Intermediate hyperglycemia in early pregnancy: A South Asian perspective
2023-05-19JohnPunnoseKomalSukhijaRashikaRijhwani
John Punnose, Komal Sukhija, Rashika M Rijhwani
John Punnose, Department of Endocrinology and Metabolism, St. Stephen’s Hospital, Delhi 110054, India
Komal Sukhija, Rashika M Rijhwani, Department of Endocrinology, St.Stephen’s Hospital, Delhi 110054, India
Abstract “Intermediate hyperglycemia in early pregnancy (IHEP)” refers to mild hyperglycemia detected before 24 gestational weeks (GW), satisfying the criteria for the diagnosis of gestational diabetes mellitus. Many professional bodies recommend routine screening for “overt diabetes” in early pregnancy, which identifies a significant number of women with mild hyperglycemia of undetermined significance. A literature search revealed that one-third of GDM women in South Asian countries are diagnosed before the conventional screening period of 24 GW to 28 GW; hence, they belong in the IHEP category. Most hospitals in this region diagnose IHEP by oral glucose tolerance test (OGTT) using the same criteria used for GDM diagnosis after 24 GW. There is some evidence to suggest that South Asian women with IHEP are more prone to adverse pregnancy events than women with a diagnosis of GDM after 24 GW, but this observation needs to be proven by randomized control trials. Fasting plasma glucose is a reliable screening test for GDM that can obviate the need for OGTT for GDM diagnosis among 50% of South Asian pregnant women. HbA1c in the first trimester predicts GDM in later pregnancy, but it is not a reliable test for IHEP diagnosis. There is evidence to suggest that HbA1c in the first trimester is an independent risk factor for several adverse pregnancy events. Further research to identify the pathogenetic mechanisms behind the fetal and maternal effects of IHEP is strongly recommended.
Key Words: Intermediate hyperglycemia; Early pregnancy; Gestational diabetes; South Asian women; Adverse events; Asian Indian
INTRODUCTION
Gestational diabetes mellitus (GDM) is the most common metabolic abnormality in pregnancy, and its prevalence varies widely depending on the population studied and the diagnostic strategy employed. GDM predisposes pregnant women to several obstetric and perinatal complications and places the mother and infant at high risk of long-term metabolic morbidity[1-3]. For many years, GDM was defined as “any degree of glucose intolerance that was first recognized during pregnancy”[4]. However, this definition fails to distinguish between women with “new onset of glucose intolerance in pregnancy” and those with preexisting undiagnosed diabetes. To circumvent this diagnostic confusion, the World Health Organization (WHO 2013) introduced the broad term hyperglycemia in pregnancy (HIP) for various dysglycemias in pregnancy[5]. Furthermore, women with HIP are subcategorized into two distinct entities: (1) Diabetes in pregnancy (DIP), those women satisfying the WHO (2006) diagnostic criteria of diabetes in a nonpregnant state (undiagnosed preexisting diabetes); and (2) GDM, women having plasma glucose values in a 75 g oral glucose tolerance test (OGTT) above the threshold values proposed by the International Association of DIP Study group (IADPSG) criteria[6] and below the threshold for diagnosis of overt diabetes at any stage of pregnancy. Screening for DIP at the first prenatal visit is accepted by several preeminent organizations, such as the International Federation of Gynecology and Obstetrics (FIGO)[7], the International Diabetes Federation (IDF)[8] and the American Diabetes Association (ADA)[4]. In contrast, the screening and diagnosis of GDM continue to be controversial. Although OGTT is generally accepted as the diagnostic test by several professional organizations, there is no agreement on the glucose load for the test, plasma glucose cut off values and the number of abnormal plasma glucose values required for GDM diagnosis. Furthermore, there is no international consensus on GDM screening strategies: Risk-based selective or universal screening, onestep or two-step screening and optimal timing of screening (Table 1).
Conventionally, GDM screening is performed between 24-28 wk of gestation (GW). The selection of this period is justified by: (1) The development of significant physiological insulin resistance by 24 GW; and (2) the availability of sufficient time in pregnancy for therapeutic intervention after GDM diagnosis. The GDM criteria proposed by O’Sullivan and Mahan[9] and subsequently modified by Carpenter and Coustan[10] were used to identify pregnant women who are prone to type 2 diabetes later in life. These criteria and the subsequent WHO 1999 criteria[11] were not validated by any obstetric or perinatal outcome studies. The landmark Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study revealed a continuous relationship between maternal glycemia between 24 wk and 32 wk and several pregnancy adverse events, which formed the basis of the glucose threshold values proposed in the IADPSG criteria[6,12]. The threshold values of the IADPSG criteria are widely accepted by several professional organizations for GDM diagnosis between 24 GW and 28 GW[5,7,8]. However, the American College of Obstetricians and Gynecologists (ACOG)[13] and National Institute for Health and Care Excellence (NICE)[14] follow different criteria for GDM diagnosis. Many countries in South Asia continue to follow modified WHO 1999 criteria to suit the behavior of their obstetric population: DIPSI criteria[15] (Table 1).
