Aqueous-soluble components of sporoderm-removed Ganoderma lucidum spore powder promote ferroptosis in oral squamous cell carcinoma
2023-05-14XiangpingWuQingnanWuYanWangYehaiLiuZhenhaoLiQingchuanLiuZhengmingHuangMingyanLiBinZhangQiminZhan
Xiangping Wu ,Qingnan Wu ,Yan Wang ,Yehai Liu ,Zhenhao Li ,Qingchuan Liu ,Zhengming Huang,Mingyan Li,Bin Zhang,5,Qimin Zhan
1Department of Otolaryngology-Head &Neck Surgery,The First Affiliated Hospital of Anhui Medical University,Hefei 230022,China;2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Laboratory of Molecular Oncology,Peking University Cancer Hospital &Institute,Beijing 100142,China;3Zhejiang Shouxiangu Pharmaceutical Co.Ltd,Jinhua 321200,China;4Beijing Weijian Jiye Institute of Biotechnology,Beijing 102488,China;5Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Head and Neck Surgery,Peking University Cancer Hospital &Institute,Beijing 100142,China
Abstract Objective:Ferroptosis is a novel cell death process which displays a promising role in cancer treatment.However,clinically available drugs targeting ferroptosis are rarely used,and yet there are no studies reporting on inducing ferroptosis via Chinese herbal extracts.Here we explored the tumor inhibition effects of Ganoderma lucidum (G.lucidum) on oral squamous cell carcinoma (OSCC).Specifically,we aimed to clarify the biological mechanism of components in the dietary,aqueous-soluble sporoderm-removed G.lucidum spore powder (A-GSP).Methods:Preliminary transcriptome analysis revealed the significant enrichment of the ferroptosis pathway.Cellular Fe2+,glutathione (GSH),malondialdehyde (MDA),reactive oxygen species (ROS) and lipid peroxide levels were measured to identify ferroptosis occurrence.Western blotting was used to measure ferroptosis-related proteins.Changes in mitochondria morphology and function were observed with transmission electron microscopy(TEM) and ATP detection assays.Ferroptosis inhibitor ferrostatin-1 was then used to verify the anti-tumor effects of A-GSP.Finally,nude mice xenograft models of oral cancer confirmed that A-GSP inhibited tumor growth.Results:A-GSP promoted ferroptosis in oral cancer cells by inducing Fe2+ influx,GSH depletion,as well as lipid peroxide and ROS accumulation.Ferroptosis-related proteins exhibited corresponding changes,particularly AcylcoA synthetase long chain family member 4 (ACSL4) increase and glutathione peroxidase 4 (GPX4) decrease.AGSP considerably lowered mitochondrial volume and ridge number,while significantly decreasing ATP production.Ferrostatin-1 reversed all of these A-GSP-induced changes.In vivo,A-GSP exerted a ferroptosismediated tumor-suppressing effect without observable adverse reactions.Conclusions:Our findings demonstrate the therapeutic potential of A-GSP for treating patients with OSCC by targeting ferroptosis.
Keywords: Ganoderma lucidum spore powder;OSCC;ferroptosis;ACSL4
Introduction
Head and neck squamous cell carcinoma (HNSCC)originates from epidermis of oral,tongue,nasal cavity,nasopharynx and throat.The cancer’s incidence is increasing globally (1-3),and approximately 40% of HNSCC are oral squamous cell carcinoma (OSCC),with most patients are in locally advanced or metastatic stage when diagnosed.Although palliative chemotherapy is the main treatment for advanced OSCC currently (4),the majority of patients are either insensitive to chemotherapeutics or suffer a series of side effects during treatment,leading to rapid disease progression and death.Thus,novel and efficient therapeutic strategies with minimal negative side effects are essential for fulfilling an urgent clinical need (5).
