Preparation of Biomanganese Oxide-biochar Composite and Its Remediation of Arsenic Contamination
2023-06-27DilixiatiAbulizi
Dilixiati Abulizi
College of Tourism, Urumqi Vocational University, Urumqi 830001, China
Abstract [Objectives] To prepare biomanganese oxide/biochar composite (BMO-BC) and examine its remediation performance on arsenic contamination. [Methods] The BMO-BC was prepared by cultivating Pseudomonas putida MnB1 in the presence of Mn2+ and biochar. [Results] The initial concentration of Mn2+ in the culture system had no significant effect on the growth rate and domestication cycle of Pseudomonas putida MnB1. The results of SEM-EDS and XRD analysis confirm that the adsorbent prepared in this experiment was a composite of biomanganese oxide and biochar particles. The results of arsenic pollution removal test in simulated environment showed that the in-situ formed BMO-BC composite had certain removal effect on As (III) and the presence of biochar particles and manganese dioxide in the reaction system and the manganese oxidation ability of microbial strain MnB1 affect its remediation performance to As (III). [Conclusions] When the initial concentration is in the range of 0-10.0 mg/L, the isothermal adsorption data of BMO-BC on As (III) conforms to the Langmuir model.
Key words Arsenic pollution, Bio-manganese oxide, Biochar, remediation
1 Introduction
Arsenic is a highly toxic and carcinogenic non-metallic substance. As long-term intake of water contaminated with arsenic can cause many serious diseases, the presence of arsenic in groundwater has become a worldwide concern[1-2]. In aquatic systems, arsenic exists mainly in inorganic forms with two predominant species: As (V) and As (III)[2]. The As (III) species is 25-60 times more toxic than As (V). Due to the increasing toxicity and flow characteristics of As (III) compared to As (V), it is necessary to use a technology that simultaneously removes and detoxifies As (III)[3].
For decades, researchers have been working on methods to remove arsenic from contaminated water at minimal cost. Chemical precipitation, membrane separation, ion exchange, electrochemical methods, adsorption, etc. are all methods for removing arsenic mentioned in the literature[4-5]. In an environment with low arsenic content, the adsorption method has advantages over other methods, including low cost, easy operation, and low dosage of chemical additives[6].
In recent years, biochar has been widely used to remove pollutants in water, which provides a low-cost, environmentally friendly solution for the immobilization of pollutants in water[7]. Due to the negative charge on the surface of most biochar, it is difficult to adsorb arsenic in anion forms such arsenite [As (V)] or arsenite [As (III)][8]. Therefore, researchers have developed a variety of methods to modify biochar to improve its ability to adsorb anions, such as the combination of biochar and manganese oxide[9-10]. According to literature reports, biochar modified by manganese hydroxyl oxide has a high adsorption capacity for arsenic pollutants in aqueous solution[11-12]. Previous studies have also shown that the adsorption capacity of biological manganese oxide (BMO) is higher than that of chemical manganese oxide[13], and BMO with high surface charge and oxidation capacity is a very effective adsorbent and oxidant[14].
Under natural conditions, the rate of microbial manganese oxidation can reach 100 000 times that of abiotic processes[15]. Nelson[16]reported that the manganese oxide produced byLeptothrixdiscophoraSS-1 showed a strong adsorption capacity for Pb2+. Toner[17]investigated the mechanisms of Zn adsorption to a biogenic Mn oxide within a biofilm produced by model soil and freshwater MnII-oxidizing bacteriaPseudomonasputida.
In view of the fact that nano-scale BMO particles are difficult to separate and purify from water, and have great toxicity to the environment, they have not been widely used in the study of adsorption and removal of pollutants in water[18]. Fixing BMO on a solid carrier that is large in size and easy to separate helps to improve the practical application of BMO in water pollution treatment. In addition, its high surface energy can prevent the aggregation of BMO nanoparticles[19].
In this paper, manganese oxide produced by manganese oxidizing bacteria is loaded on the surface of biochar, and a composite material of biochar and biomanganese oxide is prepared to study its remediation performance for arsenic pollution.
2 Materials and methods
2.1 Mn oxidizing bacteria and biocharPseudomonasputidaMnB1 was purchased from American Type Culture Collection (ATCC,number 23483), used as the Mn-oxidizing bacterium. Nutritional broth (#3 N/B, DifcoTM, Sigma-Aldrich, USA) was cultured and activated at 30 ℃ while agitating at 150 rpm for subsequent experiments. The medium used in the experiment of manganese oxidation is G/M medium.