GDM diagnosis prior to 24 GW (early GDM) by any criteria is not validated by pregnancy outcome data. Despite this limitation, many professional bodies, such as the WHO, FIGO, ACOG, and Australasian DIP Society (ADIPS), continue to recommend screening for early GDM among high-risk population groups[5,7,13,16] (Table 2). However, many organizations question the validity of mild hyperglycemia detected in early pregnancy. In 2016, the IADPSG withdrew its earlier 2010 recommendation to diagnose GDM in early pregnancy based on an abnormal fasting plasma glucose (FPG) value of ≥ 5.1 mmol/L[17]. The 2021 United States Preventive Services Task Force statement concluded that ‘the current evidence is insufficient to assess the balance of benefits and harms of screening for GDM before 24 GW[18]. The NICE guidelines (2021) restrict GDM screening in early pregnancy to women who had GDM in a previous pregnancy[14]. The ADA 2022 limits “GDM” terminology to denote impaired glucose tolerance detected in the second and third trimesters only[4]. However, it recommends screening before 15 GW to identify: (1) Undiagnosed pregestational diabetes; and (2) women at risk for adverse events,i.e., those with FPG ≥ 6.1 mmol/mol or HbA1c ≥ 41 mmol/mol (Table 2).
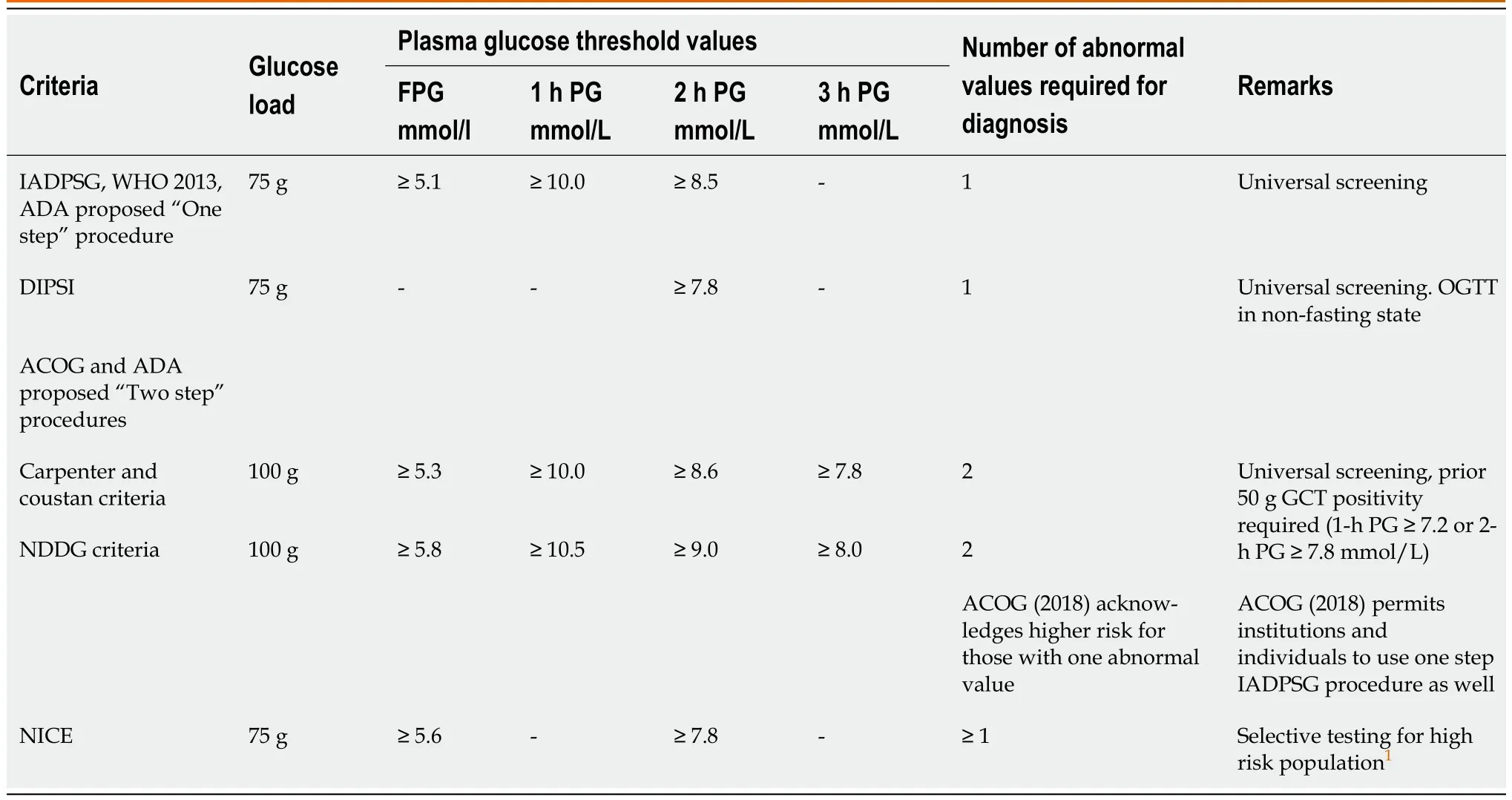
Table 1 Commonly used oral glucose tolerance test criteria for gestational diabetes diagnosis among South Asian women

Table 2 Recommendations of various organizations for “intermediate hyperglycemia” screening before 24 gestational weeks
The common practice of early GDM screening (before 24 GW) and DIP screening at the first prenatal visit among high-risk pregnant women identifies many women with milder glucose intolerance of undetermined significance: Glycemia below the threshold for overt diabetes but satisfying the diagnostic criteria for GDM. This dysglycemia in early pregnancy (before 24 GW) is referred to as Intermediate Hyperglycemia in Early Pregnancy (IHEP) and forms a significant proportion of “GDM women” in South Asian countries (India, Pakistan, Bangladesh, Sri Lanka, Nepal). This article is an update on the current knowledge on IHEP among pregnant women residing in South Asian countries.
SOUTH ASIANS AS A DIABETES RISK POPULATION
