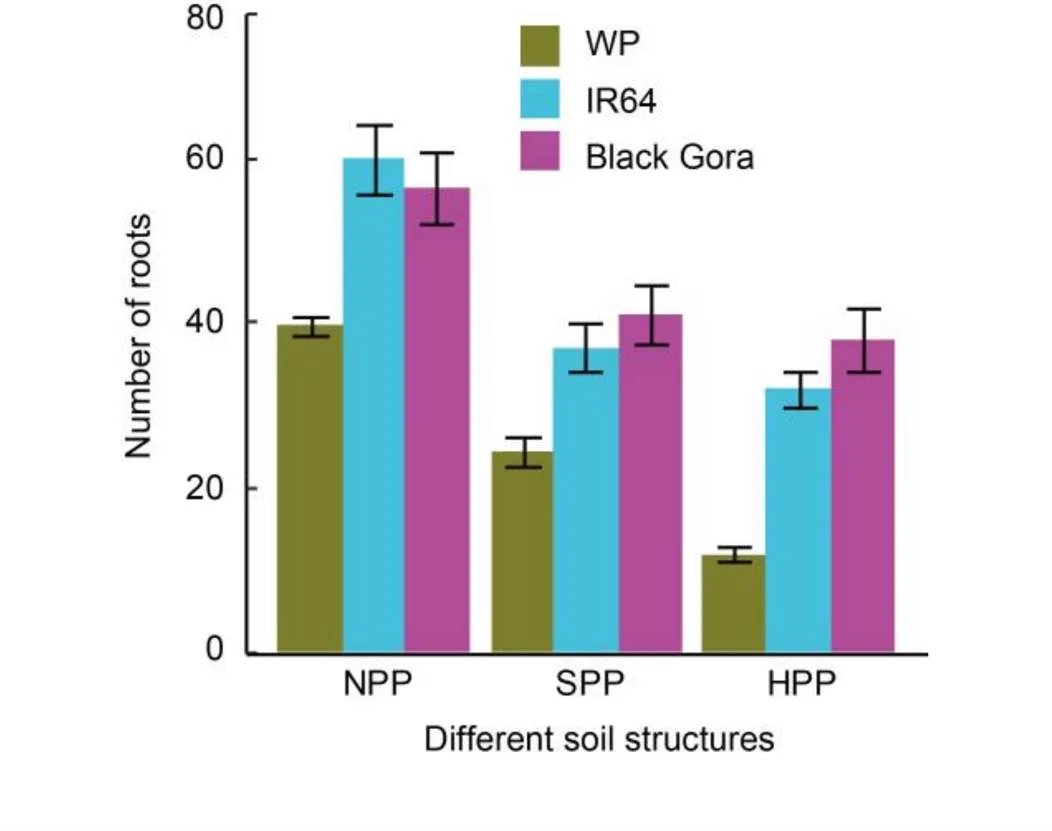
Fig. 5. Number of roots at the bottom surface of plough pan counted from camera images for BRRI Dhan 28 grown under different treatments in upland season.
Genotype means preceding season treatments. WP, Without plant; NPP, No plough pan; SPP, Soft plough pan; HPP, Hard plough pan.
Error bars are standard error of the mean (= 4).
Number of roots of BRRI Dhan 28 on lower surface of plough pan
The number of roots of BRRI Dhan 28 that reached the lower surface of the plough pan was not affected by the preceding rice genotypes (Black Gora and IR64) (Table 3). However, having no rice planted in the first flooded season resulted in a 42% decrease in BRRI Dhan 28 roots penetrating to the lower surface of the plough pan in the second upland season (Fig. 5). For BRRI Dhan 28 in the second upland season, no plough pan cores had 48% more roots than the soft plough pan treated ones and 90% more than the hard plough pan treated ones (Fig. 5).

Table 4. Root parameters of BRRI Dhan 28 grown under three different soil structures and two genotype treatments in upland season.
Data are Mean ± SE (= 4).
Other underground responses of BRRI Dhan 28
BRRI Dhan 28 following preceding rice genotype IR64 treated cores had 35% more root surface area than Black Gora treated cores in topsoil, but in subsoil, IR64treated cores had 25% less root surface area than Black Gora treated ones (Tables 3 and 4). Root surface area differed significantly between the planted and unplanted treatments for all depths combined, the plough pan and the subsoil, but not in topsoil. Plough pan strength only influenced root surface area of BRRI Dhan 28 in the plough pan (Tables 3 and 4), where it declined by 41%‒49% if either a soft or a hard plough pan was present (Table 4).