We chose biochar as a solid support for BMO. It is well known that biochar is a carbon-rich fine-grained porous material, and it has a great affinity for heavy metals due to the presence of porous structures and various functional groups such as carboxyl groups, hydroxyl groups and phenolic hydroxyl groups[20-21].
Cotton stalk biochars obtained from Xinjiang Academy of Agricultural Sciences and were treated by the following procedure before use. Briefly, the obtained biochar samples were grounded and sieved to 200 meshes, 5.0 g of biochar was weighed and treated with 1 000 mL of 0.1 M HCl solution for 4 h and repeated 3 times to remove ash. It was filtered, neutralized by washing with deionized water, dried at 110 ℃ for 24 h, sieved again, and stored in a desiccator for adsorption experiments.
2.2 Synthesis of biomanganese oxide/biochar composite (BMO-BC)The BMO-BC was prepared by incubation of MnB1 in the presence of the Mn2+and biochar. We expected that loading BMO onto biochar can improve its ability to absorb arsenic as heavy metals. The specific preparation process of BMO-BC is as follows:
Under aseptic conditions, 3.0 g biochar particles were placed in Erlenmeyer culture bottles containing 300 mL growth medium (G/M) prepared according to a previous study[18]. The composition of the G/M was 0.5 g/L yeast extract, 0.5 g/L casamino acids, 1 g/L glucose, 0.222 g/L CaCl2, 0.811 8 g/L MgSO4·7H2O, 0.001 g/L FeCl3·6H2O, 2.38 g/L 4-(2-hydroxyethyl) piperazine-1-ethanesulphonic acid (HEPES) buffer, and 1 mL/L trace element solution. The trace element solution was prepared with 6.4 mg/L CuSO4, 44 mg/L ZnSO4·7H2O, 20 mg/L CoCl2·6H2O, 13 mg/L Na2MoO4·2H2O, and DDIW. Before that, biochar and G/M were sterilized for 15 m. Then 3 mL MnB1 cell suspension was added into the culture system and placed in an incubator at 26 ℃ for incubation at a speed of 150 rpm.
HEPES buffer (final concentration is 20 mM) and MnCl2stock solution were added by filter sterilization method, After the final concentration of Mn2+was adjusted to 10 mmol /L, the culture system was moved to a shaking bed at 26 ℃ for about 5 d, and the culture system without biochar and Mn2+was used as the control.
The concentration of high valent manganese (Mn3+, Mn4+) in parallel medium was determined by Leucoberbelin Blue (LBB) dying method[22]at different time intervals. Biochar particles loaded with BMO were separated and washed with deionized water several times before purification. The BMO/biochar complex extracted in our experiment was then purified by the method described by Mandernack[23]. The purpose of purification is to remove the cells, residual organic materials and adsorbent surface adhesion of anions that may affect the adsorption and characterization of heavy metals.
2.3 Removal of arsenic byinsitusynthesized BMO-BC
Sodium arsenite was used to simulate arsenic polluted water. The 100 mg biochar sample was accurately weighed in several 250 mL conical flasks and sterilized at 121 ℃ under high pressure. The final concentration of Mn2+in the mixed solution adjusted to 10 mmol/L by pouring 100 mL growth medium (G/M) sterilized at 121 ℃ and filter sterilizide liquor of MnCl2. After 12 h of activation, the preserved strains were inoculated in 1% proportion on the aseptic operating table. The culture system was placed in a shaker at 26 ℃ for about 10 h. The As (III) reserve solution was filtered and dripped into the culture system. The experimental system with As (III) concentration of 0, 1.96, 4.76 and 9.09 mg/L was compared with the system without biochar and Mn (II) addition. In addition, the culture system without microbial inoculation with heavy metal concentration of 9.09 mg/L was used as the control group.
2.4 Isothermal adsorption of As (III) by BMO-BCAccurately weighed 50 mg BMO-BC particles in a 50 mL centrifugal tube. Different amounts of As (III) stock solution and deionized water were added to adjust the final concentration of As (III) to 0.1, 0.5, 1.0, 2.5, 5.0 and 10 mg/L, The solution volume was adjusted to 40 mL, and the pH was adjusted with 0.1 M NaOH and 0.1 M HCI. It oscillates for 24 h at a rate of 130 rpm in a rotary oscillator at 25 ℃. The adsorbed mixture was filtered by a 0.22 μm filter membrane. The concentration of heavy metal ions in filtrate was determined by atomic fluorescence spectrophotometry (AFS-810, Jitian, Beijing, China). The adsorption capacity and adsorption rate were calculated according to the difference between the concentration of heavy metal ions before and after the adsorption test. The effects of the initial concentration of heavy metal ions and the initial pH of solution on the adsorption process were also discussed.