South Asians represent approximately 2 billion people globally. A high prevalence of type 2 diabetes has been reported among South Asians residing in the Indian subcontinent as well as in its diaspora[19]. The clinical profile of type 2 diabetes among South Asians differs from that among Caucasians in various aspects: Onset at a younger age, lower body mass index (BMI), higher abdominal (visceral) obesity, greater insulin resistance and early decline in pancreatic B cell function[20]. There is an ongoing global epidemic of type 2 diabetes with its epicenter in South Asia, and India is being projected as the ”diabetic capital” of the world. The number of people with diabetes in India has increased exponentially in the past two to three decades: 19 million in 1995, 32 million in 2000, and 66.8 million in 2014, and this number is expected to increase to 79.4 million in 2025[20,21].
The ICMR-INDIa DIABetes (INDIAB) study revealed that the number of people with prediabetes (77.2 million) in India was higher than that of people with diabetes (62.4 million)[22]. The IDF estimated 76 million women aged 20 years to 39 years to have diabetes or prediabetes in the Asia-Pacific region[23]. The high prevalence of prediabetes among women of child-bearing age is mirrored by the high GDM prevalence in pregnancy in this region. India has 5.7 million women with hyperglycemia during pregnancy and ranks first in the world in this respect[8,24]. A similar higher propensity for GDM has been reported among Asian immigrants in developed countries. Asian immigrants in the United Kingdom and Norway (South, East, and West Asian immigrants) have double the odds for GDM than non-Hispanic whites residing in these countries[25]. In a recent analysis by Gamiet al[26] among the United States population, GDM rates increased significantly from 47.6 to 63.5 per 1000 live births from 2011 to 2019, and this rise was mainly observed among Asian Indian and Puerto Rican women. Additionally, women of Asian ancestry in the United States were observed to have GDM at a younger age, even with BMI within or below the normal range[27,28]. In a large study involving 10353 pregnancies at Bradford Infirmary in the United Kingdom, Farraret al[29] estimated that the glucose threshold levels in a 75 g OGTT (performed between 26-28 GW) produced a 75% or higher relative risk of large for gestational age (LGA) babies among South Asian women than among British Caucasian women. The plasma glucose threshold values for LGA babies among South Asian and British Caucasian women were FPG values of 5.2 mmol/L and 5.4 mmol/L, respectively, and 2-h post glucose load plasma glucose (2-h PG) values of 7.2 mmol/L and 7.5 mmol/L, respectively.