Similar to root surface area, root volume of BRRI Dhan 28 was 41% greater for IR64 treated cores than Black Gora treated ones in topsoil, but in subsoil, Black Gora treated cores had 50% more root volume than IR64 treated ones. Moreover, root volume was significantly greater for plants in the first season compared with the unplanted treatments at all the layers (Tables 3 and 4). There were no plough pan strength effects on the root volume of BRRI Dhan 28 in any layers of the soil cores (Table 3).
Root branching of BRRI Dhan 28 was 43% greater in topsoil if IR64 rather than Black Gora was planted in the first season. However, in subsoil, Black Gora planted in the first season resulted in 29% greater root branching of BRRI Dhan 28 compared with IR64 did. Root branching of BRRI Dhan 28 significantly differed between the planted and unplanted treatments for plough pan, subsoil and all soil layers combined. There were no soil structure effects for topsoil, subsoil and combined soil layers, but root branching in the plough pan was 59%‒70% less for soil cores without a plough pan (Tables 3 and 4).
DISCUSSION
Our results under the controlled conditions suggested that selecting deep rooting rice cultivars during a flooded season improved the growth of roots to the subsoil in a follow-on upland season under zero-tillage system in the first season. These findings supported to conduct further field verification of the impacts, including the screening of a broader range of root-trait genotypes in both the flooded and upland cropping seasons across different soils. Using rice genotypes with markedly different root-trait, a deep rooting cultivar Black Gora and a shallow rooting cultivar IR64 grown in the flooded season, our study clearly showed that the rice genotype in the flooded season affected deep root growth of BRRI Dhan 28 in the upland season. X-ray CT images showed that the deep rooting rice cultivar produced more root growth channels (biopores) through the plough pan, with surprisingly little impact caused by plough pan strength despite a > 2 MPa penetration resistance in the strongest plough pans that should restrict root growth. It is hence important to understand the mechanism of interaction of root growth between current season and preceding season.
Preceding season rice plants affect root growth in next season
Our results showed that under a simulated zero-tillage upland system, root growth of BRRI Dhan 28 benefited from bioturbation produced by preceding rice grown in a flooded paddy field, extending understanding from our earlier study that was limited to artificial macropores (Islam et al, 2021). Although the presence of biopores can alter some soil physical properties like air permeability and hydraulic conductivity in compacted soil (Stirzaker et al, 1996), penetration resistance of the soil does not change (Colombi et al, 2018). Additionally, biopores allow roots to bypass compacted soil, providing less or even no mechanical impedance. Roots usually prefer to grow towards larger pores like biopores or cracks (Dexter, 1986), following the oxygen gradient (Muthert et al, 2020). Other studies using X-ray CT images indicated that biopores can be used as preferential pathways for root growth to deeper soils for maize, soybean, wheat and barley (Pfeifer et al, 2014; Colombi et al, 2017; Atkinson et al, 2020).
Bioturbation by plant roots can also produce cracks through fracturing soil, resulting in better growth for succeeding crops (Gregory et al, 2007). An examination of X-ray CT images for cores, however, did not find evidence of cracking (data not shown). Some studies showed that in compacted soils, crop roots prefer to use existing macropores rather than penetrate the soil matrix (Gao et al, 2016; Atkinson et al, 2020), and as a result, the number of new biopores is few in compacted soils with high strength.
Our findings are consistent with Han et al (2016), who used a barley-fodder (chicory & tall fescue) rotation for two successive years in a field experiment, and found that RLD of barley is significantly greater for the successive year compared with a control with no fodder rotation. In contrast, a rhizotron study by Bauke et al (2017) found that artificial macropores in compacted subsoils have no impact on root growth of spring wheat. This contradictory result might be due to less root-soil contact in their rhizotron studies because they used larger size (6 mm) macropores than the diameter of wheat root. On the other hand, in our current study, root channels were created by the first season crop, and assuming decomposition occurred, the size of the biopores was more or less the root diameter of the succeeding rice crop. Bertollo et al (2021) reported that preceding crops (ruzigrass, oat, wheat or maize) can enhance soil macroporosity under different compaction levels in 0−30 cm soil depth that might be helpful for root growth in compacted soils. However, different types of preceding crops contribute differently to the performance of succeeding crops.
Influences of plough pan on root growth
Although a plough pan creates a physical barrier for root growth to deeper soil, we did not find any differences in the overall root growth of BRRI Dhan 28 between the plough pan and the no plough pan cores, except for root diameter. Guimarães and Moreira (2001) found in a greenhouse study that an artificially created compacted topsoil at 0‒20 cm depth causes significantly less root growth at 20‒40 cm depth compared with an uncompacted topsoil. In a field study, Ramalingam et al (2017) reported that increased penetration resistance (1.8 MPa) by compacting soil with a 3 230 kg excavator results in a 30% reduction of RLD in rice. Another field study reported that rice root growth is inhibited by 47% at the soil strength of 2.5 MPa (Hasegawa et al, 1985).