In addition to the more well-known forms of programmed cell death (e.g.,apoptosis,autophagy and pyroptosis) (6),ferroptosis is a unique form of cell death characterized by iron homeostasis disruption,glutathione peroxidase 4 (GPX4) inactivation,and lipid peroxide augmentation (7).Ferroptopsis has been implicated in cardiovascular,neurodegenerative,and lung diseases,as well as multiple cancers (8).Specifically,divalent iron ions can initiate liposome peroxidation through the Fenton reaction when the import and reduction of intracellular iron ions are out of balance (9).Acyl-coA synthetase long chain family member 4 (ACSL4) is involved in lipid peroxidation induction and acts as an important inducer for ferroptosis progression (10).Balancing the cystine/glutamate transporter system (known as system Xc-) is an important prerequisite in maintaining cellular redox homeostasis,whereas blocking the SLC7A11-glutathione(GSH) axis inactivates GPX4,inducing massive accumulation of reactive oxygen species (ROS) and triggering ferroptosis (10).Emerging evidences support the therapeutic potential of targeting ferroptosis (11-13),suggesting that the discovery of ferroptosis-targeted antitumor drugs may provide a new approach and strategy for the treatment of OSCC.
As the medicinal value of natural products are gradually approved (14,15),Ganoderma lucidum(G.lucidum) is listed as a first-class precious medicinal material in various collections of traditional Chinese medicine.In particular,G.lucidumspores contain various active ingredients such as proteins,polysaccharides,triterpenes,nucleosides and multiple trace elements (16).Nevertheless,direct consumption without any processing will hinder the absorption and utilization of internal nutrients,as the outer wall ofG.lucidumspores is composed of inactive chitin.Thus,the internal active ingredients of the spores,possessing excellent medicinal applications and safety in enhancing immunity and disease treatment,are exposed after removing the shell (17).Notably,different extraction methods may result in different anticancer mechanisms.For instance,the alcohol extract fromG.luciduminterrupts the cell cycle of breast cancer cells (18).However,the extraction time was long and organic solvent residue might be generated,which would result in unexpected cytotoxicity.Although high-pressure supercritical CO2extraction shorten the time and CO2extract could effectively inhibit the excessive activation of the Ras/Raf/MEK/ERK pathway in liver cancer cells,the necessary equipment and the cost should be taken into consideration (19).Among those extraction methods,the aqueous-soluble extraction exhibited uniquely for its convenience and good operability.And the aqueous-soluble sporoderm-removedG.lucidumspore powder (A-GSP),of which the polysaccharides and triterpenes are the main active components (20),has outstanding anti-tumor properties.A plenty of studies identified that A-GSP possesses anti-tumor effects against ovarian,lung,colorectal and urothelial carcinomas (21-24).Nevertheless,the anticancer mechanism of A-GSP remains largely unclear and requires further studying.Here,for the first time,we report that A-GSP suppresses oral tumor growth via promoting ferroptosis.Ourin vitroandin vivoexperimental evidences indicated the satisfactory anticancer efficacy with no detectable side effect of A-GSP,and highlighted the outstanding value of A-GSP as a potential anti-tumor agent in OSCC.
Materials and methods
Cell lines
Human OSCC cell lines (SCC15 and SCC25) were purchased from the Peking Union Cell Bank (Beijing,China).All cells were cultured in DMEM medium(Hyclone,USA).The incubator environment was fixed at 37 °C,95% humidity and 5% CO2.
Reagents
Sporoderm-removedG.lucidumspore powder (GSP),containing 1.81% polysaccharide and 1.31% triterpene acid(25),was provided by Zhejiang Shouxiangu Pharmaceutical Company (Jinhua,China).GSP (1 g) was dissolved in 25 mL DMEM medium to form a stock solution.Then,GSP was treated at 135 W,20 kHz sonication for 90 min to complete dissolution and diluted into working solutions of different concentrations according to the purpose of the experiment.The storage condition of the stock solution was -20 °C.Ferrostatin-1 (#HY-100579) and Liproxstatin-1 (#HY-12726) were purchased from MedChemExpress(USA).
RNA sequencing
A control group and A-GSP concentrations of 500 μg/mL,1,000 μ g/mL were set up.After exposure to A-GSP for 72 h,the SCC15 cells were collected (n=3 for each group).A library was constructed and sequenced by Novogene Biological Company (Beijing,China),and differentially expressed genes (DEGs) were analyzed (Padj<0.05,|Log2FoldChange|≥1).
