An Intestinal Trained Immunity Model Based on Drosophila melanogaster Oral Infection*
2023-05-16YANGJunXiaZHUJinTongPENGYing
YANG Jun-Xia, ZHU Jin-Tong, PENG Ying*
(1)BGI college, Zhengzhou University, Zhengzhou 450052, China;2)Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou 450052, China)
Abstract Objective To study the trained immunity effect of Drosophila melanogaster at the individual and molecular level,and provide a basis for the follow-up in-depth study of the molecular mechanism of trained immunity using genetic tools available to Drosophila. Methods Firstly, a germ-free Drosophila culture model was constructed. Subsequently, the Drosophila adult and crossdevelopmental trained immunity models were constructed. Two Gram-negative bacteria, Erwinia carotovora carotovora 15 and Pseudomonas aeruginosa, were respectively used to infect Drosophila orally. With a repeated infection elicited after the first infection completely subsided, the effects of potential trained immunity is demonstrated by comparing the survival rate and bacterial load of Drosophila melanogaster during the two infection phases. The induction of immune deficiency (IMD) pathway by Gramnegative bacteria as the expression level of corresponding innate immunity-related genes was detected by real-time quantitative PCR.Results The primary infection of during either adults or larva developmental stage can significantly improve the survival rate of secondary challenge. A higher bacterial clearance efficiency and maximum bacterial load of death is consistently observed after a second infection. The basal expression of immune response genes in IMD signaling pathway is boosted prior to secondary infection than naive animal, explaining the molecular basis of gained infection resistance. Midgut is examined to be primary anatomic site of immune response, and the effects of secondary immunization were faster and more intense than those of primary infection. The numbers of intestinal stem cells in the midgut were significantly higher during the second infection compared with the first one.Conclusion A robust trained immunity in Drosophila melanogaster intestine can be triggered by oral infection of either homologous or heterologous Gram-negative bacteria, and the immunological memory can persist across developmental stages. It may act on chromatin and store immunologic memory at relevant gene loci through chromatin modifications. A potential way for the passage of immunologic memory across developmental stages is through JNK/STAT activation of intestinal stem cells, which may carry on the immune imprint from larval to adult developmental stages in the gut.
Key words Drosophila melanogaster, intestine, trained immunity, intestinal stem cells, antimicrobial peptides, immune deficiency signal pathway
The authors dedicate this article in memory of Dr. Chen-Lu Tsou (1923-2006), a prominent protein biochemist, whose spirit will be cherished dearly by all whom had the privileges to be trained/influenced by him first hand. With no particular goal or aim back then, one of the authors (PENG Y) appreciates very much the“trained immunity” he got as a graduate student from Dr. Tsou’s group in the late 1990s, at the Institute of Biophysics, Chinese Academy of Science. As one cannot foresee what circumstance he is going to encounter in a fast evolving world, Dr. Tsou had well prepared young students to become independent thinkers, fearless fighters,steady clutchers and last but not the least human beings with dignity and heart. Without those long lasting“trained immunity”, this article and many more other significant discoveries from the authors could not have been made possible.
In classical immunology, immune response can be roughly classified into innate immunity and adaptive immunity. The innate immunity response is rapid and nonspecific, which is mainly mediated by innate immune cells (such as bone marrow derived natural killer cells and congenital lymphocytes) as well as ancient humoral systems such as defensins and complements[1]. These immune cells are activated by recognizing pathogen-associated molecular patterns(PAMPs) and damage-associated molecular patterns(DAMPs) through pattern recognition receptors(PRRs)[2]. Adaptive immunity, on the other hand,demonstrates antigenic specificity and is mainly engaged by prior exposure to antigens. T lymphocytes and B lymphocytes are the major mediators of adaptive immunity, with their antigen specificity rests on the diverse immune reportorial generated through DNA recombination at T cell receptor (TCR) locus or immunoglobulin locus. After being stimulated,antigen recognizing lymphocytes get selectively activated in a period of a few days. While most activated immune effector cells die out after the infection, a small fraction of active, proliferated clone returns to immune quiescence by acquiring a so called“memory cell” fate. Such antigen specific memory cell is largely poised to be activated quickly upon antigen stimulation, distinct from the slow activation of naive T or B lymphocytes, therefore establishing an apparent immune memory. While immunologic memory is a major feature of adaptive immunity, its specificity rests mainly on available immunologic receptor and its effective memory retrievalviachromatin modifications on effector cells by a so-called “hit and run” mechanism[3-4].