IHEP AMONG SOUTH ASIAN PREGNANT WOMEN
The screening strategies to identify IHEP/HIP are: (1) Universal or selective screening by OGTT; (2) FPG at the first prenatal visit; and (3) hemoglobin A1c (HbA1c) in early pregnancy. We performed a literature search for studies carried out between January 2004 and November 2022 on “IHEP among women residing in South Asian countries” in PubMed (medline), Cochrane Library and Google Search using the terms “gestational diabetes mellitus”, “diabetes in pregnancy”, “hyperglycemia in pregnancy”, “early diagnosis”, “first trimester”, “early pregnancy”, “South Asia”, “India”, “HbA1c”, “oral glucose tolerance test”, “fasting glucose”, and “intermediate hyperglycemia”. We identified 19 original articles that provided data on the frequency of IHEP in the South Asian region. These studies were not primarily designed to assess IHEP (early GDM) and had inadequate data for a proper systematic review or meta-analysis on this topic.
OGTT for detection of IHEP
The literature search yielded 14 GDM studies from South Asia with some data on the frequency of IHEP: Eleven from India, two from Sri Lanka and one from Bangladesh. The study design, GDM diagnostic criteria, overall GDM prevalence and frequency of IHEP in these studies are shown in Table 3[30-43]. The marked heterogeneity in the study design, the diversity of the GDM diagnostic criteria and the lack of clinical details of women with IHEP are limitations to making a comparative assessment between these studies. Five GDM diagnostic criteria were used in these studies: WHO 1999 criteria for six studies (4 studies[31,36,41,43] using both fasting PG and 2-h PG values, 2 studies[32,33] using only 2-h PG value; modified WHO 1999 criteria), DIPSI criteria for four studies (same as modified WHO 1999 criteria, but OGTT performed in nonfasting state)[34,37,38,40], IADPSG criteria for three studies[30,39,42] and Carpenter & Coustan criteria for one study[35]. As WHO-1999, modified WHO 1999 and DIPSI criteria are primarily based on 2-h PG values, the women who had GDM diagnosis by these criteria were analyzed together. The pooled data analysis of 32055 pregnant women who were screened by these criteria revealed that 4024 women had GDM, with a prevalence of 12.55%. Of 4006 women who were screened by IADPSG criteria, 1072 women had GDM, with a prevalence of 26.75%. One small study among 298 women identified 40 GDM by Carpenter & Coustan criteria, with a prevalence of 13.42%.
The number of women with GDM in different periods of gestation and their percentage in relation to total GDM women are shown in Table 3. The pooled data analysis revealed that 925 (18.5%) of 4961 GDM women in eleven studies had a GDM diagnosis in the first trimester. The combined data of seven studies showed that 1230 (32.6%) of 4961 GDM women were diagnosed before the conventional screening period of 24-28 GW. Hence, one-third of GDM women in South Asian countries belong to the IHEP category, and half of them are diagnosed in the first trimester. A selective assessment of women with IHEP diagnosis by IADPSG criteria (data from 3 studies)[30,39,42] revealed nearly the same proportions of women with IHEP in the first trimester (18.09%) and < 24 GW (35.31%) groups. The exclusion of women with DIP from the analysis[39,41,43] produced minor changes in the frequency of IHEP: First trimester, 19.55% (149 of 762 GDM women); before 24 GW, 31.03% (359 of 1157 GDM women).
The above data suggest that OGTT is widely used for the detection of IHEP among South Asian women. The Ministry of Health and Family Welfare, Government of India Technical Guideline on the Diagnosis of Gestational Diabetes (2018), recommends that all pregnant women should undergo 75 g OGTT “during the first antenatal contact as early as possible”; if the test is negative initially, a second OGTT should be done during 24-28 GW[44-46]. The FIGO endorsed this approach for hyperglycemia screening in early pregnancy in South Asian countries[7]. Similarly, the ACOG[13], ADIPS[16], and Canadian Diabetes Association[47] advocate OGTT-based screening for IHEP among the South Asian diaspora in the respective countries.