2.5 Analytical techniquesThe analysis of arsenic in samples was completed by atomic fluorescence spectrometer (AFS-810, Jitian, Beijing, China). The surface properties of BMO/BC were characterized by SEM-EDS, FTIR-ATR and XRD.
3 Results and analysis
3.1 Characterization of BMOThe growth curve of MnB1, a manganese-oxidizing bacterium, is shown in Fig.1. It can be seen that MnB1 enters a stable period when it grows to 10-15 h. It was found in the experiment that when the Mn2+concentration was set within the range of 0-600 mm, the growth rate and domestication period of MnB1 were not significantly affected by the initial concentration of Mn2+. Our experimental results are close to those reported by other scholars. MnB1 entered a stable period after 12 h of cultivation[24]. There was no significant difference in the shape of bacterial growth curves cultured at different Mn2+concentrations.

Fig.1 Growth rate of Pseudomonas putida MnB1
The effect of initial Mn (II) concentration on BMO formation is shown in Fig.2. As shown in Fig.2, the BMO concentration generated in the culture system increases with the increase of the initial Mn2+concentration. We also noted that high-valent manganese oxides such as manganese dioxide could not be detected when Mn2+was not added to the culture system. When the initial concentration of Mn2+was equal to 0, 1.0, 2.0 and 4.0 mM respectively, the content of MnOx,i.e.BMO, produced by the oxidation of MnB1 in the culture system was 0.71, 1.35, 2.07 and 2.79 mmol/L, respectively. The change trend of BMO content is close to its theoretical value[25].
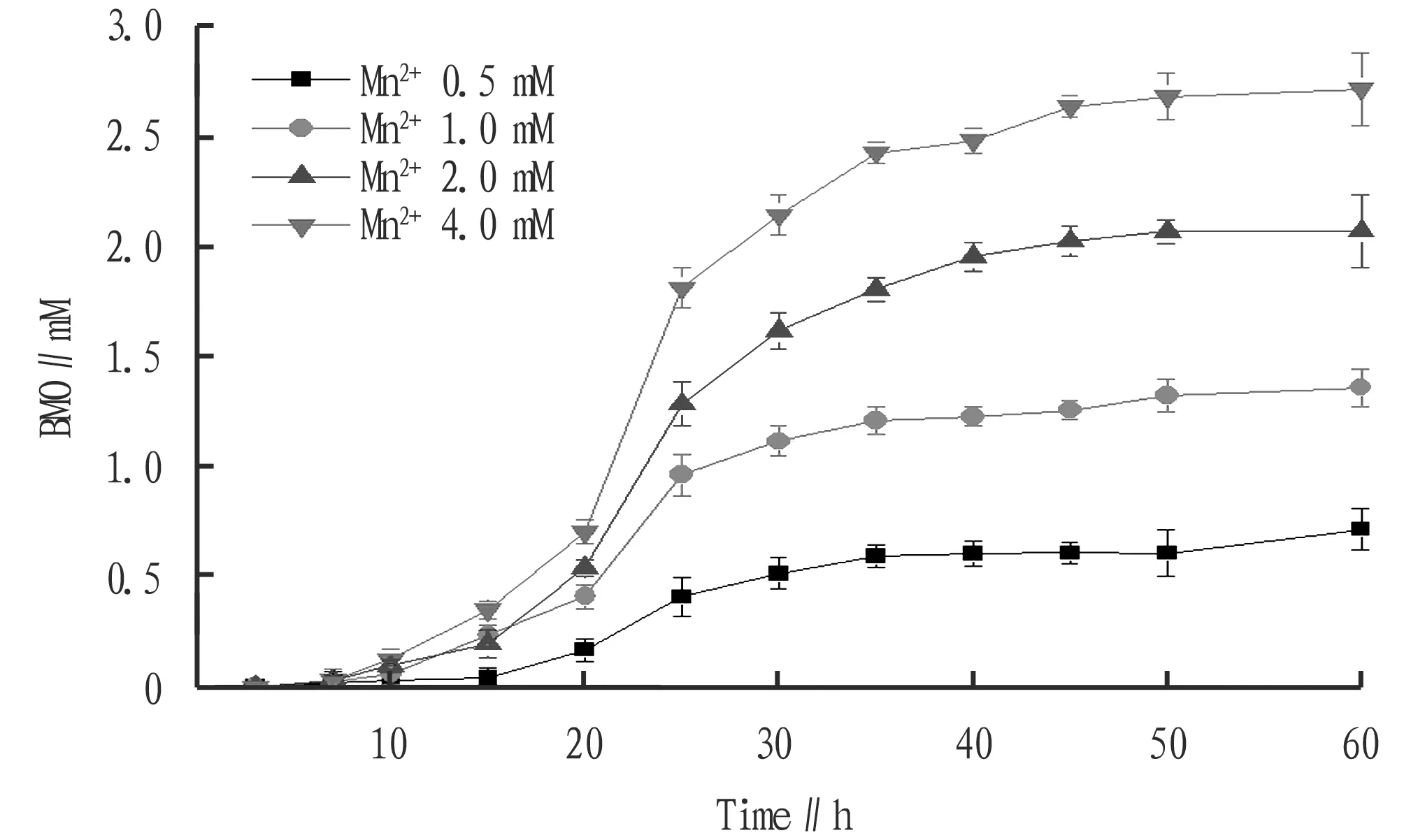
Fig.2 Effects of initial Mn (II) concentrations on the generation of BMO
3.2 Surface Properties and Characterization of BMO-BCThe results of scanning electron microscopy (SEM) comparison of biochar samples with BMO-BC are shown in Fig.3. SEM results showed that BMO loading had some effect on the surface structure of biochar (Fig.3b). Unmodified biochar (Fig.3a) has a rich and smooth pore structure that makes it easier to adsorb other substances or oxidize metals. However, the orifice and surface of BMO modified biochar are roughened by manganese oxide. The modified biochar has some impurities attached to its surface pores, which are probably manganese oxides. When the pores of the biochar are blocked, the surface area and pore diameter of the biochar will decrease.

Fig.3 SEM images of pristine biochar (a) and BMO coated biochar (b)
In order to further characterize the surface structure and microscopic characteristics of BMO-BC complex, BET specific surface area, BJH pore volume, EDS, XRD and FTIR spectra of the composite were analyzed. The results of SEM and EDS analysis of BMO-BC complex amplified 1 000, 3 000, 5 000 times are shown in Fig.4.
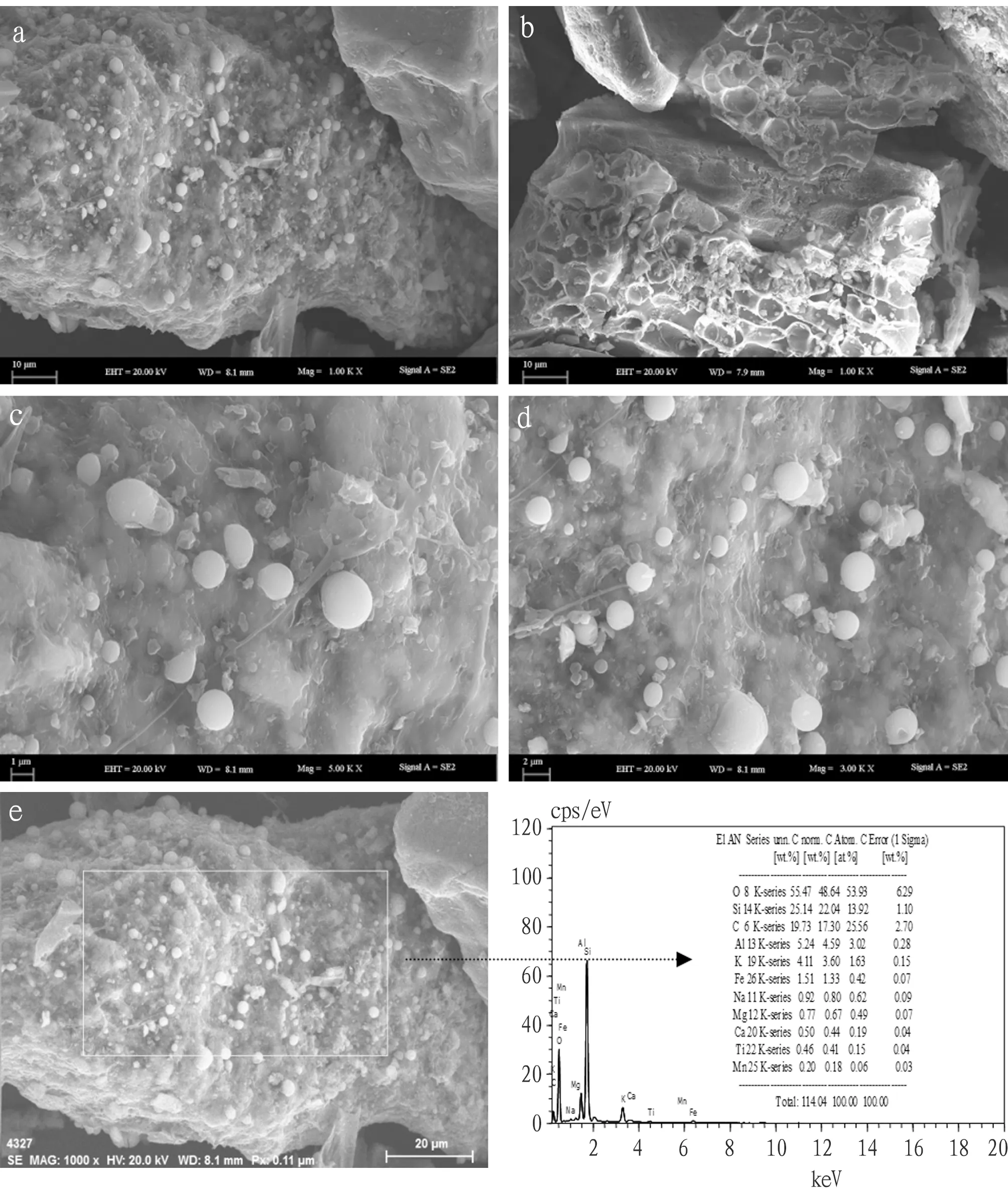
Note: a. Mag=1.00KX; b. Ma=1.00KX on different area; c. Mag=3.0KX; d. Mag=5.0KX; e. EDS analysis.Fig.4 SEM images of BMO coated biochar
The surface element content of BMO-BC measured by X-ray energy spectrometer is shown in Table 1. The weight percentages of C, O and Ca in the original biochar were 77.58%, 16.51% and 3.60%, respectively; in addition, the weight percentages of K, Mg, P and S were 0.57%, 0.70%, 0.59% and 0.44%, respectively; the weight percentages of C, O and Ca changed to 17.30%, 48.64% and 0.44% after loading of BMO, and the content of O increased from 16.51% to 48.64%; the weight percentage of Mn element in BMO-BC is 0.18%. Elements O and Mn detected by X-ray energy spectrometer prove the existence of manganese oxide.