While it has been firmly established that a memory like immune mobilization mechanism is beneficial to protect animals from repeated infections by pathogen, adaptive immunity has been long regarded as the sole keeper of such immunologic memory. On an evolutionary prospective, however,specialized lymphocyte based adaptive immunity is a relatively recent addition of pathogen resistance arsenal, appearing only in vertebrates. Closer examinations with invertebrates such asDrosophila melanogaster,Anopheles gambiaeandArtemia, which lack adaptive immunity, reveal that a more potent immune response can frequently be found when they are infected with the same or different pathogens for the second time, indicating immunological memory might be hardwired in a much more ancient and universal manner[5-9]. At the same time, antigen nonspecific immunological modulation based on previous infection was also discovered among vertebrates including humans. Studies have shown that Bacille Calmette-Guérin vaccine (BCG) can not only protect human against the infection ofMycobacterium tuberculosis, but also boost the survival rate of infants in general, and show nonspecific beneficial effects on other diseases or infections[10-12].This effect is far beyond tuberculosis and cannot be explained by the principle of adaptive immunity[13-14]. In addition, similar phenomena were observed in the clinical trials of measles and hepatitis B vaccines[15]. This phenomenon of enhanced immunity through repeated homologous or heterologous infections as observed in many organisms is often characterized by high reactivity and rapid clearance to reinfection, was known as“innate immunity memory” or “trained immunity”[16-17]. A “trained immunity” boosting vaccine is also recently developed targeting hematopoietic stem cells and demonstrated promising efficacy as a cancer therapeutic adjuvant in animal model[18]. Therefore, better understanding how immunity memory is kept and elicited in trained immunity would help us tremendously for devising next generation vaccine against cancer or emerging infectious disease.
For in-depth mechanistic investigations, a well established trained immunity model system is preferred. Since all vertebrate models come with the complexity of a combinatory action of both innate immunity and adaptive immunity, we seek to explore trained immunity in an invertebrate system with minimal cellular complexity and plenty of genetic manipulative tools.Drosophilagut is by far one of the best well understood immunity barriers characterized in invertebrates. Similar to human[19],Drosophilahost various symbiotic microbes in its gut, while its genetic circuitry of how various PRRs recognize bacteria derived PAMPs molecules to induce downstream Relish/IMP immune response has been a paradigm innate immunity discovery[20]. In addition,there are well characterized pathogens forDrosophilaenteroinfectionviaoral-route. Such infections transiently disrupt gut barrier homeostasis similar to those in human, which is accompanied by the shedding of gut enterocytes by inflammation and compensatory proliferation of intestinal stem cells[21].Since fly intestinal stem cell (ISC) is the only long lived stem/progenitor cell type in the gut[22](deriving both types of mature cells of the gut—enterocytes(ECs) or enteroendocrine cells (EEs) in a manner highly parallel to its vertebrate counterparts), we speculate that ISC might hold the key immune memory if long lasting trained immunity can be demonstrated with enteroinfection.
In invertebrate such asDrosophila, humoral responses include the pro-phenoloxidase cascade,leading to the production of reactive oxygen species(ROS), and the production of antimicrobial peptides(AMPs)[23-24]. AMPs constitute one of the most important lines of defence against infection, which can be made locally at the site of infect as well as remotely by central humoral organs such as Fat body.The Toll signaling pathway and immune deficiency(IMD) signaling pathway are the main signaling cascade responsible for AMPs generation[25], triggered by fungi, Gram-positive or negative bacteria respectively. Prior evidence have shown that trained immunity is part of the innate immune response in invertebrates and can persist after the insect passes through metamorphosis stage[9,26], but its potential occurrence and regulation mechanisms are still unclear. Researchers have proposed the following possible corresponding models for the way invertebrates respond to non-fatal infections[27-28]. The first is called “recall” phenomenon of immune memory, which can be divided into two situations.One is that when infected, the immune response is activated and the infection is cleared, resulting in increased responsiveness to subsequent homologous or heterologous infections. The other is to reduce the responsiveness to subsequent infections, which is to produce tolerance. In both cases, the main goal of establishing innate immune memory is to better protect and maintain the integrity of the body. In fact,enhanced reactivity can more effectively eliminate subsequent infection, while tolerance can limit the tissue to produce destructive sequelae or sustained immune activation. However, in the long term, this may also translate into decreased resistance to infection or increased side effects[29-30]. The second is called “immune transformation”, which mainly refers to the change of immune function in invertebrates when they are infected with homologous or heterologous secondary infection, that is, from an ordinary immune response to a more efficient immune response, so as to clear the infection more effectively.The third, called “sustained unique response”, refers to the fact that in invertebrates, trained immunity after infection can lead to a long-term state of immune activation, which is further enhanced by secondary infection[31].
Trained immunity reveals an underappreciated pathway of the body's immune response, manifested primarily by the memory characteristics of innate immune cells, and its study is of great significance as an important avenue for refining and developing classical immunology. Currently, scientists have put forward the opinion that most of the existing vaccines developed based on adaptive immunity have the advantages of strong specificity and disadvantages of being unable to be applied to the emerging, novel pathogens that have not yet emerged. Therefore, it is to the test of humans ingenuity to come up with effective vaccines prior to future global pandemic.Can a broad-spectrum vaccine based on trained immunity be developed as an alternative vaccination strategy[32]? In this study, we usedDrosophila melanogasteras a model to investigate whether trained immunity might develop greater bacterial tolerance and more efficient pathogen clearance capability, and whether trained immunity might remain after drastic metabolic responses and developmental changes have occurred, and explored the possibility that the effects of trained immunity might be memorized long term by intestinal stem cells.