Western blotting
Cells were digested,centrifuged,and radio immunoprecipitation assay (RIPA) lysis buffer was added.During this time,the sample was kept on ice and vortexed every 10 min for three times.The whole cell extracts were collected by centrifugation at 10,400 r/min at 4 °C for 15 min.The protein concentration was adjusted to a uniform level using the Micro BCA Protein Assay Kit (#23235,Thermo Fisher Scientific™,Waltham,USA).Protein (30 μ g) was separated by 10% sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) at 100 V and transferred to polyvinylidene difluoride (PVDF) membrane at 350 mA.Then,the membrane was subjected to 5% non-fat milk blocking for 2 h at room temperature.Primary antibodies were incubated for 10 h.The strips were placed in a 4 °C freezer and kept spinning slowly.The secondary antibody incubation time was 40 min at room temperature.After three-time washes in PBST,the chemiluminescence signals were detected.The information of primary antibodies is listed inSupplementary Table S1.
Intracellular iron detection
First,cells (2×107) were collected from each group and resuspended in 100 μL iron assay buffer.Cells were fully lysed using a sonicator.After high-speed (9,500 r/min)centrifugation,supernatants from all samples were retained and transferred to a 96-well plate and placed in a 37 °C incubator.Next,100 μ L iron probe (#ab83366,Abcam,Cambridge,UK) was added to each group.Incubation time was set to approximately 1 h at 37 °C.Optical density (OD)values were measured at 593 nm using infinite M200PRO(TECAN,Switzerland).
GSH detection
Cells (1×107) were collected from each group,centrifuged,and the supernatant was discarded.Protein removal reagent M solution was added at a volume thrice that of the cell pellet.Cells were lysed using quick freezing and thawed four times as follows.Briefly,the cells were placed in liquid nitrogen for 10 s and then warm water for 10 s.Cells were then immediately centrifuged at high speed (9,500 r/min)for 9 min.A GSH detection kit was used following the manufacturer’s instructions (#S0053,Beyotime,Shanghai,China),and the OD value was measured at 405 nm using infinite M200PRO (TECAN,Switzerland).
MDA detection
Cells (1×107) were collected from each group.RIPA lysis buffer (100 μ L) was added to each tube.Intracellular protein was extracted and the concentrations were determined by BCA Protein Assay Kit.An appropriate volume of MDA working solution (#S0131S,Beyotime,Shanghai,China) was chosen based on the number of cells.Absorbance values between samples were determined by a microplate reader.
Liperfluo experiment
After collecting the cells,the supernatant medium of the cells was discarded.A working solution of 20 μmol/L(#L248,Dojindo,Japan) was prepared in a serum-free medium.The solution was placed in a cell incubator for 30 min and protected from light.Methanol was added and stood for 8 min.Then,DAPI staining was performed on the nuclei and unbound DAPI was washed.Finally,80 μL of mounting medium was added.
ROS detection
Following the ROS detection kit (#S0033S,Beyotime,Shanghai,China) instructions,DCFH-DA was diluted with serum-free medium at 1:2,000 and added to the cells.Cells were placed in the incubator for 40 min and then washed with PBS.Fluorescence intensity was detected using FACS(BD Bioscience,USA) software.
Mitochondrial tracker assay
After the drug action time was reached,according to the instructions of the MitoTracker Green kit (#C1048,Beyotime,Shanghai,China),a working solution (1:10,000)was prepared with DMEM medium.The samples were placed at 37 °C,protecting from light.After 35 min,the residual reagents were washed.An appropriate amount of methanol was added,and the samples were left to stand for 10 min.DAPI (#C1002,Beyotime,Shanghai,China) was used to locate the nuclei.The fluorescence intensity of mitochondria was compared under a confocal microscope after adding mounting medium.
Cellular ATP determination
An ATP detection kit was purchased from Beyotime(#S0026,China).Cells (1×107) were taken from each group and fully lysed with RIPA first.Next,the cells were centrifuged,and the supernatant was aspirated and placed on ice.A detection working solution (100 μL) was prepared and poured into each well.The each well was rested for 5 min.The working fluid was prepared according to the instruction.Finally,20 μL of each test sample was added,and the OD values of these samples were determined.
Animal experiments
In vivotumorigenesis experiments were conducted in mice after being approved by the Animal Care Committee of Peking University Cancer Hospital.BALB/C female nude mice,4 weeks old,were used in the experiments.The weight of each mouse was in the range of 15-17 g.The mice were randomly divided into the following treatment groups: a control group (PBS),a low-concentration administration group (500 mg/kg),and a highconcentration group (1,000 mg/kg).Half of each group was inoculated with either SCC15 (5×106) or SCC25 (2×106)cells subcutaneously in the left forelimb scapula.Three days after inoculation,a 100 μ L intragastric daily administration of the A-GSP drug was started.Data of the body weight and tumor size of mice were collected every three days.The following equation was used:wherearepresents the longest distance andbrepresents the shortest distance.As soon as differences appeared,the mice were immediately terminated.Tumors were separated from the surrounding muscle and dermis.Then,tumors were weighed,and mouse serum was collected.All dissected tumor tissues were fixed,paraffin-embedded and sectioned.The tissues were fixed at 4% phosphate-buffered paraformaldehyde.
Immunohistochemistry (IHC)
The tissue sections were placed in a 65 °C oven for 90 min and dewaxed,after incubating with 3% H2O2for 15 min.The diluted EDTA solution (#ZLI-9071,Zhongshan Jinqiao,China) (1:49) was heated,boiled,and subjected to high-pressure repair for 15 min.Subsequently,the cooling time of the antigen retrieval solution was set to 45 min.The section was blocked via Goat serum (#SAP-9100,Zhongshan Jinqiao,China) for 45 min.The primary antibody was incubated for 8-10 h at 4 °C.Any unbound primary antibody was washed away with deionized water.The secondary antibody was dripped onto the tissue.After 45 min,any unbound secondary antibody was removed with deionized water as well.Next,tissues were dripped with DAB developer solution (#ZLI-9019,Zhongshan Jinqiao,China) and turned brown.Nuclei were stained with hematoxylin for 5 min,hydrochloric acid-glycolated for 10 s,and blued with ammonia for 30 s.The sample was dehydrated and mounting medium was added (#ZLI-9516,Zhongshan Jinqiao,China).The information of primary antibody is listed inSupplementary Table S1.
Scoring of immunohistochemical results
The staining results of tissue sections were independently judged positive by two doctors in the pathology department with no knowledge of the background of the study and according to the following criteria.The score was dependent on the percentage area poise for color development as follows: a score of 0 (<5%),a score of 1(5%-25%),a score of 2 (26%-50%),a score of 3(51%-75%),and a score of 4 (>75%).According to the staining intensity (negative,weakly positive,moderately positive,or strongly positive),samples were designated 0,1,2,or 3 points.The scores for percentage of positive cells were multiplied by the scores for immunostaining intensity.Points of 0-3 and 4-12 represented negative expression and positive expression,respectively.If the two doctors disagreed,a third party was consulted to determine the final result.
Transmission electron microscopy assay
Cells were collected and treated with 2.5% glutaraldehyde fixed solution (#G1102,Servicebio,Wuhan,China) for 30 min at room temperature.Cell samples were pre-coated with 1% agar solution and then fixed in 1% osmic acid for 2 h.After fixation,cell samples were dehydrated through gradient ethanol (30%-50%-70%-90%-100%) and 100%acetone.Samples were embedded in epoxy resin,cut into thin slices,and placed onto copper grid.Images were acquired using JEM-1400 PLUS transmission electron microscope (JEOL,Japan).
Statistical analysis
All data were statistically analyzed using GraphPad Prism software (Version 8.0.2;GraphPad Software,Inc.,San Diego,CA,USA).The mean comparison between the two groups was made using an independent-sample two-tail unpaired Student’st-test.A one-way analysis of variance(ANOVA) test was performed for tumor volume comparisons.Statistical significance was set at P<0.05.
