Mesoscale study on explosion-induced formation and thermochemical response of PTFE/Al granular jet
2023-05-31YuanfengZhengZhijianZhengGuanchengLuHaifuWangHuanguoGuo
Yuan-feng Zheng,Zhi-jian Zheng,Guan-cheng Lu,Hai-fu Wang,Huan-guo Guo
State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing,100081, China
Keywords: Reactive materials Shaped charge Mesoscale simulation Formation Thermochemical response
ABSTRACT The dynamic formation,shock-induced inhomogeneous temperature rise and corresponding chemical reaction behaviors of PTFE/Al reactive liner shaped charge jet (RLSCJ) are investigated by the combination of mesoscale simulation,reaction kinetics and chemical energy release test.A two-dimensional granular model is developed with the randomly normal distribution of aluminum particle sizes and the particle delivery program.Then,the granular model is employed to study the shock-induced thermal behavior during the formation and extension processes of RLSCJ,as well as the temperature history curves of aluminum particles.The simulation results visualize the motion and temperature responses of the RLSCJ at the grain level,and further indicate that the aluminum particles are more likely to gather in the last two-thirds of the jet along its axis.Further analysis shows that the shock,collision,friction and deformation behaviors are all responsible for the steep temperature rise of the reactive jet.In addition,a shock-induced chemical reaction extent model of RLSCJ is built based on the combination of the Arrhenius model and the Avrami-Erofeev kinetic model,by which the chemical reaction growth behavior during the formation and extension stages is described quantitatively.The model indicates the reaction extent highly corresponds to the aluminum particle temperature history at the formation and extension stages.At last,a manometry chamber and the corresponding energy release model are used together to study the macroscopic chemical energy release characteristics of RLSCJ,by which the reaction extent model is verified.
1.Introduction
Reactive materials (RMs) are a special category of energetic materials,which have dual properties of sufficient mechanical strength and desirable energetic characteristics [1].As a typical class of shock-initiated composites,RMs are usually pressed and sintered to fabricate reactive projectiles,which produce mechanical perforation by the kinetic energy and also release enormous chemical energy and gaseous products to enhance the damage effects [2,3].
Meanwhile,it should be emphasized that RMs are also prepared as reactive liners to enhance the damage effects of shaped charges.Generally,the reactive liner shaped charge produces a reactive jet,which penetrates the desired target mainly by its kinetic energy and then causes significant behind-armor blast effects by the corresponding chemical energy release and gaseous products [4].
Over the past two decades,significant progresses on reactive liner shaped charges have been achieved.Experimental study shows that the reactive liner shaped charge produces extremely large crater inside the concrete target,and the shaped charge with the oxygen balanced reactive liner results in the most damage [5].DE Technologies,Inc.also indicates that the lethality enhancement of shaped charge could be achieved by adopting a reactive liner[6].Then,the penetration-demolition coupled damage effects of reactive jets against typical targets are studied and discussed,and the influences of formula,particle size,stand-off on damage effects are obtained [7-10].
In fact,in addition to the above studies on damage effects,another important research object is the formation,initiation and chemical energy release of reactive jet.Take PTFE/Al reactive liner for example,the reactive jet formation behavior and the accompanied chemical process involve complicated dynamic responses and chemical kinetics.The conventional energy release measure method,such as bomb calorimeter,cannot capture the reactive jet formation and reaction processes.As such,a particular manometry chamber is often used to study the energy release characteristics of reactive jet [11].In this case,the formation and chemical energy release behaviors of reactive jet are described as three main stages,including formation,extension,and violent deflagration(see Fig.1).
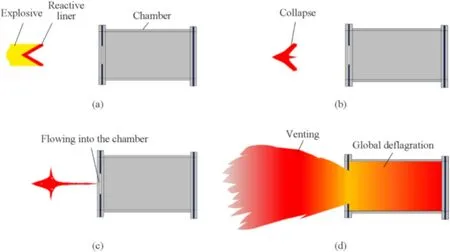
Fig.1.Typical reactive jet formation and energy release behaviors: (a) Shaped charge detonation;(b) Jet formation and initiation;(c) Jet extension and local reactions;(d)Violent deflagration.
Fig.1(b) and Fig.1(c) show the typical jet formation and extension processes: the reactive liner suffering shaped charge detonation is collapsed and deformed as a reactive jet promptly.Then,the jet extends and moves forward quickly.At the same time,what needs special emphasis is that the reactive jet is also initiated in the first two stages.For the mechanism considerations,in the first two stages,the reactive liner suffers strong shock wave,significant deformation and intense friction between PTFE matrix and aluminum particles,leading to the reactive jet temperature rising greatly.Thus,the PTFE matrix begins to decompose,and the corresponding decomposition products start to react with aluminum particles.It should also be noted that the shock-induced chemical reactions in the first two stages are local and relatively mild.Only when these local reactions gradually increase to a certain extent,the reactive jet will have a violent global deflagration reaction,shown in Fig.1(d).
In other words,the chemical reaction of reactive jet could be divided to two processes,including the shock-induced local reaction and the subsequent global deflagration.The shock-induced local reaction is also considered as the reaction growth process.Commonly,the time from the main explosive charge being initiated to the occurrence of the reactive jet global deflagration is defined as the reaction delay time τ.
It is mentioned that,in most previous studies[12-14],the local chemical reactions in the first two stages (corresponding to the reaction growth process)are usually neglected.It is often assumed that the reactive jet is completely inert before the reaction delay time τ,but reacts instantaneously when reaching the reaction delay time τ.This assumption is reasonable in dealing with the macroscopic penetration-blast coupled damage problems,especially in establishing the damage models of reactive jets against typical targets.
However,from the micro perspective,the assumption above cannot solve the reaction mechanism problems.Essentially,the shock-initiated reaction growth process is a complex mechanicalthermal-chemical coupled behavior,which highly determines the value of the reaction delay time τ and significantly influences the chemical energy release process.As such,it is necessary to reveal the shock-initiated reaction growth mechanism during the jet formation and extension processes.
As a matter of fact,some studies have concerned the reaction growth mechanism of reactive jet by means of numerical simulation method [15,16],which provide a useful reference for the present study.However,up to now,most numerical simulation studies still assume that the reactive liner is homogeneous and the real microstructure of reactive liner is ignored.In reality,it has been well known that the reactive liner is a kind of composite liner composed of different materials.Furthermore,the shaped charge effects-induced reactive jet formation behavior is accompanied by the complex coupling process of shock,temperature rise and chemical reaction.In other words,the previous simulation method with the homogeneous liner assumption is hard to essentially reveal the thermal behavior and chemical reaction mechanism.
Fortunately,although the mesoscale study on reactive jet formation is quite rare,there are some valuable mesoscale simulations on shock response and initiation behavior of reactive granular composites.For instance,Qiao et al.[17]introduce a mesoscale simulation method,which follows the real morphology distribution of composites,to investigate the shock compression response of Al/W/PTFE upon the effects of mesoscale characteristics.The results show the higher volume fraction of aluminum,the smaller volume fraction of W and the small granule size of mixtures benefit to the shock wave propagation.Tang et al.[18]investigate the shock-induced initiation behavior of Al/PTFE composites by the combination of mesoscale simulations and explosion loading experiments.It has been demonstrated that the high pressure is concentrated in the wave front,but much greater heat and larger strain exhibit along grain/matrix interfaces.The study also shows that mesoscale simulations are capable of providing the profiles of the physical and thermal processes during shock wave propagation in Al/PTFE reactive materials.
Moreover,Losada et al.[19]develop a fundamental understanding of the decomposition reactions and combustion processes of the Al/PTFE composites.The results show,under oxygen-lean conditions,CF3+Al →CF2+AlF channel is the fastest,followed by the CF2+Al →CF +AlF and CF +Al →C +AlF channels.Under oxygen-rich conditions,reactions of COF with aluminum are probed to be faster than those involving COF2species.D.J.Reding et al.[20-23]introduce a mesoscale reaction model which bridges temporal and spatial scales from the discrete particle simulation and heterogeneous media granular-level reaction model to a macroscale continuum model.The mesoscale reaction model offers significant improvement over existing homogeneous models since the new model explicitly describes nonequilibrium thermodynamics via 2D spatial heat transport.To understand shock-induced chemical reactions(SICR)for granular mixtures,Zhang et al.[24,25]have developed theoretical models to calculate the Hugoniot data and the heat released by shock temperature controlled reactions.In their study,the shock-induced temperature rise is first calculated using a constant volume and pressure adjustment.Then the Arrhenius reaction rate [26]and Avrami-Erofeev kinetic models[27]are used to calculate the reaction extent.Thus,thermochemical models for shock-induced reactions,in which the reaction efficiency is considered,are given by combining the shock temperature rise with the chemical reaction kinetics.
Based on the discussion above,it can be concluded that the mesoscale simulation and related thermochemical study should be effective methods to reveal the coupled behaviors of shock,temperature rise and chemical reaction during reactive jet formation and extension.As such,the present study mainly concerns explosion-induced formation and thermochemical response of PTFE/Al granular jet based on the mesoscale method.Firstly,twodimensional mesoscale models are established and used to analyze the aluminum particle distribution and temperature effects during the formation and extension.Then,a modified reaction extent model is developed to further gain the relationship between reacted mass of the reactive jet and the time.Finally,an energy release test is conducted by using a sealed chamber to verify the numerical and theoretical results.
2.Mesoscale simulation on formation
2.1. Reactive liner microstructure
The PTFE/Al reactive liner is prepared mainly by mixing,pressing and sintering.The fabrication process consists of the following steps.
Firstly,the mixture of PTFE and aluminum powders with the mass matched ratio (73.5/26.5) is obtained by a planetary mill machine,and then a certain amount of the mixture is put into a vacuum drying oven for 24 h.Secondly,34.7 g of well-mixed mixture is weighed and placed in a pressing mold.A cold pressing pressure is applied with a drift movement velocity about 5 mm/s,and the maximum pressure reaches 300 MPa with a duration of 30 s.Thirdly,the pressed liner sample is sintered with a maximum temperature of 380 ℃,under the protection of a nitrogen atmosphere.The detailed sintering program is given in Ref.[28].Lastly,the sintered reactive liner is re-pressed in the mold to prevent the deformation caused by the sintering process.
Fig.2 shows a typical reactive liner sample and the microstructure obtained by means of scanning electron microscopy(SEM).As can be seen,Al particles are distributed discretely and stochastically inside the PTFE matrix,without significant agglomerate.A few cracks are also observed in Fig.2(b)and the details are shown in Fig.2(c).These cracks are mainly induced by the re-press process during the fabrication.The detailed photograph shows insignificantly deformed metal Al particles and highly deformed PTFE matrix.It is noted that,although a pore is found in the SEM image,the porosity of the reactive liner is obtained as only 2.3%by the drainage test.Furthermore,the EDS analysis for the aluminum particle indicates slight carbon and fluorine elements from the adhered PTFE.

Fig.2.Specimen,microstructure,and element analysis of the reactive liner: (a) Specimen;(b) Overall microstructure;(c) Local crack in details;(d) EDS analysis.
2.2. Mesoscale model
The present study focuses on the formation and thermochemical responses of reactive jet at the granular level.Thus,a mesoscale simulation model of reactive liner,with aluminum particles encased within PTFE matrix,is needed.Meanwhile,it has been shown that the area fraction of inclusions in planar material with a sufficiently random distribution is approximately equivalent to the mass fraction of particles in the sample [29].In hence,a mesoscale simulation model could be established based on the real micro-morphology and the following assumptions: (1) the shape of aluminum particles is ideal spherical;(2) the size of aluminum particles is normally distributed;(3)the pores in the reactive liner are ignored during the modeling process,due to the insignificant porosity.
Compared with the representative volume element method,the mesoscale simulations would be more precise due to the consideration of particle size distribution based on statistical sense.For the aluminum particles,a continuous normal distribution curve with a mean μd=400 μm and a standard deviation σd=40 μm is obtained by fitting real aluminum particle size distribution,shown in Fig.3(a).Then,based on Ref.[30],a computer code is developed to generate and deliver aluminum particles into PTFE matrix randomly,without overlapping phenomenon.The detailed generation and delivery procedures are summed up in four points as follows:

Fig.3.Modeling process by particle delivery method: (a) Size distribution of Al particles;(b) Corresponding mesoscale model of reactive liner.
(1)Nrandom coordinates are firstly generated,and the area sum method is used to determine whether the coordinates are located in the liner.Then,random radii in accordance with normal distribution are also created and combined with the random coordinates generated before to form an arrayM1(x,y,R).
(2) For an arbitrary point (xi,yi,Ri) inM1,it further needs to be judged whether the point(xi,yi,Ri)with a radius ofRibeing totally within the liner boundary.Then,those points completely inside the border are allocated into an arrayM2(x,y,R).
(3) Looping in the arrayM2to judge whether the distanceKbetween any two points satisfyingK>di+dj,wheredi and djare the radii of two corresponding points.Then,all the points meeting the requirements constitute an arrayM3(x,y,R).
(4) Lastly,the mesoscale model is generated when the volume of random particles reaching the given Al particle content.Fig.3(b)shows a typical mesoscale model of PTFE/Al reactive liner in Euler area,by randomly delivering Al particles into the PTFE matrix.It can be seen the reactive liner has a cone angle of 60°,a diameter of 40 mm,a height of 40 mm,and a thickness of 6.5 mm.
Furthermore,the numerical model is developed in a Eulerian domain to analyze the jet formation and shock-induced thermoresponses(see Fig.4(a)).The Eulerian zone has macro-dimensions of 100 × 30 mm2on the left and 450 × 5 mm2on the right to decrease the calculation task.Meanwhile,the Eulerian zone is meshed by the uniform grids with the same size of 50 × 50 μm2.The boundary condition is set as“Flow out(ALL EQUAL)”along the sides of the Eulerian zone to avoid the effect of stress wave reflection.Once the simulation domain extends over the geometries,the materials are allowed to flow outward.Partial enlarged details of reactive liner show Al particles are surrounded by PTFE matrix,and the distribution of pressure and temperature inside the particle could be obtained.Fig.4(b)shows the typical setting of ten Gauss points on the reactive liner,and it is noted that all the Gauss points are placed on the center of the corresponding aluminum particles to obtain the pressure and temperature history curves.Gauss points 1-5,6-10 correspond to inner surface and outer surface,respectively.

Fig.4.Mesoscale model of reactive jet formation and extension: (a) is shown by the mirror method,①Initiation point of 8701 explosive ②8701 explosive ③Reactive liner④Air ⑤Flow out boundary (ALL EQUAL);(b) is shown by the actual 1/2 numerical model due to symmetry.
2.3. Material models
The numerical model,relating to explosive,air,and reactive liner parts,includes four materials,such as 8701 explosive,air,PTFE and aluminum.The detailed material strength models and equation of states(EOSs) are listed in Table 1.

Table 1 Material strength models and EOSs.

Table 2 Parameters of the explosive 8701.

Table 3 Parameters of the aluminum.

Table 4 Parameters of the air.
The SHOCK equation of state is employed to describe the dynamic behavior of materials,including PTFE and aluminum.The idea gas equation of state,mainly including parameters of gamma Γ,specific heat at constant pressureCp,specific heat at constant volumeCv,reference temperatureT,specific energyE0,is used to describe the material of air.The JWL equation of state is adopted to provide an accurate description of the detonation products expansion for high energy explosive material 8701 with a detonation velocity ofDand CJ detonation pressure ofPCJ,according to the following form:
whereA,B,R1,R2,ω are experimental fitting parameters;E0is detonation energy per unit volume;Vis relative volume of detonation products.
Furthermore,the Johnson-Cook strength model is used to describe the strength behavior of PTFE material subjected to large strain,high strain rate and high temperature.Such behavior is likely to arise in problems of intense impulsive loading.In this model,the yield stress,changing with strain,strain rate and temperature,is defined as follows:
whereA′,B′,C,mandnare material constants.εpis the effective plastic strain.˙ε0=1 s-1is the reference plastic strain rate.TmeltandTroomdenote the melting and room temperatures,respectively.
The Steinberg-Güinan strength model,considering the shear modulus and yield strength as functions of effective plastic strain,pressure and internal energy (temperature),is employed to describe the aluminum material during the formation of RLSCJ.The shear modulusGand yield stressYfor high strain rates are defined as
where ε is the effective plastic strain,Yis the flow stress,Y0is the yield stress at ambient conditions (P=0,T=T0),Gis the shear modulus as a function of pressure and temperature,G0is the initial shear modulus,η=V0/Vis the compression ratio,are the derivatives with respect to pressure and temperature at the reference state (T=T0,P=0,ε=0),and the constants β andnare specific parameters of materials.
The corresponding material parameters of explosive 8701 [31],aluminum [32],air [16]and PTFE [17]are listed in Tables 2-5,respectively.It is noted that ρ is the material density.
2.4. Formation phenomenon of RLSCJ
The typical formation process of RLSCJ is shown in Fig.5,where the reactive liner is represented and the explosive is hidden.As can be seen,the reactive liner,suffered collapse,closure and tension,finally forms a high-velocity jet,which has a total length of 520 mm and a tip velocity of 5680 m/s at 100 μs.

Fig.5.Mesoscale simulation results of reactive jet formation: (a) 7 μs;(b) 20 μs;(c)50 μs;(d) 80 μs;(e) 100 μs.
Furthermore,Fig.5 also reveals the unique distribution behavior of aluminum particles: at the time of 7 μs,the detonation wave sweeps to the top part of the liner,with the strong compression of the composite materials and the intense collision among aluminum particles near the top.This behavior leads to remarkable density increase,but the distribution of aluminum particles is still relatively uniform.With the further collapse of the liner,the particle distribution shows significantly different characteristics along the jet axis at the time of 20 μs,such as:at the tip of the jet,there are few aluminum particles,and in the middle of the jet,there are a large number of aluminum particles gathering near the axis;in the slug,the aluminum particles are still evenly distributed.For mechanism considerations,the density difference between aluminum and PTFEresults in velocity discrepancy for these two kinds of materials.The average material velocity versus time curves of aluminum and PTFE in reactive jet are displayed in Fig.6.It can be seen that the average velocities of aluminum and PTFE are basically identical at the initial collapse stage.Then,the aluminum average velocity gradually stabilizes at 705 m/s,while the average velocity of PTFE continues to rise to 819 m/s,which is increased by 16.2%compared with that of aluminum material.In addition,the jet formation is an instantaneous process,and there is no enough time to reach stress equilibrium between aluminum and PTFE materials.As such,the relative movement occurs between the two materials and causes remarkable unevenness of material distribution and density.When the time further reaches 100 μs,some necking points are found along the jet axis.Moreover,different from the traditional copper jet whose slug has little contribution to the damage effects,the slug of reactive jet is indispensable as it would react violently and release a lot of chemical energy and gaseous products.From the micro perspective,it can be seen that the aluminum particles are concentrated mainly in the rear of the jet at 100 μs,but the distribution density of aluminum particles at the axis decreases to some extent.Fig.7 illustrates the aluminum mass content percent along the jet axis,where the aluminum mass content percent is extracted by means of gray processing and Monte Carlo method[33].Moreover,the horizontal coordinate stands for the jet length along the axis,while the vertical coordinate represents the aluminum mass fraction,which is defined as

Fig.6.Average velocity difference between aluminum and PTFE materials.
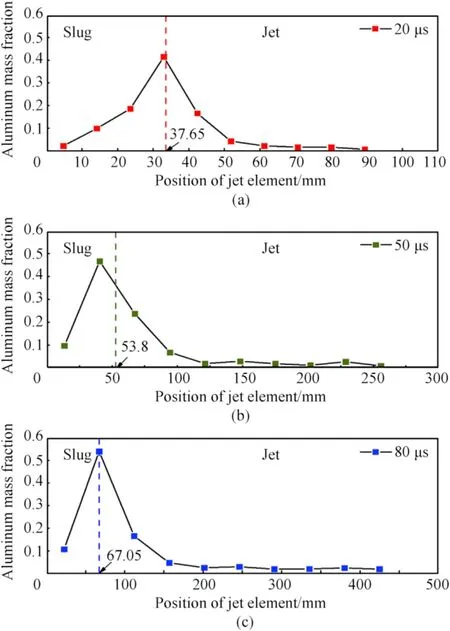
Fig.7.Aluminum mass content fraction along the jet axis:(a)t=20 μs;(b)t=50 μs;(c) t=80 μs.
where,wiis the aluminum mass in any horizontal position andWis the total aluminum mass.
For details,similar to the discussion above,the reactive jet shows a typical segmented feature: for example,at the time of 20 μs,the aluminum mass content percent is less than 5%at the jet tip,improves to 10%-20% in the middle,maintains even higher than 30% inside the rear slug.As time progresses,the aluminum particle distribution shows the same trend.
2.5. Pressure distribution characteristics
Fig.8 illustrates pressure history curves recorded by Gauss points 1-5.Take the second Gauss point as an example,it can be seen that the pressure on the inner layer firstly increases to the peak of 15.8 GPa.Then,it descends quickly within 0.3 μs.However,the pressure curve again begins to increase within 1.22 μs (tr) and maintains at 8.8 GPa approximately for about 3.1 μs (td),which indicates a secondary collision for the inner part.In fact,during the formation process,the inner part of the liner collapses and closes,and there is a secondary collision at the axis,leading to the significant secondary pressure rise.

Fig.8.Pressure on inner layer.
Fig.9 shows typical pressure history curves recorded by Gauss points 6-10 from the simulation.Take the seventh Gauss point asan example,it can be seen that the pressure on the outer layer rises steeply and gets the peak about 15.1 GPa.Then,it drops promptly with some vibrations,and the corresponding decrease stage time is about 8 μs(tdrop).From the jet formation mechanism,the outer part of the liner mainly forms the slug and there is no obvious secondary collision for the outer part.As such,after reaching the peak instantaneously,the pressure curve presents a downward trend generally.
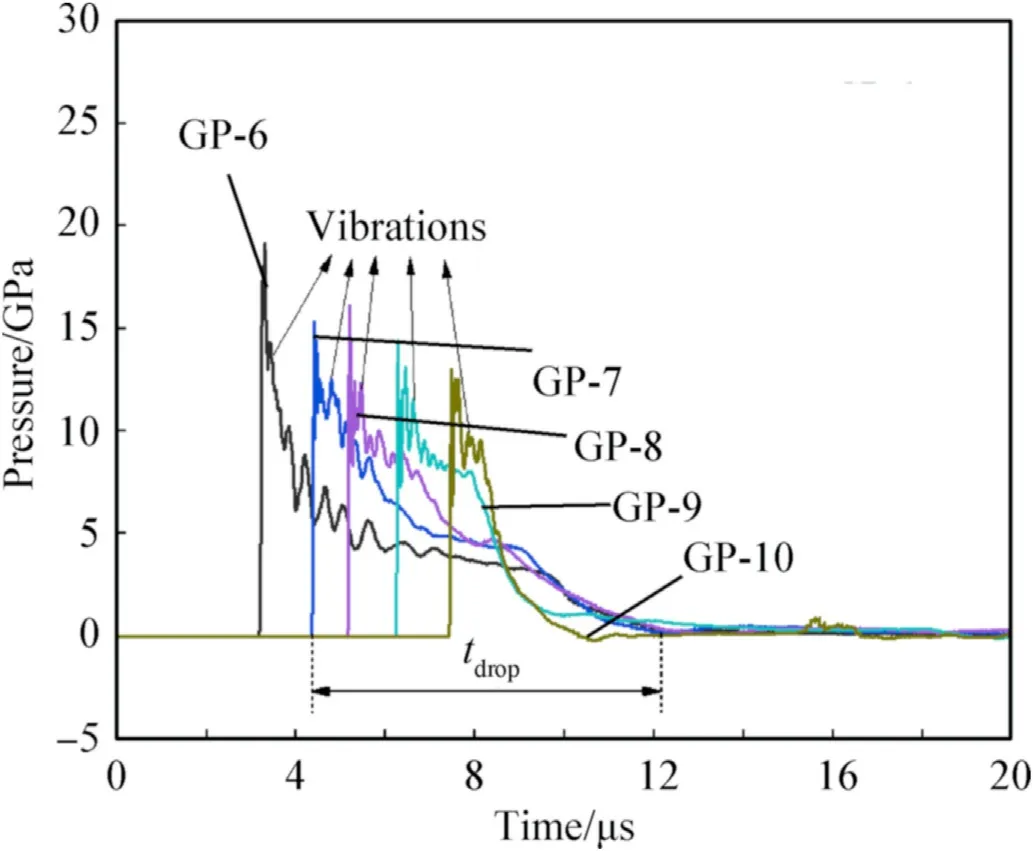
Fig.9.Pressure on outer layer.
The pressure contours are intuitively shown in Fig.10.As can be seen,the initial shock wave reaches the outer apex of the reactive liner at the time of 3 μs;At 6 μs,the shock wave is reflected at the free interface of inner apex and a corresponding tensile wave is formed.Then,the tensile wave reflected by the free surface is superimposed with the shock wave,forming a relatively low pressure region symmetrically.Moreover,Fig.10(b) and Fig 10(c)further show the inner layer of the liner starts to flow at this moment.Then,the relatively low pressure region begins to expand and move to the bottom of the reactive liner.With time goes by,the range of high pressure area in the reactive jet gradually shrinks and the jet gradually extends.

Fig.10.Evolution of the pressure field: (a) 3 μs;(b) 6 μs;(c) 8 μs;(d) 11 μs;(e) 20 μs;(f) 50 μs;(g) 80 μs.
2.6. Temperature distribution characteristics
Significantly different from traditional copper jet,the temperature response is crucial for the reactive jet.The dynamic mechanical behavior-induced temperature distribution determines the chemical reaction behavior in essence.Fig.11 shows the temperature contours of Al particles,PTFE matrix and mixed materials,respectively.It can be seen that the high temperature area is firstly observed in the PTFE matrix at 4 μs.This phenomenon indicates that most input energy is absorbed by the matrix in the early stage of jet formation,corresponding to the initial deformation concentrating in the matrix.Soon after,a wider range of high temperature area is formed in aluminum particles near the axis.Analysis from mechanism considers that the collection of aluminum particles near the axis is the prerequisite for the high temperature area.Moreover,collision among aluminum particles,friction between aluminum particles and PTFE,and severe deformation of aluminum particles are all responsible for the sharp temperature rise of the aluminum particles.Besides,it can also be found that the high temperature area in PTFE matrix also expands significantly at the time of 20 μs.
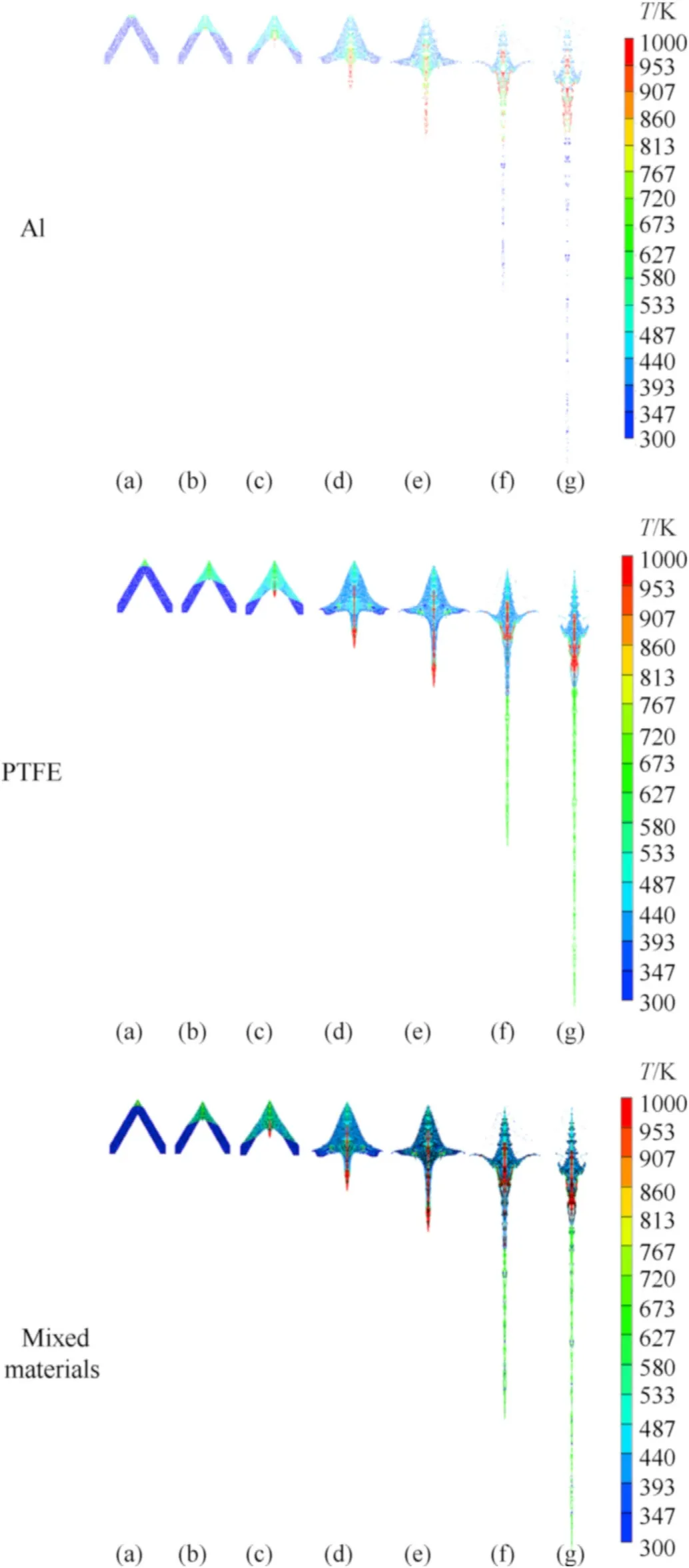
Fig.11.Evolution of the temperature field in different constituents:(a)4 μs;(b)6 μs;(c) 8 μs;(d) 14 μs;(e) 20 μs;(f) 50 μs;(g) 80 μs.
Fig.12 compares the typical temperature curves of aluminum particle and PTFE matrix near the axis.As can be seen,the maximum temperatures in PTFE and aluminum particle are 939 K and 874 K respectively,meaning that the matrix would decompose and react with aluminum powder during the jet formation and extension stages.In other words,on one hand,the aluminum particles are reacting with released C2F4;on the other hand,the reactive jet is forming,stretching and moving.It should be further emphasized that the shock-induced reaction in formation and extension stages is local and relatively mild before the time reaching the reaction delay time τ.

Fig.12.Comparison of the temperature curves of aluminum and PTFE near the axis.
3.Chemical reaction behavior and overpressure effects
3.1. Reaction extent model
The discussion above shows that the reactive jet is activated and begins to react during the formation and extension process.This peculiar formation behavior is related to mechanical-thermalchemical coupled mechanism.As time goes by,more PTFE and aluminum particles inside the reactive jet react with each other.In hence,one problem that needs to be solved is to quantitatively analyze the mass of reacted reactive jet versus time.As such,the remaining unreacted reactive jet mass can be obtained,which would be helpful to understand the chemical energy release behavior of reactive jet.
In the present study,the mechanical-thermal process-induced chemical reaction is assumed to be completely controlled by temperature rise,thus a thermochemical model could be obtained to predict the reacted reactive jet mass versus time during the formation and extension processes [24,26,27].
According to the Arrhenius model,the chemical reaction rate is expressed as
where,k,yandtare reaction constant,reaction extent,and reaction time,respectively.
Variableyis defined as
where,mandm0are mass of reacted material and mass of initial material,respectively.
By the Arrhenius law,the reaction rate constant can be expressed as
where,Ru,T,A,andEaare the universal gas constant,absolute temperature,pre-exponential factor and activation energy,respectively.
For the reactants,the pre-exponential factor can be calculated based on the differential scanning calorimetry (DSC) data:
where,β is the temperature rise rate,Tris the peak temperature of the DSC curve[34].
In the case of high rate temperature rise,the chemical reaction occurring during the reactive jet formation and extension processes could be described by then-dimensional controlled reaction model proposed by Avrami-Erofeev kinetic model,
According to Eq.(8),the pre-exponential factor and temperature rise rate can be expressed as
By substituting Eqs.(9)and(10)into Eq.(6),the reaction extent can be described as
3.2. Conduct method
Firstly,take any aluminum particleias an example,obtaining its temperature history curve by means of numerical simulation performed in section 2.In particular,due to deformation and rupture behaviors of the aluminum particle during formation and extension,the data of temperature rise and reaction extent obtained from a single point cannot well represent the whole aluminum particle.Therefore,three points are selected along the radius of the aluminum particle,and the aluminum particle is divided to inner and outer layers consequently (see Fig.13).Furthermore,the average value of the adjacent two points is taken as the reaction extent of the corresponding layer.As shown in Fig.13,y1,y2,y3are used to represent three points on the aluminum particle,then temperatures of the three points are marked asT(y1),T(y2),T(y3).After that,the chemical reaction extents for the three points are calculated by combining the temperature history curves and the theoretical model derived in section 3.1.As such,the chemical reaction extent curves of the three points can be obtained as Δ(y1),Δ(y2),Δ(y3),respectively.Thus,the reacted mass of the aluminum particlemsis given by

Fig.13.Dividing method of aluminum particle and corresponding layers.
where,m1andm2are masses of inner and outer layers,respectively.
Secondly,looping the first step and getting the reaction extent for each aluminum particle.
Thirdly,the reacted mass of each aluminum particle is obtained,and the total reacted mass of all the aluminum particlesMris also acquired.
Lastly,the total reaction extent of the reactive jet could be given byMr/M,where parameterMis the initial total mass of aluminum particles in reactive liner.It is noted that,for the case of chemical reaction with oxygen equilibrium,the reaction extent of aluminum particles represents the reaction extent of the whole reactive jet.
3.3. Reaction extent calculation
Based on the discussion above,the reaction extent curve versus time could be calculated.Table 6 lists related parameters.For an aluminum particle chosen randomly,the red lines in Fig.14 show the corresponding temperature history curves.Then,three reaction extent curves are obtained by Eq.(11)and shown by the blue lines in Fig.14.The aluminum particle temperatures display three main stages: the duration of the first stage is very short and the temperature in this stage rises sharply to about 450 K,due to the propagation of the shock wave;in the second stage,the aluminum particle temperature decreases rapidly,resulting from shock wave front leaving.In the third stage,the temperature rises again because of the friction,particle deformation and collision.Finally,the reaction extent of the aluminum particle is depicted based on the three blue reaction extent curves and Eq.(12) (see Fig.14(d)).

Table 6 Parameters used in chemical reaction extent model [24].

Fig.14.Diagram of temperature change and reaction extent of one aluminum particle: (a) Center point;(b) Intermediate point;(c) Peripheral point;(d) Reaction extent of the aluminum particle.
Similarly,the chemical reaction extent for each aluminum particle could be obtained.Thus,a graphical representation of reaction extent distribution along the initial reactive liner position is shown in Fig.15,which visualizes the status at 20 μs.As can be seen that the inner layer of the reactive liner shows a much higher reaction extent.In addition,the bottom of the liner shows a low chemical reaction extent at this moment,mainly because of the relatively weaker mechanical shock behavior in this area.By analyzing the reaction extents of all the aluminum particles,the total reaction extent of the reactive jet is calculated as 12.8%at the time of 20 μs.

Fig.15.Visualization of reaction extent distribution.
On the basis of the method above,the total reaction extent of the reactive jet versus time could be obtained,as shown with red line in Fig.16.By combining the initial reactive liner mass,the reacted mass versus time can also be obtained and shown with blue line in Fig.16.

Fig.16.Total reaction extent and reacted mass of the reactive jet versus time.
3.4. Overpressure effect
The discussion above mainly involves the reactive jet itself.If a test chamber is placed in front of the reactive liner shaped charge with a stand-off ofL,the deflagration-induced overpressure effect could be predicted theoretically.By combination of Fig.1,it is considered that the initial timet0is defined as the moment when the shaped charge begins to detonate (see Fig.1(a)),and the time for the reactive jet getting the chamber entrance ist1.Then the consumed massmof the reactive jet beforet1can be calculated according to the above discussion.Furthermore,the residual reactive materials at the time oft1could be obtained and regarded approximately as the effective reactive massme,which part enters the test chamber,deflagrates and produces overpressure effect inside.Generally,the relationship between the overpressure ΔPand the effective massmeinside the chamber is described as
where,V0is the volume of the chamber,cm3,α andfare constants determined by reaction characteristics of reactive materials [35].Besides,it should be noted thatm+me=m0.
In conclusion,with the variation of stand-offL,the timet1,when the reactive jet gets the chamber entrance,also changes.Thus,the effective massmeinside the chamber subsequently alters,and finally different overpressure would be predicted.
4.Experimental verification
4.1. Verification method
In the present study,the energy release experiment is carried out to quantitatively verify the discussion above.The experimental setup is shown in Fig.17.The test system mainly consists of a manometry chamber,a link ring,a harden steel anvil,several steel foundations,and a bottom plate.The design of the link ring is to allow for increasing chamber volume in the future study,which is not involved here.The length and diameter of the internal space of the chamber are 650 mm and 160 mm,respectively.Two pressure sensors with a measure range of 10 MPa are fixed on the wall with a distance of 120 mm.The reactive liner shaped charge is placed on the holder,towards the manometry chamber.The stand-offLis chosen as 0 mm(0 CD),50 mm(1.25 CD),100 mm(2.5 CD),200 mm(5 CD)and 400 mm(10 CD),respectively.The abbreviation CD here stands for the 8701 shaped charge diameter with a value of 40 mm.After that,the reactive liner shaped charge is initiated and the corresponding overpressure is collected.

Fig.17.Scheme of energy release experiments.
5.Results
Five experiments with differentLare conducted,respectively.The corresponding liner parameters are listed in Table 7,where the copper liner is used for contrast.Fig.18 shows both structure and photograph of the reactive liner shaped charge.Fig.19 shows the experimental setup.Fig.20 shows a sequence of photographs recorded by a high-speed camera.As can be seen,the explosive detonation products with high temperature give off a dazzling white light at the time of 2.2 ms.Then,an important venting phenomenon can be observed between 12.2 ms and 24.1 ms: the high temperature and high pressure gaseous substances are ejected from the manometry chamber,indicating that the reactive jet inside the chamber has occurred violent chemical reaction.

Table 7 Parameters of liner specimens.

Fig.18.Reactive liner shaped charge: (a) Shaped charge structure;(b) Photograph of shaped charge.

Fig.19.Experimental setup.

Fig.20.High-speed sequence of energy release behavior of reactive liner shaped charge: (a) t=0 ms;(b) t=2.2 ms;(c) t=12.2 ms;(d) t=24.1 ms.
For each test,two overpressure history curves from different channels are obtained and then averaged to characterize the overpressure behavior inside the chamber.For example,Fig.21 illustrates,at a stand-off of 2.5 CD,the overpressure curves obtained from the two channels,as well as the average overpressure curve.The result shows the three curves are relatively close,indicating it is reasonable to characterize the overpressure in the chamber by averaging method.Fig.22 shows five average overpressure history curves,corresponding to five different stand-offs listed in Table 7.The five average overpressure curves represent the same twotrends: the overpressures ascend dramatically and steeply with a time scale of several milliseconds,and then descends gradually with a time consumption of about 50 ms.In fact,the rising and falling stages correspond to the deflagration and venting processes of reactive jet,respectively.Further observation finds that the stand-offLhas an important influence on the overpressure inside the chamber: with the increase of parameterL,the peak pressure inside the chamber decreases consequently.For mechanism considerations,the increase of the parameterLmeans more reactive materials would react outside the chamber,and thus less reactive materials react inside the chamber,producing a lower pressure.
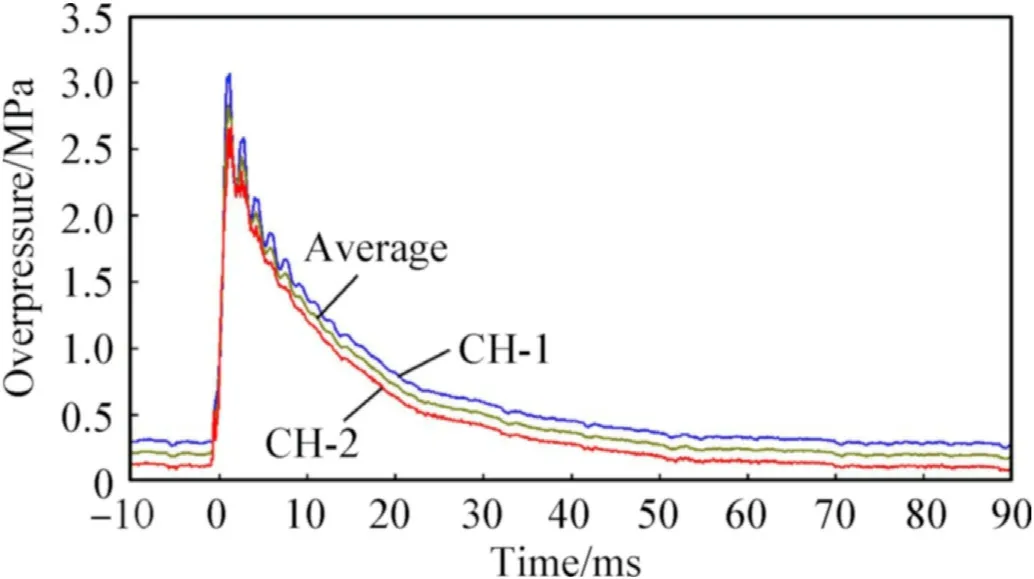
Fig.21.Typical overpressure curves at a stand-off of 2.5 CD.

Fig.22.Average overpressure history curves of reactive jets deflagrating inside the chamber.
For comparison,Fig.23 shows the overpressure formed by copper jet inside the test chamber is much lower.From the mechanism analysis,the phase state of copper jet may change to a certain extent in the chamber,resulting in the formation of a certain overpressure,but it is much lower than that induced by the chemical energy release of reactive jet.

Fig.23.Average overpressure history curve of copper jet moving inside the chamber.
For any stand-offL,it is easy to get the timet1when the reactive jet gets the chamber entrance,based on the numerical simulation method.Then,by the combination of Fig.16,the effective massmeinside the chamber is given.Finally,the theoretical predicted average overpressure could be obtained by Eq.(13).
Fig.24 illustrates the predicted variations versus the stand-off ofL,including effective massmeand average overpressure ΔPinside the chamber.At the same time,the relevant experimental results are also marked in Fig.24,by which the average overpressure prediction based on numerical simulation and theoretical model could be verified.The comparison listed in Table 8 indicates that the theoretical model has an acceptable prediction accuracy if the stand-offLless than 5.0 CD.For a larger stand-off,the error would be greater than 20%.

Table 8 Errors between experimental and predicted overpressures.
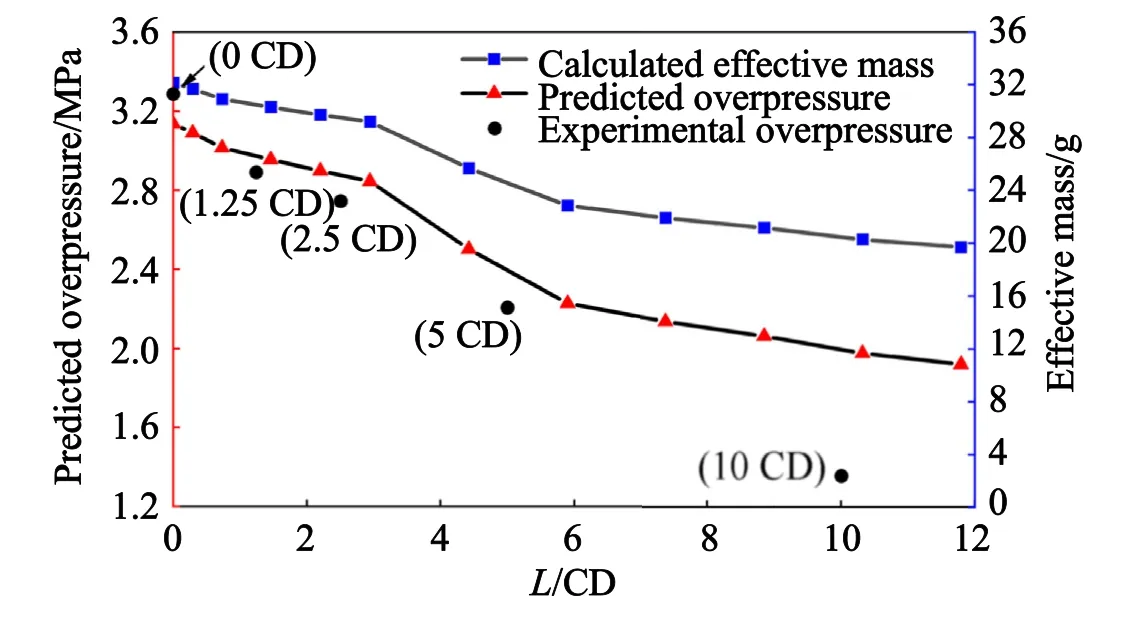
Fig.24.Comparison of experimental and predicted average overpressures.
Combined with the mechanism considerations in Fig.1,the chemical reaction of reactive jet is divided to two processes,including shock-induced local reaction and the subsequent global deflagration.Meanwhile,the present study mainly concerns on the shock-induced local reaction.That is to say,in a low stand-off and a shorter time scale relatively,the reactive jet mainly occurs the local chemical reaction,which is consistent with the derivation process of this study.Thus,the prediction accuracy is acceptable with a stand-off lower than 5.0 CD.However,the further increase of standoff indicates a longer time cost outside the chamber,and the chemical reaction in the reactive jet would change from local shock-induced reaction to global deflagration gradually in this process.The different reaction mechanism during the transition process implies that the shock-induced temperature rise would no longer be the main control mechanism of chemical reaction,and the deflagration propagation mechanism would become the main reaction mechanism subsequently.In hence,the reaction mechanism change is responsible for the non-negligible error under the conditions of large stand-offs.
6.Conclusions
The unique explosion-induced formation and thermochemical response of PTFE/Al granular jet have been researched by mesoscale simulation,theoretical analysis,and shaped charge experiment.
(1) The mesoscale simulation method is introduced to reveal the mechanical-thermal coupled mechanism during the formation and extension.The results show the density distribution of reactive jet has remarkable segmented characteristics,and the aluminum particles are likely to gather in middle and rear of the reactive jet.Further,the shock,collision,friction and deformation behaviors together lead to the temperature rise,which finally causes initiation and deflagration of the reactive jet.
(2) A thermochemical reaction model for reaction extent is developed to determine the reacted mass versus time.It shows that the inner wall of the reactive liner has a much higher reaction extent,due to a more significant temperature rise.The total reaction extent history curve of the reactive jet could be obtained.Thus,the mechanical-thermal-chemical coupled relationships are established.
(3) The energy release experiments are designed and conducted to verify the combination of numerical simulation and theoretical model.The comparison between experimentbased data and prediction shows an acceptable correspondence when the stand-off less than 5.0 CD.
(4) However,with the stand-off further increasing,the prediction error would be greater than 20%.The large error under a stand-off more than 5.0 CD is due to the reaction mechanism altering with time.Thus,more work needs to be carried out in the future to improve the mechanical-thermal-chemical coupled study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research is supported by the National Natural Science Foundation of China (No.12172052) and the China Postdoctoral Science Foundation (No.3020036722021).
杂志排行
Defence Technology的其它文章
- Numerical and experimental evaluation for density-related thermal insulation capability of entangled porous metallic wire material
- A new Ignition-Growth reaction rate model for shock initiation
- Effect of interface behaviour on damage and instability of PBX under combined tension-shear loading
- Sensitivity analysis and probability modelling of the structural response of a single-layer reticulated dome subjected to an external blast loading
- Deep hybrid: Multi-graph neural network collaboration for hyperspectral image classification
- Experimental study of polyurea-coated fiber-reinforced cement boards under gas explosions