1 Materials and methods
1.1 Drosophila strain and germ-free culture
We obtained infection sensorDipt-GFPreporter fly from BloomingtonDrosophilaStock Center(#55709) as our animal model. It is a well established sensor strain that upon Gram-negative bacteria infection. The activation of IMD pathway will lead to the expression of GFP at the site of innate immune response[33]. To exclude any contribution of existing microbiota in shaping the gut immune response, we adopted a germ-free culture methodology[34]to avoid any resident commensal bacteria in ourDrosophilaculture for trained immunity investigations.
Our protocol largely follow the published methodology by sterilizing collected eggs with bleach briefly and deposit the embryos without eggs shells with a brush into sterile standardDrosophilaculture media. We verify the effectiveness of our axenic culture condition by culture some of our embryo homogenate on antibitiotic free LB plate, as well as 16S rDNA PCR to exclude any bacteria in our culture(Figure S1). Axenic culture took 2-3 d longer for the embryos to reach wandering 3rd instar larval stage comparing with typical culture, which offers us a convenient way to exclude any vial with potential bacteria contamination by checking the developing stage one week after the start of the axenic culture.After flies reaching adulthood, we also double-check the culture condition is indeed germ-free by grinding one random fly from each culture vial and plating the fly homogenate on LB solid media.
1.2 Oral infection of Drosophila adults and the measurements of trained immunity by adult survival rate and bacterial load
We choose two known Gram-negative bacteria:Erwinia carotovora carotovora15 (Ecc15, a lab kept stock) andPseudomonas aeruginosa(PAO1, obtained from Beijing Biopreservation Center) as our infection agents, since they were studied to cause severe inflammatory reaction inDrosophilaupon feeding,leading to significant but not complete animal lethality.Ecc15bacteria culture is expanded with liquid culture in LB medium at 30℃ untilA600reaching between 0.6-0.8.Ecc15is pelleted by centrifugation 7 000 r/min at 4℃ and washed in sterile PBS twice before final re-suspension by 5%sterile sucrose solution with the finalA600reaching 100. An equivalent germ-free prep is made by diluting 5% sterile sucrose solution with sterile water until the same final volume is reached. ForPAO1, the culture expansion is done at 30℃, and the final bacterial concentration in the sucrose re-suspension is at finalA600of 25 instead of 100.
ForDrosophilaadult oral infection, a sterile filter paper is placed on top of culture vial with sterileDrosophilafood. Two loads of 0.5 ml sucrosebacteria mix each are dripped onto the filter paper to avoid liquid overflow. After the filter paper is dried briefly so that no apparent liquid come out of the paper, 25 starved adult flies were transferred from a empty sterile culture vial into the infection vial. In the subsequent days, the surviving flies were counted and transferred to a sterile culture vial every 24 h. When we quantify remaining bacteria load in surviving flies,there are barely any bacteria left after a 5 d long sequential transfer. Therefore, we can repeat the infection and recovery process with a second infection challenge afterward as the primary one.
Bacteria load in live/dead flies were done by taking 5 live or newly deceased adult flies, washed briefly in 75% EtOH for 5 s followed by three rinses in sterile PBS solution. 1 ml of sterile PBS solution is used to homogenize the flies using a beads votexer and the homegenate were serial diluted and plated on LB agar plate for overnight bacteria culture. 3 replicates were performed for each condition. The plates with colonies were imaged, and the number of colonies were counted by an ImageJ plugin.
1.3 Oral infection of Drosophila larvae and the measurements of trained immunity by adult survival rate and bacterial load
We started the oral infection of fly larvae by isolating around 200 early 3rd instar larval from axenic culture vial and starve those in a fresh empty sterile vial for 3 h. At the same time, prepare 5 g of sterile instant fly food in an empty sterile vial and stir in 1 ml of concentrated sucrose-bacteria mix until no liquid overflow is observed. We washed the starved fly larvae briefly with sterile water and dried them completely before transferring them into the infection vial onto instant fly food directly. In the next 30 min,we visually examine any larvae crawling out of the instant fly food, and pick all larval on the vial wall back to the food once every 10 min. By the end of the 30 min, if there are still larvae staying on the vial wall instead of the food, those free rangers are removed by a brush since they are likely late 3rd instar larval in development and can not be efficiently fed with bacteria.
After a 6 h feeding/infection phase, all larval in the infection vial were removed from the instant fly food. They are washed with 30% EtOH first, followed by sterile water, and then transferred to a fresh sterile culture vial with fly food to continue their development. 2-3 d after their eclosion, adult flies were infected for a second time following the standard adult oral infection method. Their survival rate and bacteria load is examined on a daily basis as the previous section.