Results
A-GSP promotes ferroptosis via Fe2+ influx,GPX4 inactivation,and lipid peroxide augmentation in oral cancer cells
To clarify the underlying effect of A-GSP treatment on tumor cells,we performed RNA sequencing in SCC15 cells treated with A-GSP for 72 h.As shown inSupplementary Figure S1,there were 233 up-regulated genes and 81 downregulated genes in treated group compared to the control group (Supplementary Figure S1A).Gene ontology enrichment analysis showed that DEGs were markedly enriched in the ferroptosis pathway (Supplementary Figure S1B).Among them,theGCLM,HMOX1,GCLC,ACSL1andACSL4were up-regulated (Supplementary Figure S1C).These results suggest that ferroptosis is the main driving event for A-GSP-induced tumor suppression.
Disruption of iron homeostasis is the primary driver of ferroptosis (26).To demonstrate whether A-GSP induces iron imbalance,we measured the intracellular Fe2+content using an iron ion kit.Interestingly,intracellular Fe2+in the A-GSP group exhibited concentration-dependent accumulation (Figure 1A).Our results also showed that GSH level was extensively decreased in A-GSP group(Figure 1B).It is reported that severe depletion of GSH is one of the important features of ferroptosis (27),given that GSH improves the levels of biological antioxidants and removes free radicals and peroxides.Furthermore,the increased production of peroxides is a key event that triggers ferroptosis (27).
MDA is an important indicator of lipid peroxidation (28).The MDA detection assay suggested that A-GSP considerably increased the intracellular MDA content(Figure 1C).Consistently,the content of lipid peroxides in the treatment groups was considerably increased in a concentration-dependent manner determined by the Liperfluo experiments (Figure 1D,E).In addition,flow cytometry analyses demonstrated that A-GSP could promote ROS accumulation in oral cancer cells (Figure 1F,G),probably further exacerbating ferroptosis (29).Moreover,we observed that the expression levels of ferroptosis-related proteins,such as NRF2,GPX4,SLC7A11,and FTH1,were dramatically decreased after AGSP treatment in a concentration-dependent manner(Figure 1H).Meanwhile,the protein levels of ACSL4,COX2,NOX1 and NCOA4 were markedly increased as determined by western blotting analysis (Figure 1H).Taken together,these results indicated that ferroptosis was induced by A-GSP treatment in OSCC cells.
A-GSP treatment induces mitochondrial morphology and function change in OSCC cells
Mitochondria are the main organelles involved in ferroptosis because of their irreplaceable role in maintaining cellular energy conversion and redox (29).To determine the corresponding effects of A-GSP,mitochondria were labeled with MitoTracker Green after 72 h of A-GSP treatment.Notably,the green fluorescence intensity of mitochondria in the A-GSP-treated group was considerably weaker (Figure 2A,B).Next,transmission electron microscopy (TEM) was used to observe the changes in mitochondrial ultrastructure.Interestingly,in the SCC15 and SCC25 cells treated with A-GSP,the mitochondrial volume markedly reduced and the mitochondrial ridges decreased or even disappeared (Figure 2C).Moreover,the cellular ATP was decreased in A-GSPtreated cells,indicating severe impairment of mitochondrial function after A-GSP treatment (Figure 2D).These results demonstrated that A-GSP dramatically altered mitochondrial morphology and function in OSCC cells.