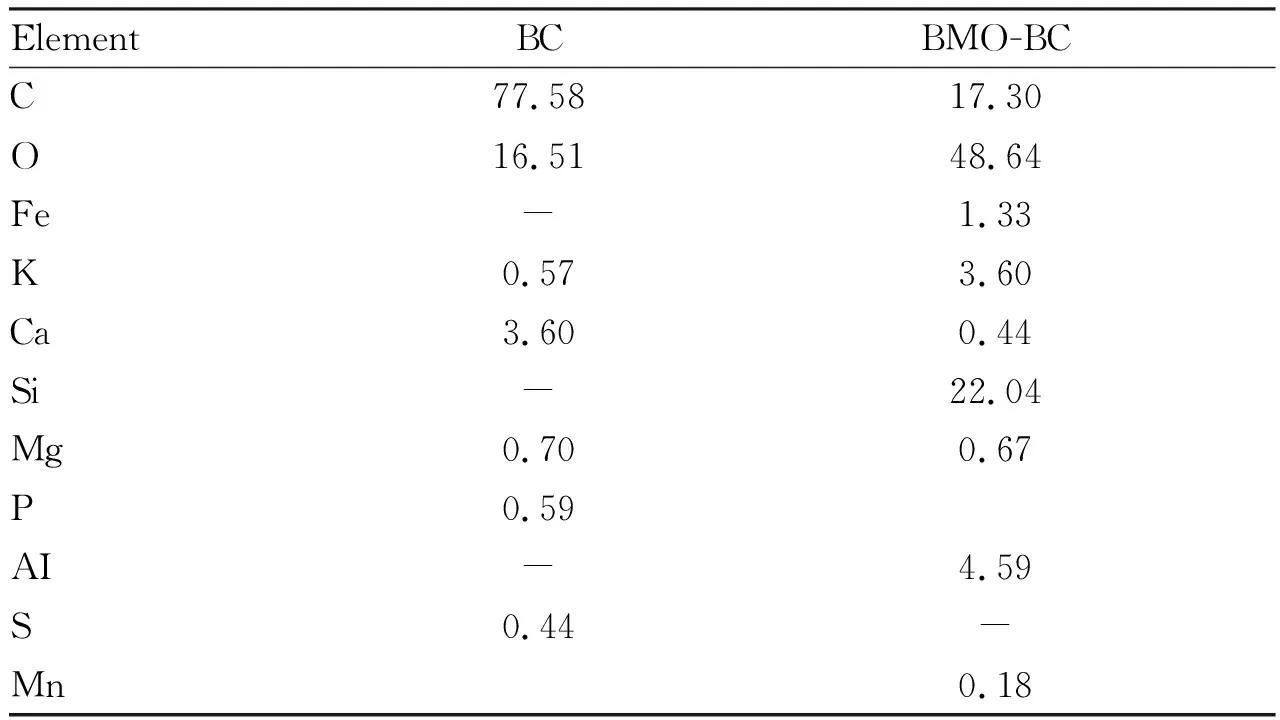
Table 1 Weight percentage of major elements in BC and GBC %
Table 2 shows the BET specific surface area and BJH porosity of BC and BMO-BC materials. It can be seen from the table that the specific surface area of biochar BET decreased from 32.365 to 26.065 cm2/g, and the pore volume decreased from 0.023 to 0.019 cm3/g. SEM image (Fig.4) shows that BMO and other impurities produced by microbial oxidation hinder the pore diameter of biochar, which can explain the decrease of surface area and pore diameter in BET and BJH measurements.

Table 2 Pore structure parameters of BC and BMO-BC
The surface properties of biochar composites were further analyzed by XRD technique in order to characterize whether the Mn oxides were successfully loaded onto the composite adsorbent. The results are shown in Fig.5. According to literature reports, the characteristic peak appears when the 2 Theta values of 12.3 and 24.88 in the XRD spectrum of natural sodium manganese minerals[24-26]. In this study, the XRD spectra of BMO-BC formed byin-situoxidation of biochar and MnB1 showed characteristic peaks consistent with those of natural sodium manganese ore. As shown in Fig.5, the relative strength and position of several crystal peaks in the XRD spectrum are very close to Mn2O3peak[17,27].
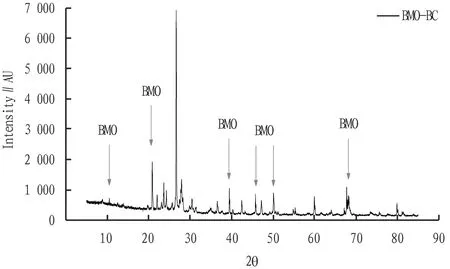
Fig.5 XRD diffraction patterns of BMO-BC
3.3 Removal of trivalent arsenic byin-situformed BMO-BC
In order to study the removal effect ofin-situformed BMO-BC on arsenic (III) in simulated polluted environment, the removal efficiency of As (III) in BMO-BC was tested at different initial concentrations of heavy metals (Fig.6.).

Fig.6 Change of As (III) concentration in the media with in situ formed BMO-BC at different As (III) initial concentration
As can be seen from Fig.6, As (III) concentration in the system gradually decreases with the passage of time. When the initial concentrations of As (III) were 1.96, 4.76 and 9.09 mg/L, the final concentrations of As (III) in the system after 72 h were 0.91, 2.62 and 5.73 mg/L, and the removal rates of As (III) were 53.41%, 44.78% and 36.89%, respectively. In the experimental group without Mn (II), the experimental group without BC and the control group (with As (III) initial concentration of 9.09 mg/L, biochar 100 mg (III), Mn (II) concentration of 10 mM, without MnB1 inoculation), the final concentration of As (III) after 72 h of shaker culture was 6.92, 8.18 and 8.10 mg/L, respectively. The removal rates were 23.85%, 9.91% and 10.87%, respectively. In the control group and the experimental group without BC, the removal rate of As (III) was only about 10%. On the one hand, the removal rate of As was related to the manganese oxidation (partial trivalent arsenic was oxidized to pentavalent arsenic from solution) produced by strain MnB1[28], and on the other hand, the microbial strain and biochar itself has limited adsorption capacity for As. The removal rate of As (III) in the Mn (II)-free group was 23.85%, indicating that in the Mn (II)-free culture system, the removal rate of about 20% of As (III) was related to the adsorption of bacteria and biochar.
From the above analysis, it can be seen that the removal rate of As (III) reaches its highest value within 12 h, and there is no significant change thereafter. In summary, BMO-BC adsorbs As (III) at a faster rate, and adsorption and desorption occur simultaneously as the experiment progresses.
3.4 Isothermal adsorption of As (III) by BMO-BCIn order to better reveal the effect of biological manganese oxide modification on improving the adsorption capacity of arsenic in biochar, we conducted an isothermal adsorption experiment usingin-situformed BMO-BC as adsorbent. Results of isothermal adsorption of BMO-BC complex on As (III) are shown in Fig.7. When the initial concentration of As (III) was 0.1, 0.5, 1.0, 2.5, 5.0, 10.0 mg/L, the maximum sorption amount and maximum removal rate of As (III) were 2.12 mg/g and 26.5% respectively after 24 h of adsorption. The maximum adsorption capacity and maximum removal rate of As (III) in the control group (with biochar and bacteria, without Mn2+) were 0.95 mg/g and 11.9%, respectively. This result reveals that, in the absence of divalent manganese, the adsorption capacity of the composite formed by biochar and strain MnB1 is very low, while the manganese oxide produced by microbial oxidation can improve the adsorption capacity of biochar.
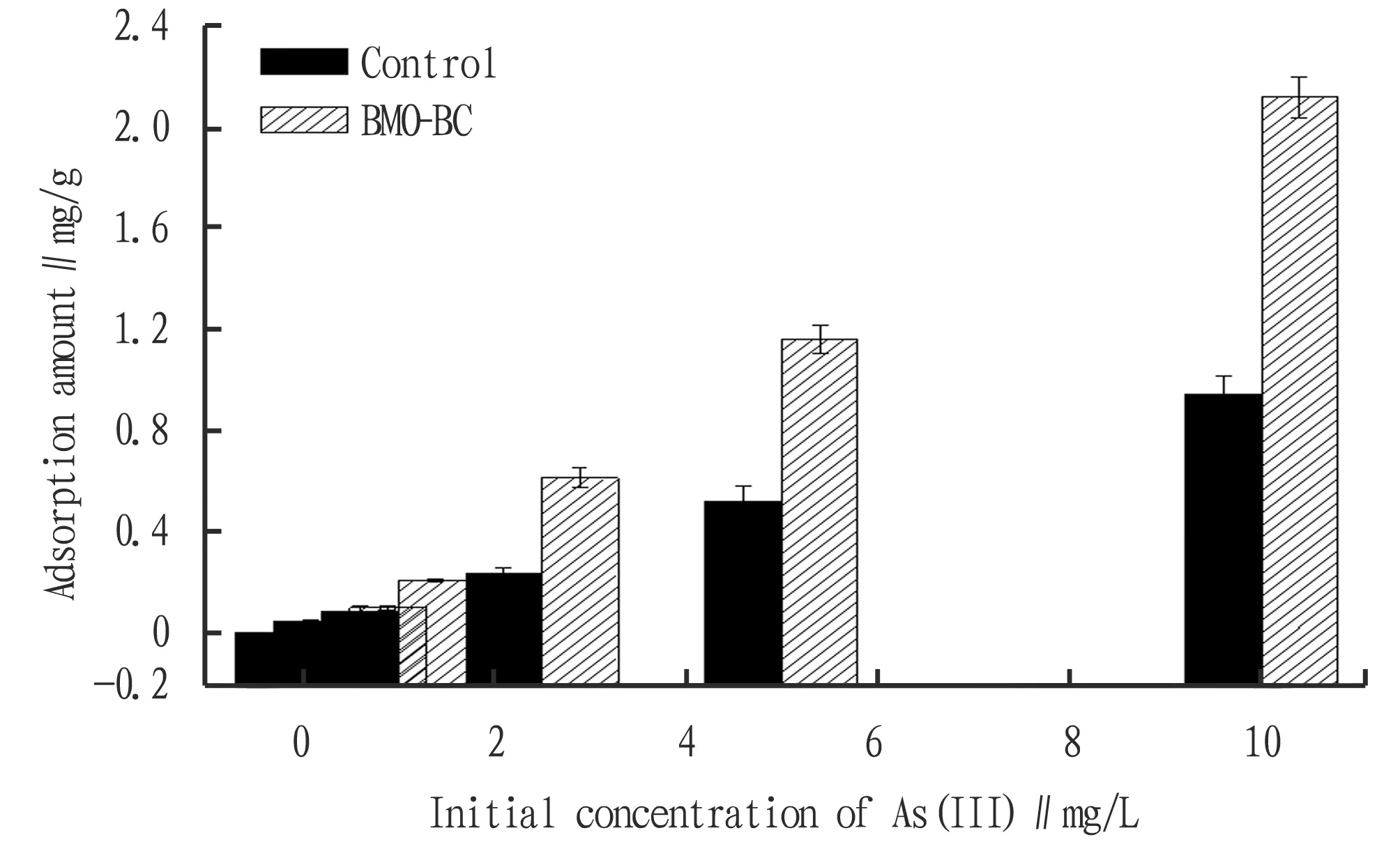
Fig.7 As (III) adsorption amount of BMO-BC at different initial concentration
The results of data fitting to the Langmuir isotherms are given in Table 3. They showed that Langmuir isotherms show a good fit to the experimental data, as indicated by the very high values of the correlation coefficient (R2>0.99). The applicability of the isotherms to the arsenic adsorption shows that monolayer adsorption on the surface of the adsorbent are possible. It is shown in Fig.7 that with the increase of equilibrium concentration, the adsorption capacity of As (III) of the control group (with biochar and strain, without Mn2+) and BMO-BC complex gradually increased. The maximum adsorption capacity Qmwas 2.77 and 5.57 mg/g, the Langmuir adsorption coefficientbwas 0.041 91 and 0.065 11, andR2was 0.997 9 and 0.999 7, respectively (Fig.8). Coating BMO on the biochar surface increased the maximum adsorption amount of As (III) on adsorbents, indicating that modifying the biochar surface with BMO enhanced its adsorption capacity for As (III).