1.4 Gene expression measurements of Drosophila gut upon primary infection or repeated infection
Total RNA were extracted from intact flies using FastPure® Cell/Tissue Total RNA Isolation Kit(Vazyme) and quantified with Nanodrop200. cDNA were synthesized from 1 μg of total RNA using HiScript® III All-in-one RT SuperMix (Vazyme),primed by Oligo-dT. Quantitative PCR were done using Taq Pro Universal SYBR qPCR Master Mix(Vazyme), using a panel of innate immunity related genes. The detailed information on primers amplifying house keeping internal control and innate immunity related genes can be found in the Table S1.
1.5 Gut immuno-staining and statistic analysis
Freshly dissected adult guts were fixed in 4%formaldehyde and rinsed with PBST. For GFP only analysis, the tissue is directly mounted in vectorshield mounting medium containing DAPI(vectorlab). For immunostaining , primary antibody(Mouse anti-Delta 9B, DSHB) were incubated with tissue pre-blocked with goat norm serum with 1∶500 dilution at 4℃ overnight first, and then washed with PBST for 5 times. Secondary antibody (Alexa Flour647 goat anti mouse, Thermo) were used at 1∶200 dilution at room temperature for 2 h. The washed tissue is then mounted in vector-shield mounting medium containing DAPI. Images were taken using a Leica STELLARIS 8 confocal microscope. GFP positive cells were scored with those ROI double positive for GFP and DAPI. ISC were scored with ROI double positive for Delta and DAPI. ImageJ were used to quantify the cells of interest at the imaging field. Student'st-test were used for statistical analysis among groups.P<0.05 (*)was considered statistically significant differences.P<0.01(**) was considered statistically very significant differences.
2 Results
2.1 Oral infection by Gram-negative bacteria elicit robust trained immunity in Drosophila adults
We setup to examine whether priorEcc15infection can protect adult flies from infection related mortality. Three experimental groups were designed(Figure 1): (1) CG (control group): during both infection windows, only sterile sucrose solution is used during bacteria feeding period. (2) ETC1 (Ecc15training control group): during the first infection windows, sterile sucrose solution is used; while for the second infection windows,Ecc15culture were used. (3) ET1 (Ecc15training group): during both the first and second infection windows,Ecc15culture were used.

Fig. 1 Schematic diagrams of repeated oral infections of Gram-negative bacteria to Drosophila
We observed a ~10% mortality rate within five d following the firstEcc15infection period, consistent with the fact thatEcc15is a well established pathogen forDrosophila(Figure 2a). Following the second infection period, we observed a similar mortality rate caused byEcc15with the naive ETC1 group, while only ~5% of trained flies (ET1 group) die out of the repeated infection over 5 d (Figure 2b). This apparent two fold decrease of bacteria lethality is consistent with a protective role the prior infection had established. To directly validate that the trained adults can resistEcc15infection with higher efficacy, we quantified the bacteria load in both the living flies in both ETC1 and ET1 group. There is a significant reduction ofEcc15load in previously trained flies,indicating a stronger bacteria clearing efficacy (Figure 2c). On the other hand, when we quantify the bacteria load upon death(BLUD), the trained group demonstrated a significantly higher load (Figure 2d),consistent with the notion that the prior training renders the recipients more tolerant to bacteria infection.
We extended our analysis with a second Gramnegative bacteria infection model withPAO1, by setting up three similar groups (Figure 1): (1) CG(control group); (2) PTC1 (PAO1training control group); (3) PT1 (PAO1training group). Oral infection withPAO1causes higher mortality rate in adult flies comparing withEcc15, such that roughly 40% of primary infected flies die within 5 d after infection.Nevertheless, there is a clear protective effect by the previous infection such that only ~25% flies die within a 5 d post infection period in the trained group(Figure 3a, b). Similar toEcc15infection, there is a stronger trend of clearing bacteria infection in the gut since PT1 group has a significant lower bacteria load comparing with naive PTC1 group (Figure 3c). BLUD counts also indicate that trained animals are tolerant to higher level ofPAO1infection (Figure 3d).