Counteracting such impacts of compaction in our study was likely the better root growth through the plough pan to the subsoil by root channels from the preceding rice plants. Not only would these root channels bypass mechanical barriers, other studies have found that they can improve nutrient content and aeration (Rasse and Smucker, 1998; Williams and Weil, 2004; Kautz, 2014; Colombi et al, 2017). Although root growth to subsoil was improved significantly if plants were present in the first flooded season when plough pans were present, we found decreased root growth at plough pan depth with increasing levels of plough pan compaction. Although bioturbation by the preceding rice crop had a positive impact on root growth through plough pans, the higher bulk density and soil penetration resistance with increasing plough pan compaction caused fewer roots to pass through the hard layer (Fig. S1), and therefore fewer biopores are used by succeeding crops (Aggarwal et al, 1995; Gathala et al, 2011).
When a plough pan was present, the increased root diameter of BRRI Dhan 28 followed other studies that found root diameters of the plants grown in compacted soils are thicker than those in uncompacted soils (Materechera et al, 1992; Guimarães and Moreira, 2001). Interestingly, thicker rice roots were observed in succeeding rice for planted cores compared with no planted ones, which may be due to the large size of root channels left by the preceding crop. Root channels and their resulting biopores, compared with compacted bulk soil, possibly exert less radial pressure on roots that would compress root tissue (Bengough, 2012; Kolb et al, 2012). However, other research found a reduction of root diameter when grown in large size pores, likely due to poor root-soil contract (Bengough, 2003).
Roots of BBRI Dhan 28 respond differently in different layers
The root growth of BRRI Dhan 28 under a simulated zero-tillage upland condition varied among different layers (topsoil, plough pan and subsoil) of soil cores depending on whether Black Gora or IR64 was grown as the preceding crop in the first flooded season. Despite limited physical constraints in topsoil, the better root growth of succeeding rice under IR64 treated cores might be due to IR64 leaving behind more decomposing roots in topsoil to produce biopores. In a field study, Han et al (2016) showed that a precrop with extensive fibrous roots creates more fine biopores that promote the growth of subsequent barley roots.
Although the number of roots passing through the plough pans differed between Black Gora and IR64 treated cores in the flooded season (Fig. 1), only a small portion of macropores (< 20%) may be utilized by the plant roots in compacted soils (White and Kirkegaard, 2010). This may have diminished the capacity of BRRI Dhan 28 to utilize Black Gora or IR64 produced biopores in the plough pan, masking differences between the treatments.
On the other hand, BRRI Dhan 28 had more RLD, root surface area, root volume, branching and root number in subsoil under Black Gora precrop treated cores than IR64 precrop treated cores, likely driven by biopores produced in the first season. Moreover, by having more roots from the first season decomposing in the subsoil from Black Gora compared with IR64, there may have been better soil physical conditions and more nutrients released in the subsoil, as suggested by the greater plant N uptake by BRRI Dhan 28 (Table S1).
This study was conducted in a highly controlled environment to explore how root growth was affected by the combined impacts of soil physical limitations and contrasting rice genotypes with different root structures grown as a first season crop. As shown by Zhang et al (2022), soil structure in the field is far more complex and heterogeneous, with a much longer developed legacy of biopores and other macropores that can be used preferentially for root growth. Although control is not good enough, the findings from our controlled greenhouse study suggested that field experiments exploring successive rice growing seasons under flooding to upland irrigation, and conventional to reduced tillage, would be beneficial. The choice of the flooded season rice genotype appeared to affect root growth of follow-on upland rice, but this need verification in the field, including impacts to yield and resource use efficiency. In our study, there was no nutrient and water scarcity which may further influence the preceding rice crop. Likewise, compaction levels in the field may be much greater than those we can simulate in the laboratory, so deep rooting cultivars may be restricted from reaching the subsoil. If zero- or reduced-tillage becomes more widely adopted, possibly the use of break crops other than rice may be needed to provide biopores for follow-on rice to exploit, so that they can reach the subsoil.
In summary, we found that root traits of different rice genotypes could have marked impact to improve rice root growth in flooded to upland rotations, whether or not a plough pan was present. The legacy of a preceding flooded paddy rice crop was evident in the root structure of the subsequent upland rice crop. A shallow rooting cultivar enhanced shallow rooting in the subsequent crop, whereas a deep rooting cultivar enhanced deep rooting. Counteracting a 50% decrease of RLD in the plough pan from soil compaction was the action of root growth from preceding rice could increase RLD by more than 90%. Further changes to root architecture were also observed, such as increased root diameter when a preceding rice crop was present. Future research should be conducted in the field, exploring more mature plants under broader soil and resource constraints. Moreover, longer decomposition times between the first and second season crops might affect root growth through biopores, so this requires further investigation.