There is no consensus on the OGTT criteria to be used for IHEP diagnosis in the South Asian region (Table 2). Considering the convenience of nonfasting state and single PG sampling, the DIPSI criteria are frequently used in India for “GDM diagnosis” in all trimesters[32]. However, there are some concerns about the validity of DIPSI criteria in the post-IADPSG era. The DIPSI 2-h PG threshold value (7.8 mmol/L) was derived from WHO 1999 criteria, a popular criteria for GDM diagnosis during the 1999-2010 period[11]. The FPG threshold value of 7 mmol/L recommended for GDM diagnosis in the WHO 1999 criteria is presently the cut off value for DIP diagnosis, and women with DIP are not considered to have GDM by any professional organization. Furthermore, with the introduction of IADPSG criteria based on the pregnancy outcome data in the HAPO study, the WHO withdrew its 1999 criteria and recommended IAPDPG criteria as the new WHO2013 criteria[5]. The DIPSI criteria were initially validated with WHO 1999 criteria, and many hospitals in India continue to use these criteria for GDM diagnosis[33] (Table 2). However, as the WHO has withdrawn its 1999 criteria and accepted the IADPSG criteria, the DIPSI criteria need to be revalidated with the WHO 2013 criteria or be validated by pregnancy outcome data. The validation of nonfasting DIPSI criteria with IADPSG criteria was attempted in two well-designed studies from India; in both studies, the sensitivity for DIPSI criteria was too low for its use as a diagnostic or screening test for GDM[48,49].
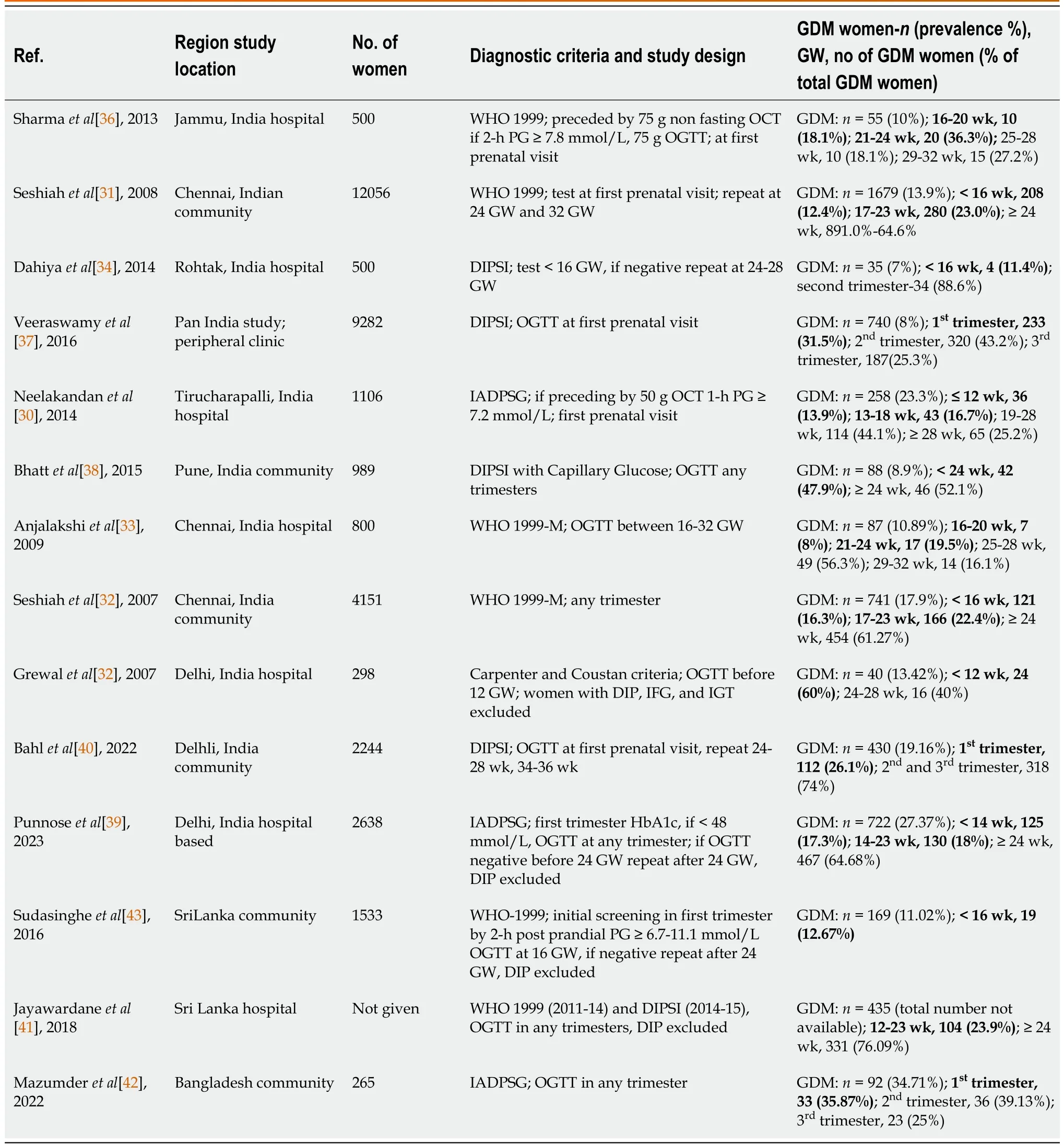
Table 3 Early Gestational diabetes among South Asian women: Oral glucose tolerance test based studies
FPG estimation for detection of IHEP
In the HAPO study on which the IADPSG criteria are based, there was heterogeneity in the frequency of abnormal FPG, 1-h PG and 2-h PG values among women diagnosed with GDM in different centers. An abnormal FPG value occurred only in 26% of women in the Hong Kong center, while the percentage in the Bellflower (California) center was > 70%. This observation led to the conclusion that FPG performed poorly in diagnosing GDM in Asians compared to Caucasian women[50]. A study in South India by Balajiet al[51] also suggested that only 24% of women who had GDM diagnosis by WHO 1999 criteria had FPG values ≥ 5.1 mmol/L (the IADPSG FPG threshold for GDM diagnosis), and the authors concluded that FPG was inadequate to diagnose GDM in the South Asian population. However, the reliability of FPG to diagnose GDM by IADPSG criteria (at least based on the available FPG and 2-h PG values) was not assessed in this paper. Subsequently, several studies among South Asian pregnant women reported FPG as a more reliable, easier test than the glucose challenge test to screen for GDM by Carpenter and Coustan criteria[52-54]. In a large North Indian study (involving 6520 pregnant women), an FPG value of ≤ 4.3 mmol/L reliably ruled out GDM in all trimesters (95.6% sensitivity), and FPG alone (≥ 5.1 mmol/L) could identify 67.9% of GDM by IADPSG criteria[55]. This study suggested that FPG can reliably “rule in and rule out GDM” and can avoid OGTT for GDM diagnosis in approximately 50% of South Asian pregnant women. The excellent area under the curve of 0.909 (95%CI: 0.898 to 0.920) for FPG in this study was contrary to the traditional belief that FPG performs poorly as a screening test for GDM in Asians.
Several studies from South Asia have tested the reliability of FPG in early pregnancy to predict GDM in later pregnancy. In a cohort of 246 pregnant women from North India, an FPG value of 4.7 mmol/L in early pregnancy reliably predicted GDM diagnosis by IADPSG criteria after 24 GW (with 94% sensitivity and 74% specificity)[56]. Another study from South India (n= 270) concluded that FPG ≥ 5 mmol/L in the first trimester reliably predicted GDM by DIPSI criteria, with an area under the ROC curve of 0.694, sensitivity of 86.6%, and specificity of 52.1%[57].
The above data suggest that FPG estimation in early pregnancy can be a reliable predictor and possibly a screening test for GDM among South Asian pregnant women. In 2013, most international professional organizations accepted the IADPSG recommendation to diagnose GDM in early pregnancy based on FPG values between 5.1 and 6.9 mmol/L[5,7,8]. Subsequently, IADPSG withdrew this recommendation[17], and some organizations supported this change[4]. Presently, FPG values between 5.1 and 6.9 mmol/L in early pregnancy are interpreted differently by many professional bodies. The WHO approves GDM diagnosis for such women and permits treatment accordingly. The IADPSG does not approve FPG use for GDM diagnosis before 20 GW. The ADA (2022) criteria approve treatment for these women, provided the FPG is ≥ 6.1 mmol/L and it is documented before 15 GW. The DIPSI and Government of India (2018) guidelines do not recommend FPG estimation at any stage of pregnancy. Obstetricians in South Asian countries follow all these guidelines, resulting in chaos in the diagnosis and management of IHEP among South Asian women.
HbA1c for detection of IHEP
Following the recommendation of the World Health Organization that HbA1c testing be used for the diagnosis of diabetes mellitus in the general population, interest in its use in pregnancy has been renewed[58]. An HbA1c level ≥ 48 mmol at booking is now accepted as a criterion to diagnose DIP or preexisting overt diabetes[5,7]. In 2011, the California state Diabetes and Pregnancy program (CSDPP) “Sweet Success” adopted a new algorithm for the diagnosis and treatment of hyperglycemia in pregnancy[59]. Accordingly, all women with HbA1c values of 39-46 mmol/mol in early pregnancy are advised to undergo GDM treatment without further confirmatory OGTT. This recommendation equates GDM to the prediabetic state of the nonobstetric population. This CSDPP proposal, although practiced in several United States centers, has not been approved by any professional body.
Considering the high prevalence of prediabetes in the background population, HbA1c can be a potential biomarker to identify high GDM risk women in early pregnancy among South Asian Women. There are limited studies among South Asian women to assess HbA1c as a diagnostic test for IHEP. In a South Indian study to assess HbA1c for screening GDM among 507 women by Balajiet al[60], a subgroup analysis revealed that all women with HbA1c ≥ 42 mmol/mol in the first trimester (n= 10) developed GDM (by WHO 1999 criteria) in later pregnancy. In another study in which HbA1c and OGTT were simultaneously tested at a mean age of 19 GW, women who had GDM had higher HbA1c (33 mmol/mol) than those without GDM (HbA1c, 30 mmol/mol)[61]. In a retrospective cohort study from our center among 2275 Asian Indian pregnant women, an HbA1c value of > 37 mmol/mol in the first trimester was found to be an independent predictor of GDM (adjusted OR 2.60, 95%CI: 1.49-4.55) by IADPSG criteria[62]. However, HbA1c in the first trimester lacked sufficient sensitivity and specificity for consideration as a diagnostic test for GDM in early pregnancy. Interestingly, we observed in this cohort that, even after exclusion of women with DIP and women who developed GDM in later pregnancy, HbA1c in the first trimester was independently associated with preterm birth and primary cesarean delivery[63]. Hence, apart from being a strong risk factor for GDM, HbA1c in the first trimester can independently predict adverse pregnancy events in South Asian pregnant women.
As HbA1c is increasingly being used to identify DIP at the first prenatal visit, it is cost effective to use the same test for the prediction of GDM and other adverse events. Furthermore, HbA1c estimation requires only a single nonfasting sample, and the test has greater preanalytic stability and reproducibility and no interference from acute stressful conditions. These factors are of special advantage for pregnant women in South Asian countries, as most of them report to hospitals in a nonfasting state and are not willing to undergo repeated blood sampling[15].
INTERVENTIONS AMONG SOUTH ASIAN WOMEN WITH IHEP
Limited data on IHEP management in South Asian countries are derived from the analysis of retrospective data. With early initiation of treatment among a small cohort of 54 women with early GDM (by WHO 1999 criteria) in South India, the birth weight of babies of GDM women was comparable to babies of non-GDM women[64]. In a retrospective study in our center among 2638 pregnant women with HbA1c < 48 mmol/mol in the first trimester, 255 women satisfied the IADPSG criteria for GDM before 24 GW (IHEP)[39]. Despite early initiation of treatment, women with early GDM (IHEP) had significantly higher adjusted odds ratios for premature birth, macrosomia, LGA babies, and neonatal intensive care unit admission and lower odds for normal vaginal delivery than non-GDM women. The highest risk for adverse events was observed among GDM women who had the diagnosis in the first trimester. A similar observation was made in a large multiethnic Australian study that revealed the highest adverse events among women who had GDM diagnosis in the first trimester, despite the best practices of management[65]. The failure to reduce adverse pregnancy events by early intervention in these studies[39,65] may be interpreted as a lack of benefit of early GDM screening. Alternatively, it can be attributed to the fetal and maternal effects of mild hyperglycemia in early pregnancy, which were not reversed with restoration of euglycemia in later pregnancy. This speculation is strengthened by the observation of an independent association of HbA1c in the first trimester with adverse events, even without the development of GDM in later pregnancy by several researchers[63,66,67].
IHEP AMONG SOUTH ASIAN PREGNANT WOMEN: CHALLENGES &RECOMMENDATIONS
The main challenges in the identification and management of IHEP are the lack of pregnancy outcomebased diagnostic criteria and the frequent changes in the recommendations of many associations and organizations of international repute. Unfortunately, the changes proposed by many professional organizations are not backed by strong research data. The withdrawal of FPG-based GDM diagnosis before 24 GW by IADPSG was based on reports that early GDM diagnosis by an abnormal FPG value was poorly predictive of later GDM at 24-28 GW[17]. This approach has the limitation of considering pregnancy as a ‘metabolically static state’, having fixed glucose threshold values for all adverse events throughout pregnancy. In contrast, the HAPO study revealed a differential effect of the gestational age of onset of hyperglycemia on adverse events: PG values between 24-32 GW were associated with abnormalities in birth weight, while the HbA1c of that period (glycemia of preceding three months) led to preterm birth, primary cesarean delivery and preeclampsia[68]. Furthermore, several studies have suggested that hyperglycemia in early pregnancyper secan lead to significant adverse pregnancy events, even without the development of GDM in later pregnancy[63,66,67]. Hence, there is a strong need to identify glucose threshold values in early pregnancy, which can reliably predict adverse pregnancy events, and not GDM development alone, in later pregnancy. The differential effect of glycemia at different stages of pregnancy on adverse pregnancy events needs to be explored further. The mechanisms behind the deleterious effects of “mild hyperglycemia in early pregnancy” on fetal development and on adverse pregnancy events have not yet been clearly identified. Further research to identify any modifiable factors in early pregnancy will help to design preventive strategies for “hyperglycemia” in the peri-conception period and to develop alternate nonglucose centric measures.
There are significant ethnic and racial differences in PG and HbA1c threshold values for adverse pregnancy events, which was evident in two well-designed studies in Europe: Lower PG threshold values for LGA for South Asians than British Caucasians in the Bradford birth cohort by Farraret al[29] and lower HbA1c (first trimester) threshold values for adverse events among South Central Asians compared to Caucasians in Spain by Mañéet al[69]. The ADA proposal of an HbA1c value of 41 mmol/mol in the first trimester to identify women prone to adverse events is derived from a New Zealand study involving predominantly Caucasian women[67]. The ADA-proposed HbA1c and FPG threshold values (≥ 6.1 mmol/L) for adverse pregnancy events were tested in a cohort of 2638 pregnant South Asian women in our center[4,39]. The percentage of women with adverse events identified by the ADAproposed FPG and HbA1c threshold levels was significantly lower than the percentage of women having these events in the group of women with a diagnosis of IHEP by IADPSG criteria. Hence, an IHEP diagnosis identifies more South Asian pregnant women who are prone to adverse pregnancy events than those detected by the ADA-proposed FPG and HbA1c threshold values.
The trimester-related variations in the effect of hyperglycemia on fetal and adverse events and ethnic differences in the threshold for these adverse effects are major areas for future research. Despite having the highest number of women with HIP in the world, no center from South Asia (Indian subcontinent) was included in the HAPO study. There is a strong need for a HAPO-like study in early pregnancy among pregnant women of this region to identify the PG threshold values for various adverse events. Furthermore, the benefit of early intervention should be assessed in a randomized control trial. However, in obstetric practice, where early GDM screening and early initiation of GDM treatment have been common practices for several decades, withdrawing GDM treatment from women who have a diagnosis of “early GDM or IHEP” is a major challenge to researchers.
CONCLUSION
A significant number of pregnant women in South Asian countries have intermediate hyperglycemia in early pregnancy. The current estimates suggest that one-third of GDM women among South Asian countries are diagnosed before the conventional screening period of 24-28 gestational weeks. The guidelines of regional professional bodies such as DIPSI and the local governmental guidelines strongly recommend screening for IHEP at the first prenatal visit. There is no consensus on the test or the criteria used for IHEP diagnosis in this region. Despite the controversies on the diagnostic threshold values, the OGTT is the preferred test for IHEP diagnosis in South Asia. Other tests, such as FPG and HbA1c, are routinely performed to detect DIP and hence can be considered potential tests for IHEP detection. The frequent changes in international guidelines on IHEP detection and management, without strong research data to justify these changes, have led to major confusion in obstetric practice in South Asian countries. The intervention studies among women with IHEP have yielded conflicting results, which is partly attributable to the heterogeneity in study design. However, there is some suggestion in these studies of a possible fetal effect of mild hyperglycemia in early pregnancy that may not be reversible with the normalization of blood glucose in later pregnancy. Further research to identify the exact pathogenetic mechanisms of maternal and fetal effects of IHEP is recommended.
FOOTNOTES
Author contributions:Punnose J, Sukhija K, and Rijhwani RM contributed equally to this work; all authors have read and approved the final manuscript.
Conflict-of-interest statement:The Authors have no conflict of interest to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:John Punnose 0000-0001-9883-6188; Komal Sukhija 0000-0002-0543-214X; Rashika M Rijhwani 0000-0002-2904-3712.
S-Editor:Chen YL
L-Editor:A
P-Editor:Yu HG
杂志排行
World Journal of Diabetes的其它文章
- Early diabetic kidney disease: Focus on the glycocalyx
- Inter-relationships between gastric emptying and glycaemia:Implications for clinical practice
- Cardiometabolic effects of breastfeeding on infants of diabetic mothers
- Efficacy of multigrain supplementation in type 2 diabetes mellitus: A pilot study protocol for a randomized intervention trial
- Association of bone turnover biomarkers with severe intracranial and extracranial artery stenosis in type 2 diabetes mellitus patients
- Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes
