Determination of Lead in Lime-preserved Egg by Microwave Digestion and Flame Atomic Absorption Spectroscopy
2023-08-02MimiZHANG
Mimi ZHANG
Anhui Institute for Food and Drug Control, Hefei 230051, China; National Agriculture vice-processing Food Surveillance Test Center, Hefei 230051, China
Abstract [Objectives] To establish a method for determining the lead content in lime-preserved eggs, to provide a theoretical basis for the quality control of production enterprises and the sampling and testing of supervision departments. [Methods] The lead content in lime-preserved eggs was measured by the microwave digestion and flame atomic absorption spectroscopy. [Results] The method had a correlation coefficient of r2=0.998 in the injection concentration range of 0-4 mg/L. The detection limit of the method was 0.008 2 mg/kg. In the range of 0.2 to 1.0 mg/kg addition concentration, the recovery rate of the method was 92.5%-108.0%, and the relative standard deviation (RSD) was <5%. [Conclusions] This method is accurate and reliable, simple and efficient, and is suitable for the detection of lead in lime-preserved eggs.
Key words Microwave Digestion, Flame atomic absorption spectroscopy, Lime-preserved eggs, Lead
1 Introduction
Lime-preserved eggs, also known as Pidan or pine-patterned eggs, have a long history in China and are a traditional food loved by Chinese people. The ancient processing technology requires the use of lead oxide[1]. If improperly controlled, the lead content in lime-preserved eggs will easily exceed the standard. Excessive lead exposure will damage the human nervous system, heart, and respiratory system, reduce concentration, and have a significant impact on hematopoietic, digestive, and reproductive functions, and it may also affect brain metabolism, leading to decreased intelligence and cognitive function in children[2-4]. Although most of the current lime-preserved eggs production technologies have been improved and lead oxide is no longer used, some producers and small workshops still use traditional techniques to make lime-preserved eggs, resulting in a higher content of lead in lime-preserved eggs[5]. Therefore, monitoring the content of lead in lime-preserved eggs has high reference value for consumers to purchase lime-preserved eggs products.
At present, the main methods for determining the lead content in food include flame atomic absorption spectroscopy, graphite furnace atomic absorption spectroscopy, atomic fluorescence spectroscopy, inductively coupled plasma atomic emission spectroscopy, inductively coupled plasma mass spectrometry, anode dissolution voltammetry,etc.[6]. Among them, the atomic absorption spectroscopy method of graphite furnace has high sensitivity, but the stability is greatly affected by the type of graphite tube and the number of uses. It is more suitable for the detection of low-concentration samples, and the graphite tube is expensive as a consumable material and the cost of use is higher. The stability of atomic fluorescence spectroscopy is lower. Inductively coupled plasma atomic emission spectroscopy and inductively coupled plasma mass spectrometry equipment are expensive and difficult to popularize. The anode dissolution voltammetry method requires the use of a pure metal anode, which is expensive, and the dissolution rate of the substance on the anode is affected by factors such as potential, temperature, and electrolyte concentration, and needs to be strictly controlled. In addition, it also requires complex data processing and analysis. By contrast, the flame atomic absorption equipment is cheap, low cost of use, simple and efficient operation. In this study, it is intended to establish a method for the determination of lead content in lime-preserved eggs by microwave digestion-flame atomic absorption spectroscopy, so as to provide a theoretical basis for the quality control of production enterprises and the sampling and testing of supervision departments.
2 Materials and methods
2.1 Materials
2.1.1Lime-preserved eggs for test. The lime-preserved eggs for test were purchased from a large supermarket in Hefei City, Anhui Province.
2.1.2Main reagents. Standard solution of lead unit element (1 000 mg/L standard product, National Institute of Metrology of China), nitric acid (guaranteed reagent, Merck Group, Germany), experimental water (ultra-pure:25 ℃ resistivity ≥18.2 MΩ·cm, self-made).
2.1.3Instruments and equipment. Shimadzu Atomic Absorption Spectrophotometer (Shimadzu, Japan); DuoPUR acid sub-boiling distillation purification system (Beijing LabTech Instrument Co., Ltd., China); AL204 electronic analytical balance (sensitivity 0.000 1 g) (Mettler, Switzerland); microwave digestion system (Anton Paar, Austria).
2.2 Methods
2.2.1Digestion of samples. Weighed 0.5 g of lime-preserved eggs samples with uniform sample preparation, placed in a microwave digestion tube, added 8 mL of concentrated nitric acid and 1 mL of hydrogen peroxide, mixed evenly, closed the lid and put in the digestion tank for digestion. After the digestion was completed, cooled to room temperature, removed the digestion tube and placed it in an electric heating sleeve. After waiting for the acid to dry out, transferred to a 25 mL volumetric flask, rinsed the digestion tube with ultrapure water at least 3 times and transferred to the volumetric flask, and finally used ultrapure water to set the volume to the scale, and conducted the blank test at the same time.
2.2.2Preparation of standard solution. Accurately pipetted 1.0 mL of lead standard solution mother liquor into a 10 mL volumetric flask, and used 1% nitric acid solution to fix the volume to obtain 100 mg/L of lead standard intermediate solution.
2.2.3Instrument conditions. (i) Microwave digestion procedure. The first stage:120 ℃, the heating time was 10 min, and the holding time was 5 min; the second stage:150 ℃, the heating time was 5 min, and the holding time was 5 min; the third stage was 180 ℃, the heating time was 15 min, and the holding time was 20 min. (ii) Atomic absorption instrument conditions. Flame type:air-acetylene; measurement wavelength:283.3 nm; slit width:0.7 nm; gas flow rate:2.0 L/min; auxiliary gas flow rate:15.0 L/min; combustion head height:7 mm; combustion head width:10 mm; combustion head angle:0 degree; lamp current:10 mA; buckle background method:deuterium lamp.
2.2.4Linear relationship and detection limit. Separately pipetted 0, 0.5, 1.0, 2.0, and 4.0 mL of lead standard intermediate solution in a 100 mL volumetric flask, and fixed the volume to the scale with 1% nitric acid solution to obtain 0, 0.5, 1.0, 2.0, and 4.0 mg/L of lead standard solution. Adjusted the instrument to the optimal condition, injected samples in turn, measured the light absorption intensity and plotted a calibration curve. The standard blank was continuously measured 11 times to calculate the detection limit of lead by flame atomic absorption.
2.2.5Precision and recovery tests. The lime-preserved eggs sample with a background value of zero was divided into 3 parts, and the lead standard solution was added separately, so that the added concentration separately reached 0.2, 0.5, and 1.0 mg/kg. Accurately weighed 0.5 g of samples, and set the volume to 25 mL after the microwave digestion was complete. The atomic absorption conditions were set to be optimal, and the concentration of the standard sample was measured in turn, and the measurement was repeated 6 times for each sample.
3 Results and analysis
3.1 Plotting of the standard curveThe standard solutions with concentrations of 0, 0.5, 1.0, 2.0 and 4.0 mg/L were separately injected, and a standard curve for the absorbance intensity was plotted. The results are shown in Fig.1.
From Fig.1, it can be seen that in the injection concentration range of 0-4.0 mg/L, the regression equation of the measured standard curve wasy=0.025 3x-0.000 3. The linear correlation coefficientR2=0.998 8, showing a good linear correlation. Through 11 times of blank sample tests, the standard deviation of the blank sample signal value multiplied by 3 and then divided by the slope of the standard curve was taken as the minimum detection limit of this method, and the result was 0.008 2 mg/kg, indicating the sensitivity of the method is very high.

Fig.1 Standard curve
3.2 Precision and recovery rateTaking the limit of lead in lime-preserved eggs stipulated inNationalFoodSafetyStandardMaximumLevelsofContaminantsinFoods(GB 2762-2017)[7]0.5 mg/kg as the reference, three levels below, equal to and above the limit value (0.2, 0.5 and 1.0 mg/kg) were selected for precision and recovery tests. Each level was repeated for 6 times, and the precision and recovery rate at different addition levels were calculated. The results are shown in Table 1.