METHODS
Plant and soil materials
This was a double season experiment. Two contrasting rice genotypes were used for the first season, which was under a paddy flooded system. These were deep rooting cultivar Black Gora (antype) (Zhao et al, 2011) and shallow rooting cultivar IR64 (antype) (McNally et al, 2009). The second season was under upland conditions and only BRRI Dhan 28 was used because it is a widely used cultivar in the dry season (Boro) in Bangladesh. Black Gora and IR64 cultivars differ in root angle and root depth (Shrestha et al, 2014; Munasinghe and Price, 2016), and the root depth of BRRI Dhan 28 is more or less similar to IR64, but the roots have a steeper angle than IR64 (Islam, 2016). Rice plants were grown in a sandy loam soil collected from the plough layer to 20 cm depth at a commercial farm in Insch, Aberdeenshire, UK. This soil has been used in numerous studies exploring rice root growth and more details can be found in Islam et al (2021).
Experimental design
The study was conducted in a greenhouse under tropical conditions with day/night temperatures of 28 ºC/24 ºC, light intensity of 464 μmol/(m2·s) and 11 h photoperiod. The rice was grown in soil-filled PVC tubes (48 cm height and 10.2 cm diameter) packed to a depth of 43 cm, consisting of a topsoil (20 cm) and subsoil (23 cm) (Fig. S2). A 5-cm plough pan was formed at the top of the subsoil layer in some treatments. Three soil structure treatments were created: No plough pan (bulk density of 1.12 g/cm3), soft plough pan (bulk density of 1.40 g/cm3) and hard plough pan (bulk density of 1.50 g/cm3).
Soil preparation
The soil collected from the field was dried to approximately 0.18 g/g gravimetric moisture content to ease sieving. After drying, the soil was first broken up by hand, homogenized and passed through a 4-mm sieve. The moisture content of the proctor density (maximum bulk density) was measured from a subsample of soil. A 4.5-kg proctor compaction rammer (ELE International, Leighton Buzzard, UK) was dropped for 20 times onto loose soil that was in a mould. This was done for soil at different moisture contents, starting at 0.18 g/g. The soil was broken after each test and wet using a spray bottle, and then the wet soil was mixed and packed again in the mould. A small sample was taken after measuring soil weight and volume to determine water content. The maximum bulk density (1.53 g/cm3) of the soil was found at 0.20 g/g water content.
Core packing
The soil was wet to 0.20 g/g moisture content by gently spraying before packing. All the soil cores were packed using the same procedure described in Islam et al (2021). In brief, there were: (i) 5 cm empty space at the top, (ii) 20 cm topsoil, (iii) a 5-cm plough pan below topsoil, and (iv) a 18-cm subsoil layer at the bottom. To ease soil removal, an acetate sheet lined the inner surface of the soil core. The plough pan was packed first, then the subsoil and finally the topsoil layer using a metal packer. To form the plough pan, soil was packed in three layers (1.67 cm thickness of each layer) using a 4.5-kg proctor hammer. Dropping the proctor hammer 20 times produced a hard plough pan of 1.50 g/cm3bulk density, and dropping it 14 times produced a soft plough pan of 1.40 g/cm3bulk density. The topsoil and subsoil were then packed to 1.12 g/cm3bulk density in four layers each with a metal packer. For the no plough pan treatment, the whole column was packed at 1.12 g/cm3bulk density.
On separate soil cores equilibrated using a tension table (Ecotech, Bonn, UK), water contents of the topsoil and subsoil were found to be 0.36 g/g at -5 kPa and 0.29 g/g at -20 kPa. The penetration resistance at plough pan depth was recorded for each water potential by a cone penetrometer with an angle of 30º cone opening (Table 5). The base area of cone penetrometer was 1.87 mm2. The penetrometer fitted to a Z05 mechanical test frame (Zwick GmbH, Ulm, Germany), which recorded the applied force and controlled insertion into soil at a speed of 1 mm/min to a depth of 4 mm.
Planting rice seedlings and growth conditions
The same approach was used for both the simulated flooded and upland seasons. Rice seeds were germinated on wet filter paper at 25 ºC for 48 h in a controlled environment. No fertilizers were applied during planting or over the whole growing season. Two germinated seedlings were planted in the centre of each core at a depth of 4 mm from the soil surface and after 7 d, the weakest plant was removed. For the first week of the first flooded season, soil water content was maintained at -5 kPa and then a flooded condition was maintained up to the 5th week of rice growth, followed by a final week of growth with no added water. The rice stems were then cut at the soil surface and the cores were left in the greenhouse to decompose for one month, with water content maintained 0.36 g/g (i.e. -5 kPa water potential). We selected this timeframe as it is similar to the delay between cropping seasons typically found in practice for some areas in Bangladesh (Banglapedia, 2012). The second season upland rice had soil water content maintained by watering once every day to 0.36 g/g for 5 weeks, followed by one week with no added water. During the growth and decomposition period, the soil cores were kept in the tropical greenhouse.
