Characterization and antimicrobial, antioxidant, and anti-proliferative activities of green synthesized magnesium oxide nanoparticles with shoot extracts of Plicosepalus curviflorus
2023-08-07ReemHamoudAlrashoudiManalAbudawoodAyeshaMateenHajeraTabassumNouraIbrahemAlghumlasSabihaFatimaBasmahAlmaarikFarahMaqsoodNawalAlMusayeibMusaratAmina
Reem Hamoud Alrashoudi, Manal Abudawood, Ayesha Mateen✉, Hajera Tabassum, Noura Ibrahem Alghumlas,Sabiha Fatima, Basmah Almaarik, Farah Maqsood, Nawal M.Al Musayeib, Musarat Amina
1Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh 12372, Saudi Arabia
2Department of Optometry and Vision Science, College of Applied Medical Science, King Saud University, Riyadh 11451, Saudi Arabia
3Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh 11495, P.O.Box 22452, Saudi Arabia
ABSTRACT
Objective: To synthesize magnesium oxide nanoparticles using ethanol extract of shoots of Plicosepalus curviflorus (PC-MgONPs)and evaluate the antimicrobial, antioxidant, and anti-proliferative activities of PC-MgONPs.
Methods: The green synthesized PC-MgONPs were characterized by ultraviolet-visible (UV), Fourier-transform infrared spectroscopy,zeta potential, energy dispersive X-ray, and scanning electron microscopy.Furthermore, we investigated total antioxidant capacity and antimicrobial and anti-proliferative activities using breast cancer cell lines (MDA-231).
Results: The UV spectrum of PC-MgONPs showed a sharp absorption peak at 300 nm.The presence of magnesium, oxygen,and sodium was confirmed by energy dispersive X-ray analysis.Scanning electron microscopy revealed PC-MgONPs as roughly spherical granular structures with sizes ranging from 20.0 to 76.4 nm.PC-MgONPs showed considerable antimicrobial activities against Escherichia coli, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans with zones of inhibition of 11-17 mm.In addition, total antioxidant capacity and anti-proliferative activity of PC-MgONPs against MDA-231 cells were dose-dependent.
Conclusions: The synthesized PC-MgONPs could be a potent antimicrobial, antioxidant and anti-cancer agent, which needs further investigation.
KEYWORDS: PC-MgONPs; Plicosepalus curviflorus; FTIR;Antimicrobial; Cell viability; Antioxidant
1.Introduction
Magnesium oxide nanoparticles (MgONPs) are interesting nanomaterials with excellent surface reactivity, promising biomedical applications, remarkable stability under harsh conditions,and less harmful effects, and can be employed as economical precursors[1].These nanomaterials have shown potential biomedical features, including, antioxidant, antimicrobial, anticancer, antiinflammatory, anti-diabetic, bone regeneration, and analgesic effects[2-4].MgONPs are utilized as ointments for the treatment of heart burns, wounds, and bone regeneration.These nanoparticles also exhibited excellent toxic properties against a wide range of human diseases that are resistant to many drugs[5].Compared to other metal nanoparticles, MgONPs have many benefits, including non-toxicity,biocompatibility, cost-effectiveness, and stability, possessing important medical uses and potent antibacterial properties[6].The Food and Drug Administration has determined that magnesium oxide (MgO) is generally recognized as safe for use in human food[7].Moreover, recently, the Food and Drug Administration has authorized the use of magnesium sulphate injection in the treatment of preeclampsia in order to prevent seizures in patients[8].
Significance
Magnesium oxide nanoparticles (MgONPs) have emerged as attractive nanomaterials with diverse biomedical applications due to their distinctive physicochemical features, including biocompatibility, high stability, biodegradability, cationic capacity, and redox properties.The current study reports the synthesis of MgONPs using ethanol extract of Plicosepalus curviflorus shoot and MgONPs show antibacterial, antioxidant,and anti-proliferative activities.