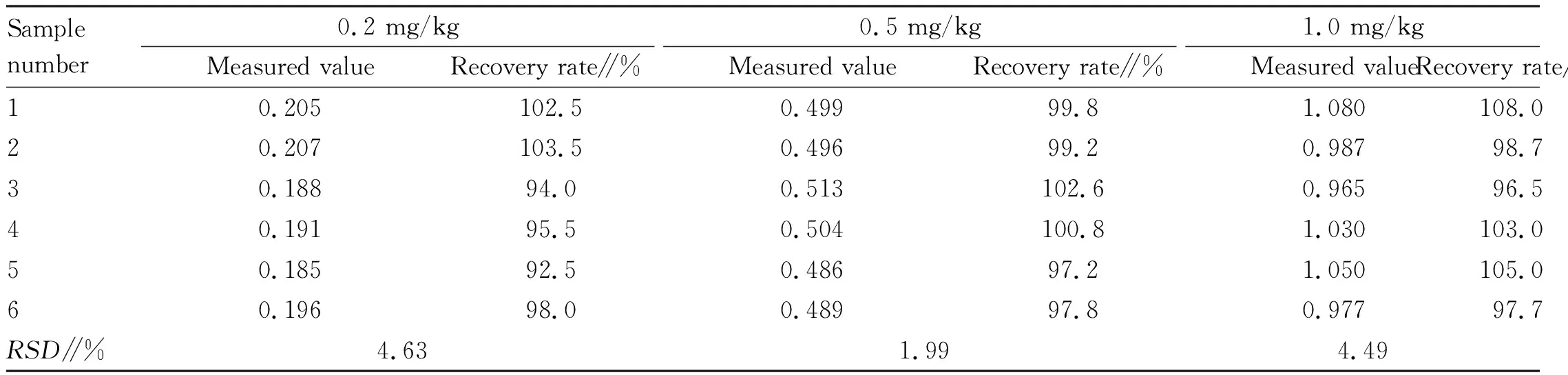
Table 1 Results of precision and recovery tests at different addition levels
As shown in Table 1, the recovery rate was 92.5%-108.0% and theRSDwas less than 5% when the limit level was lower than, equal to and higher than the national standard, indicating that the method has good accuracy and stability and is very suitable for the determination of lead in lime-preserved eggs.
3.3 Determination of lead in lime-preserved eggs samples
Ten samples of lime-preserved eggs were purchased from a large supermarket in Hefei City, Anhui Province, processed and injected according to the method stated above, and the content of lead in the samples was measured as shown in Table 2. The results showed that the content of lead in 6 samples of lime-preserved eggs purchased from supermarket was lower than the detection limit, and the method of determining the content of lead in lime-preserved eggs by microwave digestion instrument flame atomic absorption spectroscopy was feasible, simple and efficient.
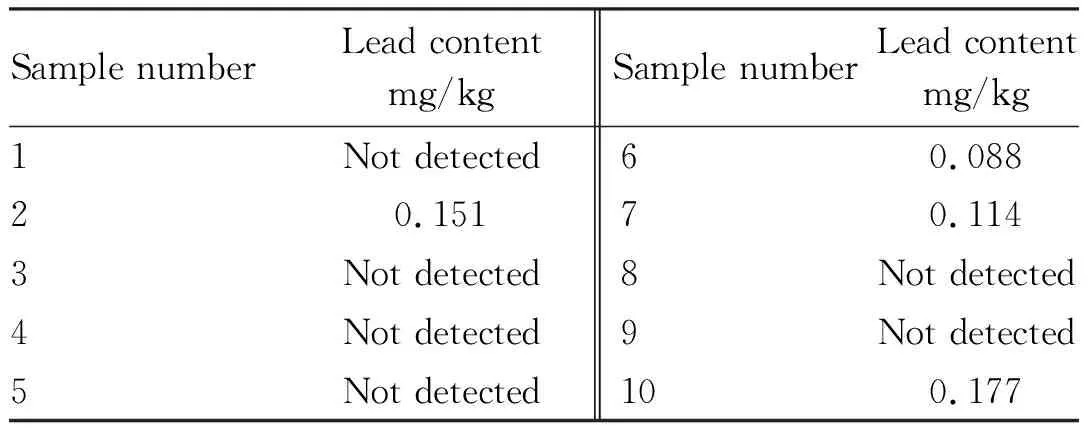
Table 2 Test results of lead in lime-preserved eggs
4 Conclusions and discussion
In this study, we established a method for the determination of lead in lime-preserved eggs by microwave digestion-flame atomic absorption spectroscopy. The method showed good linearity in the range of 0-4 mg/L, and the detection limit was 0.008 2 mg/kg, which was much lower than the national standard of 0.5 mg/kg for lead in lime-preserved eggs. Taking this limit value as a reference, the applicability of the method was investigated at low, medium and high concentration levels, and the results showed that the recovery rate of the method were in the range of 92.5%-108.0%. The method was used to test the lead content of 10 groups of lime-preserved eggs sold in the market, and the results were reliable, demonstrating that the method had very good applicability for the detection of lead in lime-preserved eggs.
杂志排行
Asian Agricultural Research的其它文章
- Analysis of Application Status and Recommendations for Shade-Tolerant Lawns and Ground Cover Plants in the West Lake Scenic Area
- Cloing and Bioinformatics Analysis of ndk Gene from Vibrio alginolyticus
- Design of Diversified Intelligent Control System for Energy-saving Optimization of Solar Greenhouse in North China
- Analysis of Land Use Change and Driving Factors in Mojiang County Based on PLUS Model
- Intellectual Property Protection and New Development Pattern Construction of Xinjiang Jujube
- Current Situation, Reasons and Suggestions of Income and Consumption of Rural Residents