Harvesting
Plant height and the numbers of leaves and tillers were recorded. Shoots were then cut at the soil surface and the shoot fresh weights were recorded in both seasons. After that, fresh shoots were dried in an oven at 70 ºC for 48 h. The soil was then removed from the cores by removing the base cap and gently pushing soil in the plastic liner of the PVC cylinder using a metal plunger. Afterwards, the plastic liner was pealed away from the surface of intact soil. The intact soil column was separated into topsoil, plough pan and subsoil by cutting with a hacksaw. Intact soil at the plough pan layer was frozen at -18 ºC for 3 weeks. After washing away a thin bottom layer of the frozen soil from the plough pan with a jet of warm water, the surface was photographed with a digital single-lens reflex (DSLR) camera (smc PENTAX-D FA MACRO, f 8 aperture, ISO of 200 and resolution of 6 016 × 4 000 pixel, Pentax, Tokyo, Japan) to capture roots that penetrated in the second season. Two photographic lights were used when capturing root images to provide good quality images. The rice roots in each of the topsoil, plough pan and subsoil layers were washed from the soil by first breaking apart and then gently washing over a 2-mm sieve. During root washing, some partially decomposed roots from the first season plants were found, evident from their darker colour and decomposed appearance (Fig. S1). These roots were separated manually with tweezers by spreading the roots on a large glass tray.

Table 5. Penetration resistance at plough pan for different treatments at different water contents. MPa
Data are Mean ± SE (= 4).
Root measurement
The rice roots in different layers (topsoil, plough pan and subsoil) were washed carefully using tap water over a 0.5-mm sieve. Then, roots were immersed in 50% ethanol solution in a plastic container and stored in a refrigerator at 4 ºC. Immersed washed roots in different layers of the cores were placed on a large plexiglass tray filled with 4‒5 mm deep water and spread out using tweezers before scanning on an A3 flatbed scanner fitted with a transparency unit (Expression 10000XL scanner, Epson, Suwa, Japan) at 800 dots per inch (DPI). Different root parameters such as root length, surface area, root volume, average diameter, branch number and tip number were measured by the WinRhizo (Version 2013e) software. From the DSLR images of the base of the plough pan, the number of roots was counted using the ImageJ software.
C and N analyses
After the first season growth, small subsamples of soil at 0‒3 cm depth were taken to measure C and N contents. In the second season, the soil was collected during destructive root separation from different layers. Shoots were dried at 70 ºC and soils were dried at 105 ºC for 48 h, respectively. The dried shoots and soil samples were powdered with a ball mill machine (Reisch, MM 400, Düsseldorf, Germany). Around 4‒8 mg powdered shoot and 10‒12 mg powdered soil were weighed into a tin capsule. The total N and C contents were determined using a CNS (carbon, nitrogen and sulphur)-Analyzer (CE Instruments, NA 2500, Milan, Italy). The total N uptake by the plant from soil was measured by multiplying shoot dry weight and shoot N content.
X-ray CT scanning, image processing, and data collection

Statistical analysis
All statistical analyses were carried out using R (version 4.0.3) in the RStudio (version 1.3.1093) environment (RStudio Team, 2020). The normality of data was verified by the Shapiro-Wilk tests, prior to any further statistical tests. First, differences between treatments were determined with two-way analysis of variance (ANOVA) with genotype (Black Gora, IR64 and without plant) and soil structure (no plough pan, soft plough pan and hard plough pan) as factors. Second, two-way ANOVA was performed to measure the differences between the first season Black Gora and IR64 treatments (without plant treatment was deleted from the data for analysis). Post hoc analysis was performed by the Fisher’s least significant difference (LSD) test at the 95% confidence level.
ACKNOWLEDGEMENTS
This study was funded by the Commonwealth Scholarship Commission in the UK. We thank Annette Raffan, Yehia Hazzazi, Licida Maria Giuliani, Faraj Elsakloul and Luke Harrold for their constructive discussion during and after setting up the experiment. We are also thankful to Dr. Stewart J. Chalmers for the X-ray CT Scanning and to Jaime Buckingham for laboratory assistance.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Root (separated roots) images after harvesting BRRI Dhan 28.
Fig. S2. Design of soil core with different plough pan treatments.
Table S1. Carbon (C) and nitrogen (N) contents in plant and soil in flooded season under different treatments.
Table S2. Carbon (C) and nitrogen (N) contents in plant and soil in upland season under different treatments.