Fig. 2 Oral infection by Ecc15 elicit robust trained immunity in Drosophila adults
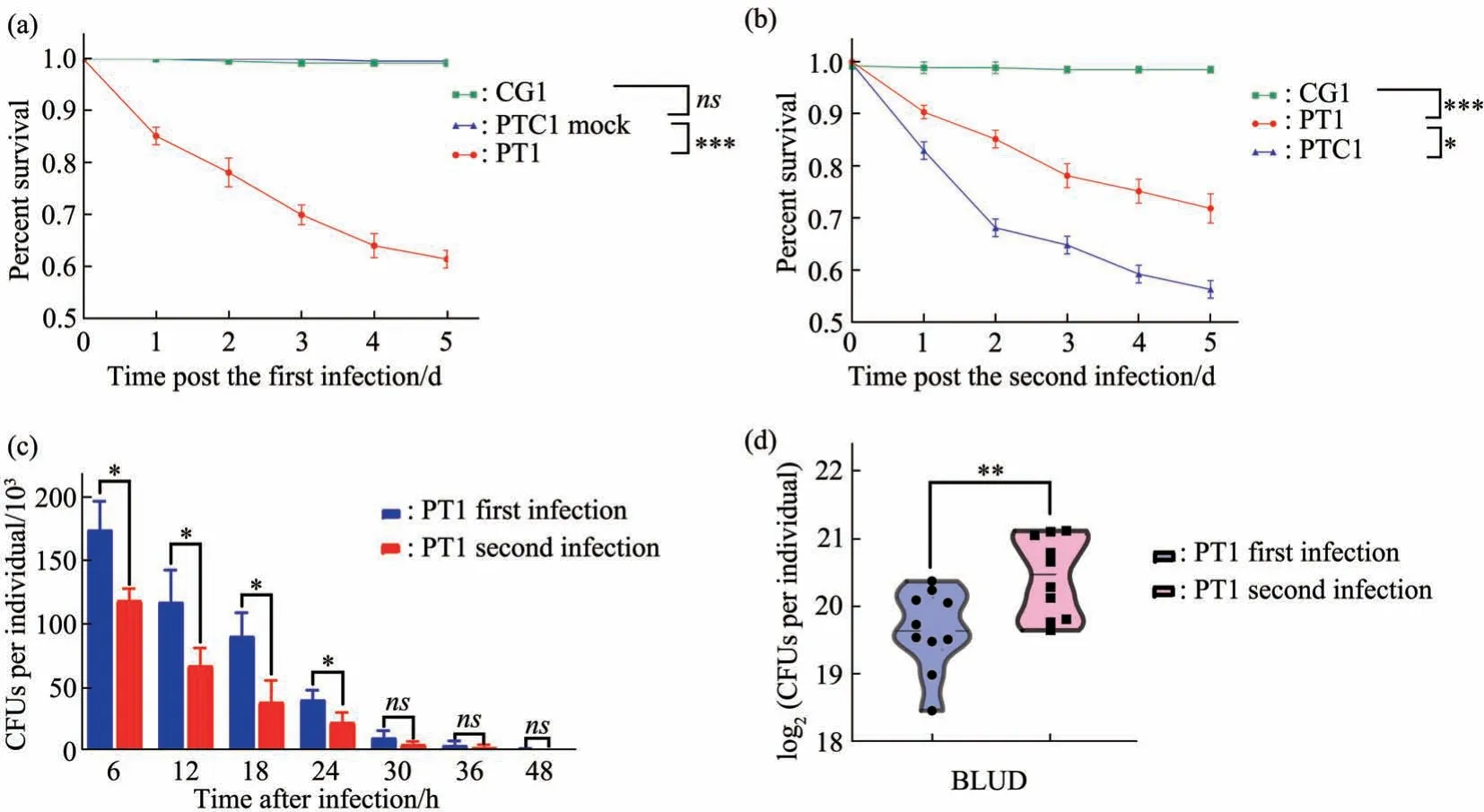
Fig. 3 Oral infection by PAO1 elicit robust trained immunity in Drosophila adults
2.2 Trained immunity obtained from oral infection by Gram-negative bacteria is non-specific and can protect infection by unrelated pathogen
The robust boost of infection resistance caused by eitherEcc15orPAO1inspires us to wonder whether the gained resistance is specific to any particular pathogen. In another word, whether flies with trained immunity can “remember” the specific bacteria they first encounter. To answer this question,we setup a cross infection treatment group (EPT1) by first infecting the flies withEcc15and then challenge those survived withPAO1infection (Figure 1). The results are compared with untrained (PTC1) and those trained (PT1) with the same pathogen. The results clearly demonstrated priorEcc15infection clearly protect flies againstPAO1challenge, since heterogeneous training group (EPT1) is similar to homogeneous training group (PT1) in every regards,including decreased mortality, lower bacteria load in live animals, and higher BLUD counts. (Figure 4d-f).
We also repeat the heterogeneous training scheme by switch the order of different pathogens.PET1 group is first infected withPAO1and then withEcc15, compared with untrained (ETC1) and those trained (ET1) with the same pathogen (Figure 1). We observed equivalent protective effects, comparing heterogeneous training group (PET1) with homogeneous training group (ET1) (Figure 4a-c).Therefore, we concluded that the trained immunity gained byDrosophilaoral infection is not pathogen specific, at least for the two well characterized Gramnegative bacteria we used.

Fig. 4 Trained immunity in Drosophila adults can be triggered by heterologous bacteria
2.3 Immunological memory gained by trained immunity can persist through different developmental stages
For this purpose, we setupEcc15infections across different developmental stages. ET2 group is infected withEcc15at early 3rd instar larval stage and reinfected as adult (Figure 1). ETC2 group is the training control group which is only fed with sucrose at early 3rd instar larval stage. Compared with uninfected control group (CG2), around 10% ET2 group die within 5 d after infection withEcc15at adulthood. Although this mortality rate is higher than those when the primary infection happens in adulthood (ET1), the protection from larval infection is significant, comparing with ETC2 control group in term of lower mortality, decreased bacteria load after infection, as well as higher BLUD counts (Figure 5 a-c).
We repeated this cross developmental infection scheme by usingPAO1as the infection agent, and also observed a significant protective effect againstPAO1infection if the initial infection is induced at the larval stage (Figure 5d-f). Therefore, immunological memory established in the larval stage can be long lasting, at least for weeks and persist over the major metamorphosis transition in fly development. We notice that the mortality rate decrease by those cross developmental stage memory (Figure 5a, d) are not as striking as when both infections occur in adulthood(Figure 2a, 3a). This discrepancy might suggest the integrity of gut might be a contributing factor in maintaining a “resistant” state for subsequent infections. Nevertheless, this cross developmental stage retention of immunological memory suggest intestinal stem cells might be a critical memory carrier, since it's the only major cell type retaining through metamorphosis in fly gut. Such long term memory might be encoded/decoded in the genome of ISC through epigenetic mechanisms, warranting further in-depth investigations.

Fig. 5 Trained immunity in Drosophila can persist across metamorphic developmental stages
2.4 Immunological memory is accompanied by boosted widespread immunity related transcriptional responses
Following the protective role a prior infection confers toDrosophilain term of combating Gramnegative bacteria infection, we reason that such resistance might be due to more robust induction of immune response genes inDrosophilagut. Therefore,we examined selected immune related genes from three distinct functional categories to assess whether during a second challenge, stronger immune response can be elicited in term of gene expression.
Firstly, we profile six genes from Peptidoglycan recognition proteins (PGRPs) family, which are the major innate immune recognition molecules inDrosophilafrom bacteria and fungal cell wall components[35]. InDrosophila, distinct PGRPs bind to peptidoglycans on Gram-positive or Gram-negative bacteria and provide essential signals upstream of the Toll and IMD pathways, while a number of PGRP genes were direct targets of Toll or IMD signaling,functioning to titrate Pathogen-associated molecular patterns molecules to prevent exaggerated activation of innate immunity response[36-39]. We used both theEcc15andPAO1infection model and compared the level of induction between PGRP genes within first 12 h after pathogen infection between naive and trained animals. PGRP-LB, PGRP-SD, PGRP-LE,and PGRP-LC all are induced by eitherEcc15orPAO1infection within the first 12 h, although with different extent regarding distinct pathogens (Figure 6a, b). PGRP-LB and PGRP-SD are more robustly induced, which parallels with a significantly stronger induction among the trained animals. A similar trend of increased induction level in trained flies can be observed in PGRP-LE and PGRP-LC as well,although the lower overall induction fold might contribute to the diminished statistic significance comparing the naive animal and trained adults.
Beyond PGRP sensors, AMPs are the major arsenal produced by the insect innate immunity to restrict pathogens proliferation in its gut. Many AMPs are induced by IMD pathways and therefore are reliable readouts for insect innate immunity response[40]. Dipt, AttA, AttD, CelA1 expression levels peak at 6 h afterEcc15infection, indicating they are early response genes (Figure 6c). For those,we observed a significantly more robust induction for Dipt, AttA and AttD in the trained animals compared with naive animals, while the increase for CelA1 is not significant. Mtk and Def peak at 12 h afterEcc15infection, suggesting they are late responders, for which the trained animals demonstrated significantly higher induction level (Figure 6c). ForPAO1infection, Dipt, AttA, AttD, CelA1 similarly are early responders, although CelA1 is induced more robustly comparing withEcc15. For all 4 early responding genes, trained flies demonstrated significantly higher expression level comparing with naive individuals(Figure 6d). For late responding AMPs, Mtk and Def,Mtk is more robustly induced byPAO1comparing withEcc15. Comparing with trained flies with naive ones, trained flies demonstrated significantly higher induction level besides the 12 h time point for Def(Figure 6d).
Finally, we profiled three members as representatives of the Tot gene Family[41]. All Tot genes are induced under stressful conditions such as bacterial infection, and play a role inDrosophilastress tolerance. Tot A, Tot C, Tot M expression level are strongly induced at 6 h after eitherEcc15orPAO1infection (Figure 6e), consistent with their known immediate induction by inflammation related JNKSTAT signaling[42]. We observe a stronger induction of Tot A, Tot C, Tot M afterPAO1infection compared with those afterEcc15infection, consistent with a higher mortality rate associated withPAO1oral infection (Figure 6f), thus possibly stronger inflammatory response in the gut. Nevertheless, a consistent more robust induction level of all Tot genes examined were found in the trained animals,compared with their naive counterparts. All of our gene expression profiling indicate the trained immunity related stronger resistance to repeated bacteria infection can be attributed to stronger immunity related transcription responses, including at least IMD signaling, AMPs production, as well as JNK/STAT triggered stress tolerance.
2.5 Immunological memory in the gut can be contributed by both enterocytes and intestinal stem cells

Fig. 6 Immunological memory is accompanied by boosted widespread immunity related transcriptional responses
Our gene expression analysis demonstrate more robust collective immune related transcriptional inductions, although our analysis could not distinguish the specific cell types where those responses are triggered. Therefore, we take advantage of the live-cell fluorescent Dipt-GFP sensor offering by our fly model, to explore whether stronger immune response can be observed in the fly gut comparing trained animals and naive animals. We compared Dipt-GFP fluorescence before and after infection along the whole digestive track. As expected, there is no Dipt-GFP positive cell in germ-free gut, while there are patches of green cells appearing in the gut after 6 h of pathogen infection (Figure S2, S3). While this is consistent with the known fact that Dipt-GFP signal reflects IMD signaling triggered AMPs production,the patchiness of the green cells reflect cells along distinctive anatomical location in the gut might have distinctive immune responses. We consistently observed a distinctive patch of Dipt-GFP positive cells in the midgut afterEcc15infection (Figure S2).Therefore, we focus on this region for comparison of immune responses after the second pathogen challenge, comparing the trained animals with naive ones. Most Dipt-GFP positive cells have large nuclei as revealed by strong Dapi staining, suggesting those are polyploid mature enterocytes (Figure S2, S3).When we count the number of Dipt-GFP positive cells in this patch, we consistently observed a higher cell count comparing theEcc15trained animals with naive ones, on two different time points post infection(Figure 7a). Similarly, this Dipt-GFP positive patch of midgut cells shows up afterPAO1infection. The higher count of Dipt-GFP cells comparing withEcc15infection is consistent with higher morbidityPAO1causes. Nevertheless, the same significant increase of GFP positive cell counts are observed comparingPAO1trained animal with naive ones (Figure 7b). We also repeat the experiment by training the flies withPAO1infection first and examine the Dipt-GFP positive patch on the midgut afterEcc15challenge.Again, we observed more Dipt-GFP enterocytes compared with naive flies (data not shown). Since most of the Dipt-GFP positive enterocytes are unlikely to be freshly differentiated from ISC within the 6-12 h post infection time window we examined,our results suggested enterocytes lining the gut epithelium can be more immunological active after training with prior infection.

Fig. 7 Immunological memory in the gut can be contributed by both enterocytes and intestinal stem cells
Our observation that immunological memory can persist through different developmental stages also strongly indicate the potential role of ISC in conveying long term memory. Although there is no evidence that ISC participate in IMD signaling related innate immunity response[43-45], there are numerous reports on elevated mitogenic activities of ISC upon inflammation to maintain the gut homeostasis[46-48].This is triggered by JNK/STAT pathway ligands released by stressed enterocytes[49]. We used delta immunostaining as a reliable means to quantitate the number of ISCs in the midgut. Delta staining reveals patch signal on few cells in the gut. Their small DAPI positive nuclei is consistent with the diplod genome ISCs maintain, comparing with large nuclei the neighbouring differentiated enterocytes process(Figure S4). We first measured delta positive ISC in the midgut withEcc15infection. We clearly observed a more than doubling effect of ISC counts 12 h followingEcc15infection (Figure 7c), consistent with previously known inflammation induced ISC cell division. This increase of ISC count is less pronounced 24 h postEcc15infection compared with uninfected control, suggesting an immediate boost of ISC activity early in the infection cycle. When we count ISC number on flies previously trained withEcc15infection, we do observe an elevated ISC counts on both time points, suggesting trained ISC are more mitogenic active after pathogen challenge(Figure 7c). We observed a similar immediate boost of ISC activity withPAO1infection, although the number of ISC increase by more than 4 fold at 12 h post infection, consistent with its higher inflammatory activity compared withEcc15(Figure 7d).Importantly, pre-training withPAO1also boost the ISC cell counts compared with naive animals (data not shown). Therefore, elevated ISC proinflammatory response is a general feature for trained immunity with distinct Gram-negative pathogens. Our anatomic analysis of cellular responses in fly gut identify both enterocytes and ISC to be more active upon repeated inflammatory challenge. We speculate they might be differential tuned by trained immunity to execute distinctive aspects in resisting the infection of future pathogens.
3 Discussion
We used the fast developing model organismDrosophilato establish a robust trained immunity model. Our model is advantageous for two main reasons: firstly,Drosophilalacks the adaptive immune system, therefore, immunological memory can be 100% attributed to the innate immunity system. The availability of a large number of genetic homogeneous individuals for a large number of repeated experiments as we demonstrated in our study improves the reliability of the findings[50]. In addition,the significantly gained resistance toward same or different pathogen infection through the oral route as we demonstrated with two different Gram-negative bacteria pathogens offers a robust platform to evaluate the efficacy of immunological memory. Our current study used an easy-to-establish germ-free fly culture system to exclude any influence of prior exposure to other bacteria. This ideal case clearly demonstrated a primary infection event “vaccinate” the flies nonspecifically, possibly by fine-tuning the gut to be more robustly responsive in limiting a repeated bacteria infection, as well as providing more stress resistance mechanisms. The latter effect can be seen as the trained flies demonstrated higher BLUD counts in general, and more robust induction of stress resistant Tot genes. The more efficient pathogen clearing effect in trained animals is reflected by robust gains of induction for peptidoglycan recognition proteins, as well as antimicrobial peptides. Where there might be additional mechanisms for the increase of infection resistance that our current study fail to pinpoint, our limited transcriptional profiling suggests immunological memory might not be limited to only a few gene locus. Instead, our data is more consistent with a genome wide chromatin modification model that many sites were “poised” to be more active, a state of chromatin changes established by the prior infection. Although our study did not go in such detail to establish the underlying chromatin changes for a more robust induction of immune related genes we discovered, recent epigenetic profiling comparing naive T cells and antigen-stimutated T cells found T cell activation enables thousands of DNaseIhypersensitive sites in the T cell genome, which are inducible chromatin priming sites associated with the establishment of immunological memory in T cells[51].Further cell specific, genome wide epigenetic profiling is needed to validate our hypothesis that the“immunological memory” in both innate immunity and adaptive immunity can be recorded and retrieved at the level of site specific chromatin modifications in different organisms.
The simple anatomical structure of fly gut also offers us opportunity to reveal which cell types can be“trained” with prior infection. Our Dipt-GFP reporter is mainly activated in differentiated enterocytes.Therefore, using this reporter, we did observed enterocytes in trained fly gut are activated to a higher extend. This is consistent with the notion that enterocyte is the major cell type for the gut barrier immunity function and it's the major site for bacteria triggered IMD-relish innate immunity response.However, enterocytes are known to have limited lifespan and can not self regenerate under normal circumstances[44]. Furthermore, enterocytes are known to undergo massive inflammation induced cell death and shedding in fly gut[21]. Therefore, although the enterocytes might contribute significantly to the adult stage 5-d apart immune training scheme we used in our current study, it is unlikely to persist long term due to continuous cellular turnover. On the other hand, ISC would be a cell type conferring long term memory due to its unique self renewal and differentiation potential in the gut[45]. Indeed, through a cross developmental stage infection training scheme, we found significant trained immunity in fly gut can be maintained through tissue modeling metamorphosis process. Although parallel comparison of the protective effects though prior infection in either larval or adult stage is hard to achieve, we did observe a weaker “protection” cross developmental stages regarding mortality rate in general. This decline could be explained by either the lack of enterocytes component of the memory, a temporal decline of“memory” recorded due to a longer lag period between the two bouts of infections, or both. When we examined the anatomy of fly guts under infection,ISCs demonstrate increased inflammation induced proliferation in the trained gut. Therefore, this observation is consistent with ISCs themselves are differentiable responsive to inflammation cues dependent on their prior infection history, which is consistent with a recent discovery[45]. Whether ISCs and enterocytes record infection history differentially in their genome remains a long term question to be addressed further. When stem cell confers long term immune memory through differentiating into mature enterocytes, it remains to be seen whether those newly generated enterocytes function as stronger immune barrier by retaining some aspects of immune memory encoded into the infected ISCs.
The similar phenomenon of trained immunity has been demonstrated in other invertebrates (Anopheles gambiae[52],Aedes aegypti[26],Bombus terrestris[53],etc.) and in vertebrate models (mouse[54], rhesus macaques[55],etc.). In sum, the proposal of trained immunity provides a new direction for the study of immunological mechanisms, and can be used in the future to develop novel broad-spectrum vaccines based on innate immune memory[56]and to open up new therapeutic strategies to modulate trained immunity. Mechanistic studies using a well suited model as our current study demonstrates will be tremendously helpful in this endeavor by elucidating how the memory is “recorded”, “stored” and“retrieved” on a single cell basis.
4 Conclusion
A robust trained immunity inDrosophila melanogasterintestine can be triggered by oral infection of either homologous or heterologous Gramnegative bacteria, and the immunological memory can persist across developmental stages. It may act on chromatin and store immunologic memory at relevant gene loci through chromatin modifications. A potential way for the passage of immunologic memory across developmental stages is through JNK/STAT activation of intestinal stem cells, which may carry on the immune imprint from larval to adult developmental stages in the gut.
AcknowledgmentsGraphical abstract is created by BioRender.com with the help of XU Hong-En from Zhengzhou university. We are grateful for the assistance and support provided by the Henan Key Laboratory for Pharmacology of Liver Diseases and the Henan Engineering Technology Research Center for Accurate Diagnosis Neuroimmunity for the smooth implementation of this study.
Supplementary materials Available online (http://www.pibb.ac.cn or http://www.cnki.net):
PIBB_20230134_Figure_S1.pdf
PIBB_20230134_Figure_S2.pdf
PIBB_20230134_Figure_S3.pdf
PIBB_20230134_Figure_S4.pdf
PIBB_20230134_Table_S1.pdf