Plicosepalus curviflorus (P.curviflorus) grows natively in Saudi Arabia, Yemen, East Africa, and Northeast Africa[9].In Saudi Arabia,the entire plant has been used in traditional medicine to heal diabetes and in Yemen, the stem and heated twigs of this plant have been used in cancer treatment and as a poultice on the chest to cure pneumonia,respectively[10].The flavane gallates, triterpenes, quercetin,catechin, and sterols found in the shoot extracts have antioxidant,hypoglycemic, antibacterial, cytotoxic, and anti-diabetic effects[11].
The aerial portions of P.curviflorus have been selected for green synthesis of MgONPs as the shoot part of the selected plant possesses active phenolic compounds (catechin, curviflorside, and curviflorin) with strong antibacterial and anti-proliferative activities,which we have isolated in our previous study[12].Green synthesis of plant-based nanoparticles from metals, especially magnesium,and MgO, was used for numerous purposes[13,14].The study aims to synthesize MgONPs using shoot extracts of P.curviflorus (PCMgONPs) as the above-mentioned compounds are involved in the reduction, capping, and stabilization of formed nanoparticles as well as enhancement of biological properties of biosynthesized MgONPs.The antimicrobial, antioxidant, and anti-proliferative effects of MgONPs were also evaluated.
2.Materials and methods
2.1.Preparation of P.curviflorus ethanol extracts
The ethanol extract was prepared by macerating dried powder of P.curviflorus shoots (500 g) in ethanol (2 L × 3, each) for 8 h using a Soxhlet apparatus at room temperature.The solvent was chosen based on its solubility, and safety, and solvents with polarity values close to solute polarity perform better extraction.The resultant ethanol extract was filtered and subsequently liberated from the organic solvent by rotary evaporation at 50 ℃ under decreased pressure, yielding dark green ethanol extract (14.0 g).
2.2.Green synthesis of MgONPs using biomass of P.curviflorus
The green synthesis of PC-MgONPs was carried out by adding freshly prepared 1.0 mM magnesium nitrate (1.02 g of magnesium nitrate dissolved in 100 mL of distilled water) solution into 100 mL of P.curviflorus solution (5 g dissolved in 200 mL of ethanol) and stirred continuously at 600 rpm at 80 ℃ for 6 h with a magnetic stirrer (HJ-3 Thermostatic Magnetic Stirrer, Jiangsu, China).Afterward, 10 mL of NaOH (1.0 M) was poured dropwise into the reaction mixture to form a precipitate.The color change from green to dark brown was used to monitor the creation of nanoparticles.The reaction mixture was then centrifuged at 5 000 rpm for 15 min,the recovered precipitate was washed several times with ethanol to remove impurities, and dried PC-MgONPs (7.5 g) were calcined at 400 ℃ in a furnace.The mechanism of formation of PC-MgONPs was represented by the following equation:
2.3.Spectroscopic and microscopic characterization of PCMgONPs
The formation of PC-MgONPs was confirmed by measuring the typical peak through a UV-Vis spectrophotometer at an absorption wavelength range of 200 to 800 nm (Shimadzu Corporation, Kyoto,Japan).FTIR analysis was performed to detect functional moieties and operated in a 400-4 000 cm-1spectral range (Spectrometer-vector 22, Bruker, Germany).The crystalline structure of pre-synthesized PC-MgONPs was confirmed by X-ray diffraction (XRD) using Miniflex 600, X-ray diffractometer, Holland and it was operated at 40 kV with a current of 30 mA using CuKα radiation at 2θ angle ranging from 20 ℃ to 80 ℃.The stability and size distribution of the formed PC-MgONPs were assessed by zeta potential (ZP) and dynamic light scattering (DLS) using a particle size zeta potential analyzer based on laser light scattering (Zetasizer NS-3000,Malvern Analytical Ltd, Malvern, United Kingdom).The surface morphological features, including size, shape, and composition of PC-MgONPs, were monitored by scanning electron microscopy(SEM) equipped with energy-dispersive X-ray (EDX) spectroscopy using Zeiss SEM (Zeiss MultiSEM-505, Jena, Germany) with spectral images.Also, the elemental composition of the PC-MgONPs was estimated through EDX.
2.4.Preparation of stock solutions
The working stock solution was prepared by weighing 100 mg of P.curviflorus ethanol extracts and dissolved in dimethyl sulfoxide at a concentration of 100 mg/mL.PC-MgONPs was weighed and dissolved in a solution containing sterile distilled water and nitric acid (4.8 mL water + 0.2 mL of nitric acid) to get the concentration of 10 mg/mL.The stock solution prepared were used to evaluate cell viability, antimicrobial and total antioxidant activity.
2.5.Microbial cultures
In the present study, five microbial strains were used to evaluate antimicrobial activity and minimum inhibitory concentration (MIC)of P.curviflorus ethanol extracts and PC-MgONPs.Three ATCC(American Type Culture Collection) microbial strains were used including Escherichia coli (E.coli, ATCC 25922), Staphylococcus aureus (S.aureus, ATCC 29213) and Candida albicans (C.albicans,ATCC 10231).Two clinical isolates [methicillin-resistant S.aureus(MRSA) and Pseudomonas aeruginosa (P.aeruginosa)] were used for the antimicrobial sensitivity.
2.6.Evaluation of antimicrobial activity
2.6.1.Agar well diffusion assay
The antimicrobial activity of PC-MgONPs was performed by agar well diffusion method.The bacterial cultures were grown in Muller Hinton broth (MHB) for 12-18 h and the turbidity of bacterial cultures was adjusted to a 0.5 McFarland standard (1×108CFU/mL).One mL of bacterial culture (1×108CFU/mL) was pipetted into the center of a sterile Muller Hinton agar (MHA) petri dish and spread on the plate using a spreader and wells were made into agar plates containing inoculums using a sterile cork borer (6 mm in diameter).Then, 100 μL of stock solution of the plant extract (100 mg/mL) and PC-MgONPs (10 mg/mL) were added to agar wells as test samples,and a disc of standard antibiotic (gentamycin) was used as a standard control.
The culture plates were incubated at 37 ℃ for 18-24 h.The zone of inhibition (including the diameter of the wells) was measured to detect antimicrobial activity[15].
2.6.2.MIC assay and IC50 calculation
The MIC of the plant extract (100 mg/mL) and PC-MgONPs (10 mg/mL) stock solution was determined by broth microdilution method, using sterile 96-well polystyrene cell culture plates.The microdilution plate was prepared by adding 100 μL MHB from well 2 to well 12.The first well contained 200 μL of the plant extract and PC-MgONPs and serial double dilution from wells 1 to 10 was performed by transferring 100 μL from each well.Finally, 10 μL of bacterial suspension was added to all the wells except well 12.Well 11 was kept as a positive control containing MHB and bacteria suspension, while well 12 was marked as a negative control containing only MHB.Next, the plates were incubated at 37 ℃ for 18-24 h.After incubation, a freshly prepared 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent in sterile water at 0.5 mg/mL stock solution of volume 40 μL was added to each well and incubated at 37 ℃ for 30 min, and plates were measured at an absorbance of 600 nm in a microplate reader(SpectraMax plus 384).The experiment was done in triplicate[16,17].
The IC50calculations were performed using the online QuestGraph™IC50Calculator online link (https://www.aatbio.com/tools/IC50-calculator)[18].
2.7.Determination of total antioxidant capacity (TAC)
The in vitro antioxidant potential of PC-MgONPs was performed by TAC assay TAC using a total antioxidant capacity kit, MK-187,Sigma.Briefly, the test involves the reduction of Cu2+ion to Cu+by small molecules and proteins.The reduced Cu+ion chelates with a colorimetric probe, giving a broad absorbance peak at 570 nm,which is proportional to TAC[19].
2.8.Determination of cell viability/proliferative activity
The cell viability of PC-MgONPs against a breast cancer cell line(MDA-231) was evaluated using the MTT assay.The cancer cells were grown in Dulbecco’s Modified Eagle Medium supplemented with 1% antibiotic and 10% of fetal bovine serum solution in a humidified incubator with 5% CO2at 37 ℃.To measure cell inhibition, an MTT assay was performed.The cancer cells were seeded in 96-well plates at a density of 1×105and incubated at 37 ℃ for 1 d.During the incubation time, cells were treated with PC-MgONPs (500, 250, 125, 62.5, 31.25, and 15.62 μg/mL) and P.curviflorus (5 000, 2 500, 1 250, 625, 312.5 and 156.25 μg/mL)for additional 24 h at 37 ℃.Then, 10 μL of MTT reagent solution was added to each well, and the plates were incubated at 37 ℃ for 4 h.With the addition of 100 μL of dimethyl sulfoxide, the purplecolored formazan crystals were dissolved.The absorbance was measured at 570 nm using a multiplate reader[20].
The cell inhibition percentage was calculated with the equation:Cell inhibition (%) = 100 - (ODtest/ODcontrol× 100).
2.9.Statistical analysis
Statistical analyses were performed in triplicates using one-way ANOVA and data were presented as mean ± standard deviation.A P-value < 0.05 was considered significantly different.The GraphPad Prism Software 5th version for Windows, La Jolla, CA was used to perform statistical analysis.
3.Results
3.1.UV-Vis spectral analysis
The formation of PC-MgONPs was confirmed by UV-Vis spectroscopy (wavelength range: 200-800 nm).A characteristic SPR absorbance peak was observed at 300 nm which verified the formation of PC-MgONPs (Figure 1).

Figure 1.UV-Vis spectra of magnesium oxide nanoparticles using shoot extracts of Plicosepalus curviflorus (PC-MgONPs).
3.2.FTIR spectroscopy
FTIR profile of P.curviflorus extract (Figure 2A) illustrated 13 peaks positions at 3 440.58, 2 995.75, 2 912.76, 2 194.79, 2 015.64,1 660.45, 1 436.25, 1 406.56, 1 310.31, 1 018.46, 951.96, 697.67 and 667.22 cm-1, whereas the PC-MgONPs reaction mixture elucidated 6 absorbance peaks sites at 3 292.18, 2 165.33, 2 150.41, 1 992.65,1 633.88, and 1 338.33 cm-1(Figure 2B).The absorbance peaks located between 3 000-3 600 cm-1were allocated to the stretching vibrations of hydroxyl (-OH) amine (-NH) and (-CH) groups where-NH was characterized by a lower peak value than -OH.

Figure 2.FTIR analysis of (A) Plicosepalus curviflorus and (B) PC-MgONPs.
According to FTIR data, the amide linkage of protein had a higher potential to bond with magnesium, resulting in the formation of a protein coating around MgONPs that prevents agglomeration and stabilizes the medium.In this study, the involvement of hydroxyl groups, amide group, and alkene functional groups in the bioreduction process could be confirmed through the shift of the deformation vibration of O-H, -N-H, and -C=C groups positioned at 3 440.58 cm-1, 2 194.79 cm-1, and 1 660.45 cm-1to 3 292.18 cm-1,2 165.33 cm-1, and 1 633.88 cm-1, respectively.Thus, it is reasonable to conjecture that hydroxyl, amine alkene, and alkyne functional groups present in the extract of P.curviflorus possibly perform both the capping and reduction process of nanoparticle formation.
XRD spectra analyzed the crystallinity and purity of biogenicformed PC-MgONPs (Figure 3A), and the major XRD peaks of 2θ values were 36.8°, 42.4°, 61.8°, 74.9°, and 78.6°.The characteristic Bragg reflections were indexed location to (111), (200), (220),(311), and (222) lattice planes of face-centered cubic structure as compared with the standard reference (JCPDS file no.39-7746)[21]and these peaks confirmed the existence of MgONPs.The sharp diffraction pattern of pre-synthesized PC-MgONPs confirmed its high crystallinity and nano-size.Debye Scherrer’s equation was applied to calculate the size of biogenic PC-MgONPs by using the width of the strongest peak (200) located at 42.4° (2θ value).The results revealed that the average crystallite size was 25 nm.

Figure 3.(A) X-ray diffraction spectrum and (B) size distribution analysis of PC-MgONPs.
The average size distribution of the PC-MgONPs in the solution and the thickness of the capping or stabilizing compound enveloping metallic particles was determined by DLS.The results showed average particle size and polydispersity index (PDI) were 126.2 nm and 0.287 respectively (Figure 3B).The formed PC-MgONPs had a PDI of less than 0.7 demonstrating their high quality and reasonably well-defined dimensions with high monodispersity (PDI).The surface charge of pre-synthesized PC-MgONPs and the magnitude of charge were calculated by zeta potential and found to be 86.79 value.
3.3.Microscopic characterization of PC-MgONPs
SEM images of PC-MgONPs revealed that the particles had a spherical form and well-dispersed nanorods of PC-MgONPs that lacked any aggregation, with sizes ranging from 20.0 to 76.4 nm.In addition to producing a spherical shape that was well distributed and had a high ratio of surface area to volume, larger clusters were generated (Figure 4A).EDX spectra were used to analyze the elemental composition of PC-MgONPs (Figure 4B).The EDX graph revealed that the photomediated PC-MgONPs were exceedingly pure, displaying weight and atomic percentages of Mg (31.01%, 38.1%), O (55.1%, 51.3%), and Na (13.8%, 10.6%).

Figure 4.(A) Scanning electron microscopy and (B) energy dispersive X-ray analysis of biogenic PC-MgONPs.The arrow in the image represents the size of the particles (nm).
3.4.Antimicrobial activity, TAC, and anti-proliferative activity of PC-MgONPs
3.4.1.Antimicrobial activity
The antimicrobial activity of P.curviflorus extract and green synthesized PC-MgONPs was evaluated against E.coli, S.aureus,MRSA, P.aeruginosa, and C.albicans.PC-MgONPs were found to be effective against E.coli and C.albicans with a zone of inhibition of 17 and 16 mm, respectively (Figure 5 and Table 1).

Table 1.Diameter of zone of inhibition of PC and PC-MgONPs (mm).
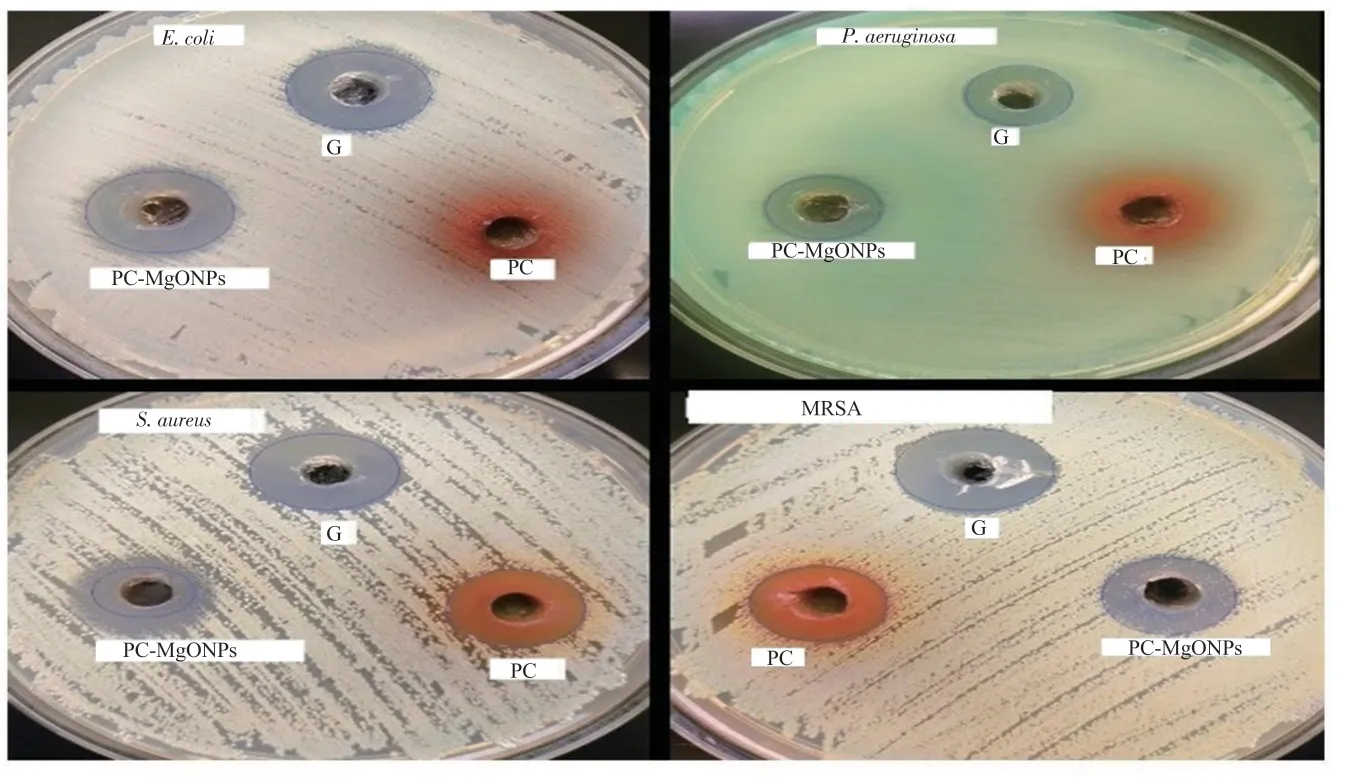
Figure 5.Antimicrobial effect of PC-MgONPs on the microbial strains by Agar well assay.G: gentamicin.
In addition, S.aureus showed the least MIC value of 15.62 μg/mL and IC50value of 7.17 μg/mL, whereas E.coli and C.albicans showed MICs of 31.25 and 62.50 μg/mL with IC50values of 10.59 and 15.84 μg/mL, respectively (Table 2).P.curviflorus extracts showed the highest MIC values (1 250 and 2 500 μg/mL) against S.aureus and MRSA and showed a partial zone of inhibition.

Table 2.Minimum inhibitory concentration (MIC) and IC50 values of PCMgONPs and PC (μg/mL).
3.4.2.TAC
The results of TAC assay are illustrated in Figure 6A.The green synthesized PC-MgONPs displayed excellent antioxidant potential.As reflected in the figure, a dose-dependent pattern was observed in the antioxidant activity ranging from 0.042-0.360 nmol/mL.Moreover, 10 mg/mL of PC-MgONPs exhibited the highest TAC.

Figure 6.(A) Total antioxidant capacity and (B-C) anti-proliferative activity of PC-MgONPs and PC against breast cancer MDA-231 cells.*P<0.05.
3.4.3.Anti-proliferative activity
PC-MgONPs and P.curviflorus extract showed a concentrationdependent decline in cell viability (P < 0.05).In addition, PCMgONPs exhibited more significantly inhibitory effects against MDA-231 cell viability compared to P.curviflorus extract (Figure 6B-C).
4.Discussion
A biological approach for the synthesis of metal and metal oxide nanoparticles can be employed as an alternative to chemical and physical methods[22].The benefits of biological synthesis,including its low cost, environmental friendliness, biocompatibility,scalability, and evasion of tough synthesis conditions like high temperature and pH, can be credited with this phenomenon[22].Among biological entities, plants are recognized as promising entity for the biogenic synthesis of nanoparticles due to the presence of chemical components and high metal tolerances[23].MgONPs are acknowledged by the FDA as a safe alternative to antibiotics with highly efficient antibacterial properties[7,24].The ethanol extract of P.curviflorus shoots is a rich source of different types of bioconstituents including flavonoids, phenols, terpenes, steroids,proteins, and carbohydrates[12].These phytocomponents might have contributed to the reduction, stabilization, and capping of produced PC-MgONPs, which plays a crucial role in the reduction of Mg(NO3)2·6H2O to MgO-NPs.The hydroxyl and carbonyl groups of phytoconstituents serve as reducing and stabilizing substances[25].The formation of biogenic PC-MgONPs initiates once the P.curviflorus extract is introduced into 1.0 mM Mg(NO3)2solution.The gradual color change of Mg(NO3)2/P.curviflorus solution from dark green to dark brown indicates the formation of PC-MgONPs.This color change can be attributed to the role of P.curviflorus metabolites in the reduction of NO3-to NO2followed by the reduction of Mg2+to Mg(OH)2by liberated electrons.The maximum color intensity was attained due to the reduction of a large number of metal ions.The as-prepared Mg(OH)2was calcinated at 400 ℃ to form PCMgONPs[26].The formation of PC-MgONPs was further confirmed by different spectroscopic (UV-Vis, FTIR, XRD, ZI, DLS) and microscopic (SEM and EDX) techniques.
The UV-Vis spectroscopy analysis showed a characteristic SPR absorbance peak at 300 nm, which satisfies the standard MgO absorption pattern because all oxide nanomaterials have wide band gaps and a propensity for shorter wavelengths.The formation of PC-MgONPs was confirmed by UV-Vis spectroscopy (wavelength range: 200-800 nm)[27].
FTIR analysis was performed to identify the potential biomolecules that contributed to the bioreduction of magnesium oxide and stabilization of PC-MgONPs[28].The presence of peaks between 2 100-2 250 cm-1corresponds to alkyne (C≡C, C≡N) functional groups, and the peak appearance at 2 194.79 cm-1in P.curviflorus extract and 2 165.33 cm-1in PC-MgONPs indicates the presence of unsaturated hydrocarbons in the extract as well as in biogenic PCMgONPs[29].The appearance of prominent peaks at around 1 662-1 626 cm-1is characteristic of the C=C stretching vibration type for disubstituted alkene.The peak shifting in the spectral profile of PC-MgONPs could be attributed to the interactions between those chemical functional groups and MgONPs[30].It is reported in the literature that free amide groups in the protein molecules allow them to interact with MgONPs[31].Our results exhibited broad distinctive spectral bands at 3 292.18-3 440.58 cm-1are characteristics of the O-H stretching vibration type of hydroxyl functional moiety in polyphenols and N-H stretching vibration in primary and secondary amines (amino acids, proteins, and peptides) as well as hydrocarbons[32].The results also indicate that some organic residues, such as hydroxyl and carboxyl groups, are present on the surface of the prepared PC-MgONPs.
The surface charge of pre-synthesized PC-MgONPs and the magnitude of charge were calculated by zeta potential and found to be 86.79 value, which illustrates the nanoparticle stability in dispersion by developing specific charge groups on their surface[33].The result revealed that PC-MgONPs produced by P.curviflorus shoot extracts exhibited a charge on the surface of the PC-MgONPs,possibly due to free nitrate ions in the reaction mixture that provides repulsive force as electrostatic force stabilization[34].Furthermore,no particle agglomeration was seen, as the formed PC-MgONPs were well dispersed.These results indicate that phytocomponents of the plant extract served not only as a reducing agent but also as a stabilizer, which protects the aggregation of MgONPs.
SEM analysis displayed spherical and well-dispersed nanorods of PC-MgONPs with sizes ranging from 20 to 76.4 nm[35].The EDX graph revealed that the PC-MgONPs were exceedingly pure,displaying weight and atomic percentages of Mg (31.01%, 38.1%),O (55.1%, 51.3%), and Na (13.8%, 10.6%), which corresponds to the synthesis of MgO via ethanol extract of P.curviflorus[36].
PC-MgONPs were also found to be effective against E.coli and C.albicans with a zone of inhibition of 17 and 16 mm in contrast to other studies of MgONPs antibacterial activity which showed zero zones of inhibition against E.coli and S.aureus[37].PC-MgONPs exhibited very effective effects in comparison with MgONPs[37].Whereas, in our previous study, the biogenic green synthesis of MgONPs using Saussurea costus biomasses exhibited the highest MIC values for E.coli and P.aeruginosa in contrast to those of PCMgONPs in our present research work[38].
Among the Gram-negative bacteria, E.coli was most sensitive to PC-MgONPs, because the Gram-negative bacteria are coated with lipopolysaccharide molecules, which have a negative charge and bind with positively charged MgO ions on the surface of PCMgONPs, possibly leading to increased uptake of ions that damages the intracellular structures of Gram-negative bacteria[39].
As reported in the previous research, three compounds quercetin,catechin, and flavane gallate 2S, 3R-3,3′,4′,5,7-pentahydroxyflavane-5-O-gallate were isolated from the aerial parts of P.curviflorus[16],flavane gallate and catechin possess antimicrobial activity against various Gram-positive and Gram-negative pathogenic bacterial strains by binding to the lipid bilayer which damages bacterial membrane leading to inactivation and inhibition of intracellular and extracellular enzymes synthesis[40].
Numerous studies indicate that the antimicrobial activity of MgO is generally attributed to the production of reactive oxygen species(ROS), causing membrane breakdown and cellular content leakage.It has been shown that bacterial cells treated with MgONPs produce deep craters on their membrane surface, indicating that structural damage to the membrane leads to cellular content leakage.Whereas,our results showed PC-MgONPs had better antimicrobial activity than the plant extract which only showed antibacterial effects against S.aureus and MRSA.Nanoformulation of catechin and other active plant components had strong antibacterial activity, but in the present study, the plant extract exhibited less antimicrobial activity against the pathogenic microbial strains[41].
Although cellular oxidation is essential for cell proliferation, it also has some negative side effects due to the production of free radicals and ROS.Overproduction of these free radicals has severe consequences on the protective antioxidant system, which in turn causes cellular damage by oxidizing essential macromolecules,ultimately resulting in apoptosis/cell death.TAC analysis confirmed PC-MgONPs possess superior antioxidant capability, which may be due to the capping effect of phytochemicals like flavonoids, which have several hydroxyl groups and phenolic functional groups on their surface, making synergistic effects.Our results both confirm and extend the work of previous researchers[42].
Moreover, curviflorin, curviflorside, flavonoids, and naphthalene were found in shoot parts of P.curviflorus[11], which possess strong antioxidant activity.Previous studies on the extracts of P.curviflorus and PC-MgONPs support our present findings.PC-MgONPs exhibited strong antioxidant activity which involves the redox potential of phytochemicals and the active compounds found in the P.curviflorus extract and the MgO antioxidant capability[43].
In our results, a decrease in cell viability of MDA-231 cells was observed as the concentration of PC-MgONPs and P.curviflorus extract increased, which may be attributed to the generation of ROS by PC-MgONPs, resulting in damages to mitochondrial membrane integrity and thus activation of the apoptotic pathway leading to cell death.The result was consistent with that found in a previous study in which P.curviflorus extract showed the expression of apoptosisregulating genes including caspase-3, -8, -9, p53, Bax, and Bcl-2[29].Contrary to what has been shown in the past, the toxicity of PC-MgONPs may be directly related to their size, with smallersize PC-MgONPs being more hazardous because they generate more ROS that interact with cellular components and penetrate cell membranes, releasing Mg+ions[36,44].Among the phytochemicals,phenolic compounds such as catechin showed an anti-proliferative effect against lung cancer A549 cells by inhibiting cyclin E1 and p-AKT and induction of a potent cyclin kinase inhibitor.Moreover,epicatechin induces apoptosis in breast and prostate carcinomas[45].
To conclude, PC-MgONPs were successfully synthesized using P.curviflorus shoot extract.PC-MgONPs showed antimicrobial,antioxidant, and anti-proliferative properties.However, in vivo studies are needed to further verify their effects.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
The authors extend their appreciation to the Research Supporting Project number (RSPD2023R656), King Saud University, Riyadh,Saudi Arabia.
Funding
This work was funded by the Researchers Supporting Project Number (RSPD2023R656), King Saud University, Riyadh, Saudi Arabia.
Authors’ contributions
RHA theorized and designed the research study, reviewed the literature, acquired data, and critically commented on the original draft of the manuscript.MA reviewed and edited the final draft of the manuscript.AM contributed to the conceptualization and design of the study, interpreted the results, and wrote the manuscript.HT designed the study and contributed to the discussion of the study.NIA managed data collection and analysis.SF interpreted the results.BA critically revised and edited the final version of the manuscript.FM critically commented on and edited the final version of the manuscript.NMA managed data collection.MA interpreted the results.All the authors have read and agreed with the published version of the manuscript.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Myricetin alleviates ovalbumin-induced allergic rhinitis in mice by regulating Th1/Th2 balance
- Role of flaxseed (Linum usitatissimum L.) in disease prevention and treatment
- Cryptotanshinone ameliorates cladribine-induced cognitive impairment in rats
- Epigallocatechin-3-gallate exerts antihypertensive effects and improves endothelial function in spontaneously hypertensive rats