Aggarwal G C, Sidhu A S, Sekhon N K, Sandhu K S, Sur H S. 1995. Puddling and N management effects on crop response in a rice-wheat cropping system., 36(3/4): 129–139.
Ambassa-Kiki R, Aboubakar Y, Boulama T. 1996. Zero-tillage for rice production on Cameroonian Vertisols., 39(1/2): 75–84.
Atkinson J A, Hawkesford M J, Whalley W R, Zhou H, Mooney S J. 2020. Soil strength influences wheat root interactions with soil macropores., 43(1): 235–245.
Banglapedia. 2012. Rice. National Encyclopedia of Bangladesh. [2023-01-20]. https://en.banglapedia.org/index.php/Rice.
Bauke S L, Landl M, Koch M, Hofmann D, Nagel K A, Siebers N, Schnepf A, Amelung W. 2017. Macropore effects on phosphorus acquisition by wheat roots: A rhizotron study., 416(1): 67–82.
Belder P, Bouman B M, Spiertz J J, Peng S, Castañeda A R, Visperas R M. 2005. Crop performance, nitrogen and water use in flooded and aerobic rice., 273(1): 167–182.
Bengough A G. 2003. Root growth and function in relation to soil structure, composition, and strength.: de Kroon H, Visser E J W. Root Ecology. Berlin, Heidelberg: Springer: 151–171.
Bengough A G. 2012. Root elongation is restricted by axial but not by radial pressures: So what happens in field soil?, 360(1): 15–18.
Bertollo A M, de Moraes M T, Franchini J C, Soltangheisi A, Balbinot A A Jr, Levien R, Debiasi H. 2021. Precrops alleviate soil physical limitations for soybean root growth in an Oxisol from southern Brazil., 206: 104820.
Bingham I J, Glyn Bengough A, Rees R M. 2010. Soil compaction- N interactions in barley: Root growth and tissue composition., 106(2): 241–246.
Bouman B A M, Lampayan R M, Toung T P. 2007. Water Management in Irrigated Rice: Coping with Water Scarcity. Los Baños, the Philippines: International Rice Research Institute.
Chen S, Xia G M, Zhao W M, Wu F B, Zhang G P. 2007. Characterization of leaf photosynthetic properties for no-tillage rice., 14(4): 283–288.
Colombi T, Braun S, Keller T, Walter A. 2017. Artificial macropores attract crop roots and enhance plant productivity on compacted soils., 574(1): 1283–1293.
Colombi T, Torres L C, Walter A, Keller T. 2018. Feedbacks between soil penetration resistance, root architecture and water uptake limit water accessibility and crop growth: A vicious circle., 626: 1026–1035.
Cresswell H P, Kirkegaard J A. 1995. Subsoil amelioration by plant-roots: The process and the evidence., 33(2): 221.
Dexter A R. 1986. Model experiments on the behaviour of roots at the interface between a tilled seed-bed and a compacted sub-soil: II. Entry of pea and wheat roots into sub-soil cracks on JSTOR., 95(1): 135–147.
Elkins B C, Sickle V K. 1984. Punching holes in plowpans., 28: 38–41.
Fukai S, Cooper M. 1995. Development of drought-resistant cultivars using physiomorphological traits in rice., 40(2): 67–86.
Gao W, Hodgkinson L, Jin K, Watts C W, Ashton R W, Shen J, Ren T, Dodd I C, Binley A, Phillips A L, Hedden P, Hawkesford M J, Whalley W R. 2016. Deep roots and soil structure., 39(8): 1662–1668.
Gathala M K, Ladha J K, Saharawat Y S, Kumar V, Kumar V, Sharma P K. 2011. Effect of tillage and crop establishment methods on physical properties of a medium-textured soil under a seven-year rice-wheat rotation., 75(5): 1851–1862.
Gathala M K, Timsina J, Islam M S, Rahman M M, Hossain M I, Harun-Ar-Rashid M, Ghosh A K, Krupnik T J, Tiwari T P, McDonald A. 2015. Conservation agriculture based tillage and crop establishment options can maintain farmers’ yields and increase profits in South Asia’s rice-maize systems: Evidence from Bangladesh., 172: 85–98.
Gregory A S, Watts C W, Whalley W R, Kuan H L, Griffiths B S, Hallett P D, Whitmore A P. 2007. Physical resilience of soil to field compaction and the interactions with plant growth and microbial community structure., 58(6): 1221–1232.
Guimarães C M, Moreira J A A. 2001. Soil compaction on upland rice., 36(4): 703–707.
Han E, Kautz T, Köpke U. 2016. Precrop root system determines root diameter of subsequent crop., 52(1): 113–118.
Haque M E, Bell R W, Islam M A, Rahman M A. 2016. Minimum tillage unpuddled transplanting: An alternative crop establishment strategy for rice in conservation agriculture cropping systems., 185: 31–39.
Hasegawa S, Thangaraj M, O’Toole J C. 1985. Root behavior: Field and laboratory studies for rice and non-rice crops.: Soil Physics and Rice. Manila, the Philippines: International Rice Research Institute: 383–396.
Huang C P, Ding D L. 1995. The effects of paddy upland rotation on crop yield and soil physical and chemical characteristics., 7: 448–450. (in Chinese with English abstract)
Huang M, Ibrahim M, Xia B, Zou Y. 2011. Significance, progress and prospects for research in simplified cultivation technologies for rice in China., 149(4): 487–496.
Islam M D D, Price A H, Hallett P D. 2021. Contrasting ability of deep and shallow rooting rice genotypes to grow through plough pans containing simulated biopores and cracks., 467(1/2): 515–530.
Islam M S. 2016. Genetic mapping of rooting in rice: Exploiting a high throughput phenotyping in plants. Aberdeen, UK: University of Aberdeen.
Kamboj B R, Yadav D B, Yadav A, Goel N K, Gill G, Malik R K, Chauhan B S. 2013. Mechanized transplanting of rice (L.) in nonpuddled and no-till conditions in the rice-wheat cropping system in Haryana, India., 4(12): 2409–2413.
Kautz T. 2014. Research on subsoil biopores and their functions in organically managed soils: A review., 30(4): 318–327.
Kautz T, Köpke U. 2010.endoscopy: New insights to root growth in biopores., 144(2): 440–442.
Kolb E, Hartmann C, Genet P. 2012. Radial force development during root growth measured by photoelasticity., 360(1): 19–35.
Ladha J K, Dawe D, Pathak H, Padre A T, Yadav R L, Singh B, Singh Y, Singh Y, Singh P, Kundu A L, Sakal R, Ram N, Regmi A P, Gami S K, Bhandari A L, Amin R, Yadav C R, Bhattarai E M, Das S, Aggarwal H P, Gupta R K, Hobbs P R. 2003. How extensive are yield declines in long-term rice-wheat experiments in Asia?, 81(2/3): 159–180.
Ladha J K, Pathak H, Tirol-Padre A, Dawe D, Gupta R K. 2015. Productivity trends in intensive rice-wheat cropping systems in Asia.: LadhaJ K, HillJ E, DuxburyJ M, GuptaR K, Buresh R J. Improving the Productivity and Sustainability of Rice- Wheat Systems: Issues and Impacts. Madison, WI, USA: American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: 45–76.
Li C K. 1992. Paddy Soils of China. Beijing, China: Science Press: 156–162. (in Chinese)
Linh T B, Sleutel S, Guong V T, Khoa L V, Cornelis W M. 2015. Deeper tillage and root growth in annual rice-upland cropping systems result in improved rice yield and economic profit relative to rice monoculture., 154: 44–52.
Lipiec J, Hatano R. 2003. Quantification of compaction effects on soil physical properties and crop growth., 116(1/2): 107–136.
Materechera S A, Alston A M, Kirby J M, Dexter A R. 1992. Influence of root diameter on the penetration of seminal roots into a compacted subsoil., 144(2): 297–303.
McNally K L, Childs K L, Bohnert R, Davidson R M, Zhao K Y, Ulat V J, Zeller G, Clark R M, Hoen D R, Bureau T E, Stokowski R, Ballinger D G, Frazer K A, Cox D R, Padhukasahasram B, Bustamante C D, Weigel D, MacKill D J, Bruskiewich R M, Rätsch G, Buell C R, Leung H, Leach J E. 2009. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice., 106(30): 12273–12278.
Mishra V K, Saha R. 2008. Soil physical behaviour and rice () yield under different sources of organics, methods of puddling and zero tillage., 78: 399–404.
Munasinghe M, Price A H. 2016. Genetic and root phenotype diversity in Sri Lankan rice landraces may be related to drought resistance., 9(1): 24.
Muthert L W F, Izzo L G, van Zanten M, Aronne G. 2020. Root tropisms: Investigations on earth and in space to unravel plant growth direction., 10: 1807.
Narang M K, Chandel R, Dogra B, Manes G S. 2020. Development of mat nursery raising and uprooting techniques for paddy (L.) crop and their field evaluation with mechanical transplanter for South East Asia., 51(2): 79–90.
Peng S B, Bouman B, Visperas R M, Castañeda A, Nie L X, Park H K. 2006. Comparison between aerobic and flooded rice in the tropics: Agronomic performance in an eight-season experiment., 96(2/3): 252–259.
Pfeifer J, Kirchgessner N, Walter A. 2014. Artificial pores attract barley roots and can reduce artifacts of pot experiments., 177(6): 903–913.
Piron D, Pérès G, Hallaire V, Cluzeau D. 2012. Morphological description of soil structure patterns produced by earthworm bioturbation at the profile scale., 50: 83–90.
Ramalingam P, Kamoshita A, Deshmukh V, Yaginuma S, Uga Y. 2017. Association between root growth angle and root length density of a near-isogenic line of IR64 rice withunder different levels of soil compaction., 20(2): 162–175.
Rasse D P, Smucker A J M. 1998. Root recolonization of previous root channels in corn and alfalfa rotations., 204(2): 203–212.
Rosolem C A, Foloni J S S, Tiritan C S. 2002. Root growth and nutrient accumulation in cover crops as affected by soil compaction., 65(1): 109–115.
Sanchez P A. 1973. Puddling tropical rice soils: 2. Effects of water losses., 115(4): 303–308.
Shrestha R, Al-Shugeairy Z, Al-Ogaidi F, Munasinghe M, Radermacher M, Vandenhirtz J, Price A H. 2014. Comparing simple root phenotyping methods on a core set of rice genotypes., 16(3): 632–642.
Singh S P, Jain A, Anantha M S, Tripathi S, Sharma S, Kumar S, Prasad A, Sharma B, Karmakar B, Bhattarai R, Das S P, Singh S K, Shenoy V, Chandra Babu R, Robin S, Swain P, Dwivedi J L, Yadaw R B, Mandal N P, Ram T, Mishra K K, Verulkar S B, Aditya T, Prasad K, Perraju P, Mahato R K, Sharma S, Anitha Raman K, Kumar A, Henry A. 2017. Depth of soil compaction predominantly affects rice yield reduction by reproductive-stage drought at varietal screening sites in Bangladesh, India, and Nepal., 417: 377–392.
Steduto P, Faurès J M, Hoogeveen J, Winpenny J, Burke J J. 2012. Coping with Water Scarcity: An Action Framework for Agriculture and Food Security. Rome, Italy: Food and Agriculture Organization of the United Nations: 38.
Stirzaker R J, Passioura J B, Wilms Y. 1996. Soil structure and plant growth: Impact of bulk density and biopores., 185(1): 151–162.
RStudio Team. 2020. RStudio: Integrated Development Environmentfor R. RStudio, PBC, Boston, MA, USA. http://www.rstudio.com/.
Timsina J, Jat M L, Majumdar K. 2010. Rice-maize systems of South Asia: Current status, future prospects and research priorities for nutrient management., 335(1): 65–82.
Weller S, Janz B, Jörg L, Kraus D, Racela H S U, Wassmann R, Butterbach-Bahl K, Kiese R. 2016. Greenhouse gas emissions and global warming potential of traditional and diversified tropical rice rotation systems., 22(1): 432–448.
White R G, Kirkegaard J A. 2010. The distribution and abundance of wheat roots in a dense, structured subsoil: Implications for water uptake., 33(2): 133–148.
Williams S M, Weil R R. 2004. Crop cover root channels may alleviate soil compaction effects on soybean crop., 68(4): 1403–1409.
Yuan S, Stuart A M, Laborte A G, Rattalino Edreira J I, Dobermann A, Kien L V N, Thúy L T, Paothong K, Traesang P, Tint K M, San S S, Villafuerte M Q, Quicho E D, Pame A R P, Then R, Flor R J, Thon N, Agus F, Agustiani N, Deng N Y, Li T, Grassini P. 2022. Southeast Asia must narrow down the yield gap to continue to be a major rice bowl., 3(3): 217–226.
Zhang Z B, Yan L, Wang Y K, Ruan R J, Xiong P, Peng X H. 2022. Bio-tillage improves soil physical properties and maize growth in a compacted vertisol by cover crops., 86(2): 324–337.
Zhao K Y, Tung C W, Eizenga G C, Wright M H, Ali M L, Price A H, Norton G J, Islam M R, Reynolds A, Mezey J, McClung A M, Bustamante C D, McCouch S R. 2011. Genome-wide association mapping reveals a rich genetic architecture of complex traits in., 2: 467.
Zhou W, Lv T F, Chen Y, Westby A P, Ren W J. 2014. Soil physicochemical and biological properties of paddy-upland rotation: A review., 2014: 856352.
Copyright © 2023, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2023.03.017
27 January 2023;
31 March 2023
Md. Dhin Islam (dhinislam@bsmrau.edu.bd)
(Managing Editor: Wu Yawen)
杂志排行
Rice Science的其它文章
- Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
- Effect of GW8 Gene Editing on Appearance Quality of Erect-Panicle Type (dep1) Japonica Rice
- Transcriptome Analysis of oserf922 Mutants Reveals New Insights into Rice Blast Resistance
- ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice
- LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice
- Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa