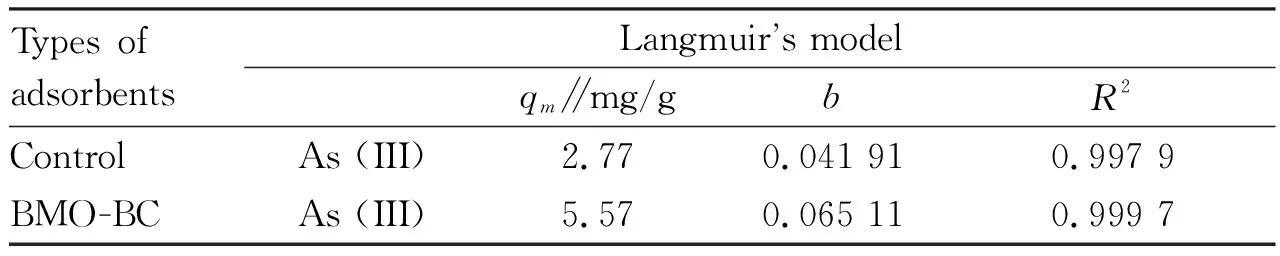
Table 3 Parameters of the isotherms for As (III) adsorption onto BMO-BC

Fig.8 Adsorption isotherms for As (III) onto adsorbents
4 Conclusions
BMO-BC, a new adsorption material, was synthesized using a manganese-oxidizing bacterium and biochar to investigate its removal performance of arsenic pollution. We reached the following conclusions. (i) The growth rate and domestication period ofPseudomonasputidaMnB1 were not significantly affected by the initial concentration of Mn2+in the culture system when the concentration of Mn2+changed from 0 to 600 mM. (ii) The changing trend of BMO content produced by Manganese Oxidizing Bacteria MnB1 is close to its theoretical value. (iii) The results of scanning electron microscopy (SEM) and X-ray energy dispersive spectrometer (EDS) indicate that the signals of O and Mn on the surface of the composite prove the existence of manganese oxide. XRD analysis further confirmed that the adsorbent prepared in the experiment was biochar-biomanganese oxide complex. (iv) Simultaneous removal of arsenic in simulated polluted environment was carried out. The results showed that thein-situsynthesis of BMO-BC complex had a certain removal effect on As (III) at different initial heavy metal concentrations. Its adsorption performance was affected by the presence of biochar particles and manganese dioxide in the reaction system and the manganese oxidation ability of microbial strain MnB1. (v) When the initial concentrations of As (III) were 0-10.0 mg/L, the isothermal adsorption of As (III) on BMO-BC were described in accordance with Langmuir model.
杂志排行
Asian Agricultural Research的其它文章
- Construction of Industrialization Development Model for "1+4+X" Community-Based Elderly Care in Gannan Old Revolutionary Base Area in the Context of Population Aging
- Amplification and Bioinformatics Analysis of vscN Gene from Vibrio alginolyticus
- Flower Characteristics and Resource Evaluation of Fruit Mulberry