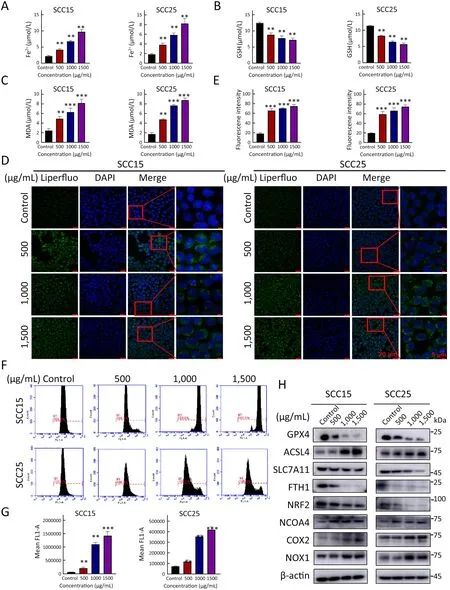
Figure 1 A-GSP induces Fe2+ accumulation,GPX4 inactivation and lipid peroxide increase in OSCC cells.(A-C) Changes in intracellular Fe2+ content (A),GSH content (B) and intracellular MDA content (C) after 72 h of A-GSP treatment in SCC15 and SCC25 cells;(D)Content of intracellular lipid peroxides in each group was observed under a confocal microscope (Scale bars are 20 μ m and 5 μm,respectively);(E) Statistical analysis of Liperfluo experimental results;(F) Flow software detected the ROS generation in each group of cells;(G) Statistical results of ROS fluorescence intensity;(H) Fold changes of proteins encoded by ferroptosis-related genes after 72 h of A-GSP treatment in OSCC cells.A-GSP,aqueous-soluble sporoderm-removed G.lucidum spore powder;OSCC,oral squamous cell carcinoma;GSH,glutathione;MDA,malondialdehyde;ROS,reactive oxygen species.**,P<0.01;***,P<0.001.
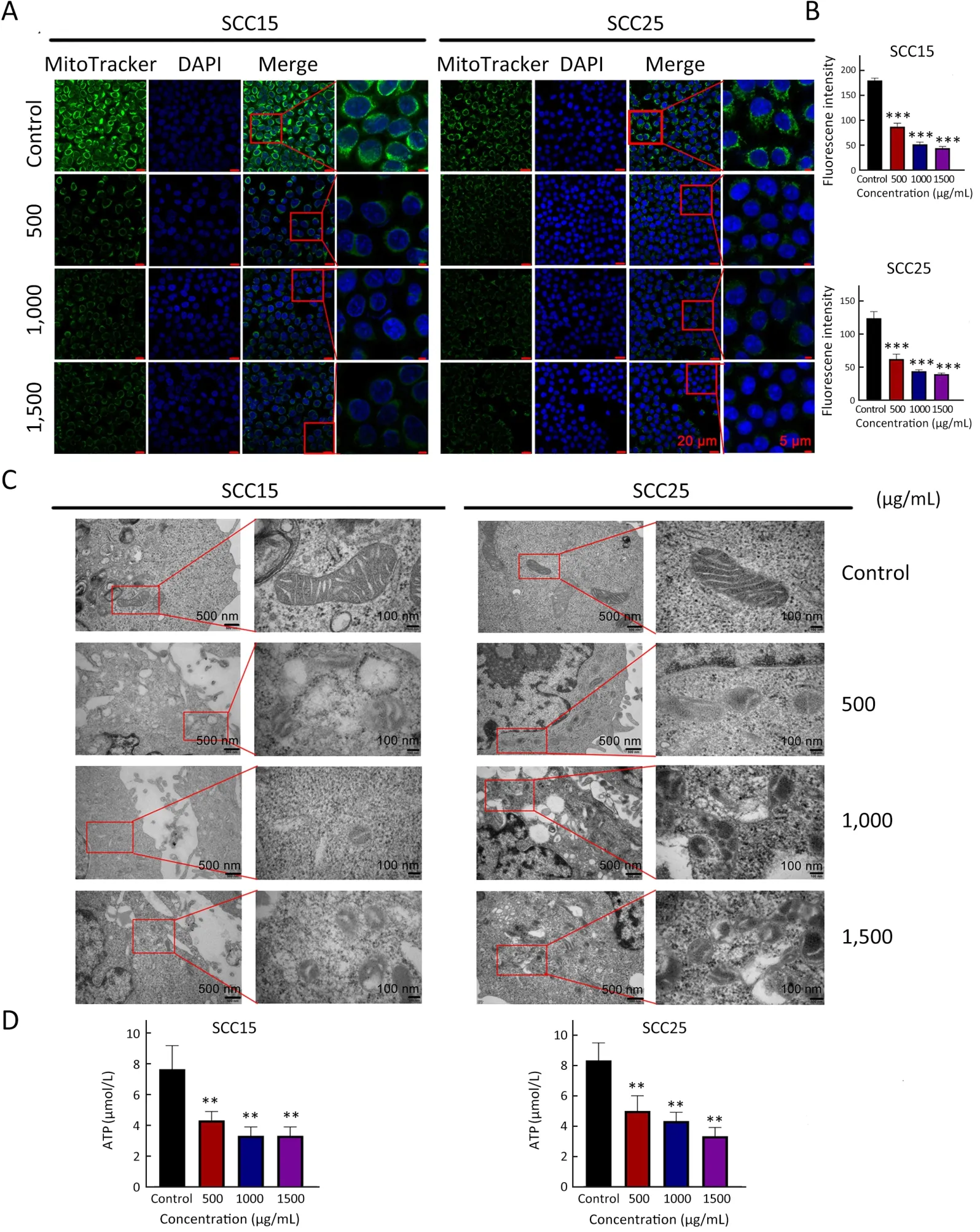
Figure 2 A-GSP alters mitochondrial morphology and function in OSCC cells.(A) Changes of MitoTracker Green-labeled mitochondria under different treatment conditions (Scale bars are 20 μm and 5 μm,respectively);(B) MitoTracker Green experiment,statistical results of mitochondrial fluorescence expression intensity;(C) TEM images of mitochondrial ultrastructural changes (Scale bars are 500 nm and 100 nm,respectively);(D) ATP content of SCC15 and SCC25 cells treated with A-GSP for 72 h.A-GSP,aqueous-soluble sporoderm-removed G.lucidum spore powder;OSCC,oral squamous cell carcinoma;TEM,transmission electron microscopy.**,P<0.01;***,P<0.001.
Ferroptosis inhibitors reverse A-GSP-induced ferroptosis
To further confirm that tumor suppression following AGSP treatment was induced by ferroptosis,the ferroptosis inhibitors ferrostatin-1 and liproxstatin-1 were introduced.As expected,the cell viability of A-GSP-treated group was substantially improved in presence with ferroptosis inhibitors (Figure 3A,B).Additionally,the colony formation ability of A-GSP-treated group was notably enhanced after treating by ferroptosis inhibitors (Figure 3C,D).As shown inFigure 3E,the mitochondrial green fluorescence signal in the ferroptosis inhibitor-treated cells was significantly enhanced compared with the A-GSP group (Figure 3E,F).Moreover,A-GSP-induced intracellular accumulation of Fe2+and lipid peroxide levels were also alleviated in ferrostatin-1-treated cells (Figure 4A-D).The A-GSPinduced decrease in intracellular GSH level was recovered to some extent in the ferroptosis inhibitor-treated group(Figure 4E).Furthermore,impaired mitochondrial function was slightly alleviated by ferroptosis inhibitors (Figure 4F).Correspondingly,ferrostatin-1 treatment markedly reversed A-GSP-induced increase in ROS levels (Figure 4G,H).The above data revealed that ferrostatin-1 could rescue the ferroptosis-induced damage in OSCC.Taken together,these data strongly supported the hypothesis that ferrostatin-1 can rescue ferroptosis-induced damage in OSCC.

Figure 3 A-GSP-induced reduction in OSCC cells viability can be rescued by ferroptosis inhibitors.(A) Cells were treated with a mixture of ferrostatin-1 and A-GSP at concentrations of 10 μ mol/L and 1,000 μ g/mL,respectively,for 72 h (All related experiments with the addition of ferrostatin-1 in this figure have the same treatment conditions).Rescue of cell viability was detected;(B) Cells were treated with a mixture of the ferroptosis inhibitor Liproxstatin-1 and A-GSP at concentrations of 15 μmol/L and 1,000 μg/mL,respectively,for 72 h.The recovery of cell viability was detected;(C) Ferrostatin-1 reversed the loss of self-cloning ability of oral cancer cells;(D) Statistical results of clone formation experiments;(E) Changes in MitoTracker Green-labeled mitochondria after adding ferrostatin-1 to the drug(Scale bars are 20 μm and 5 μm,respectively);(F) Statistical analysis results of MitoTracker Green experiments.A-GSP,aqueous-soluble sporoderm-removed G.lucidum spore powder;OSCC,oral squamous cell carcinoma.***,P<0.001.
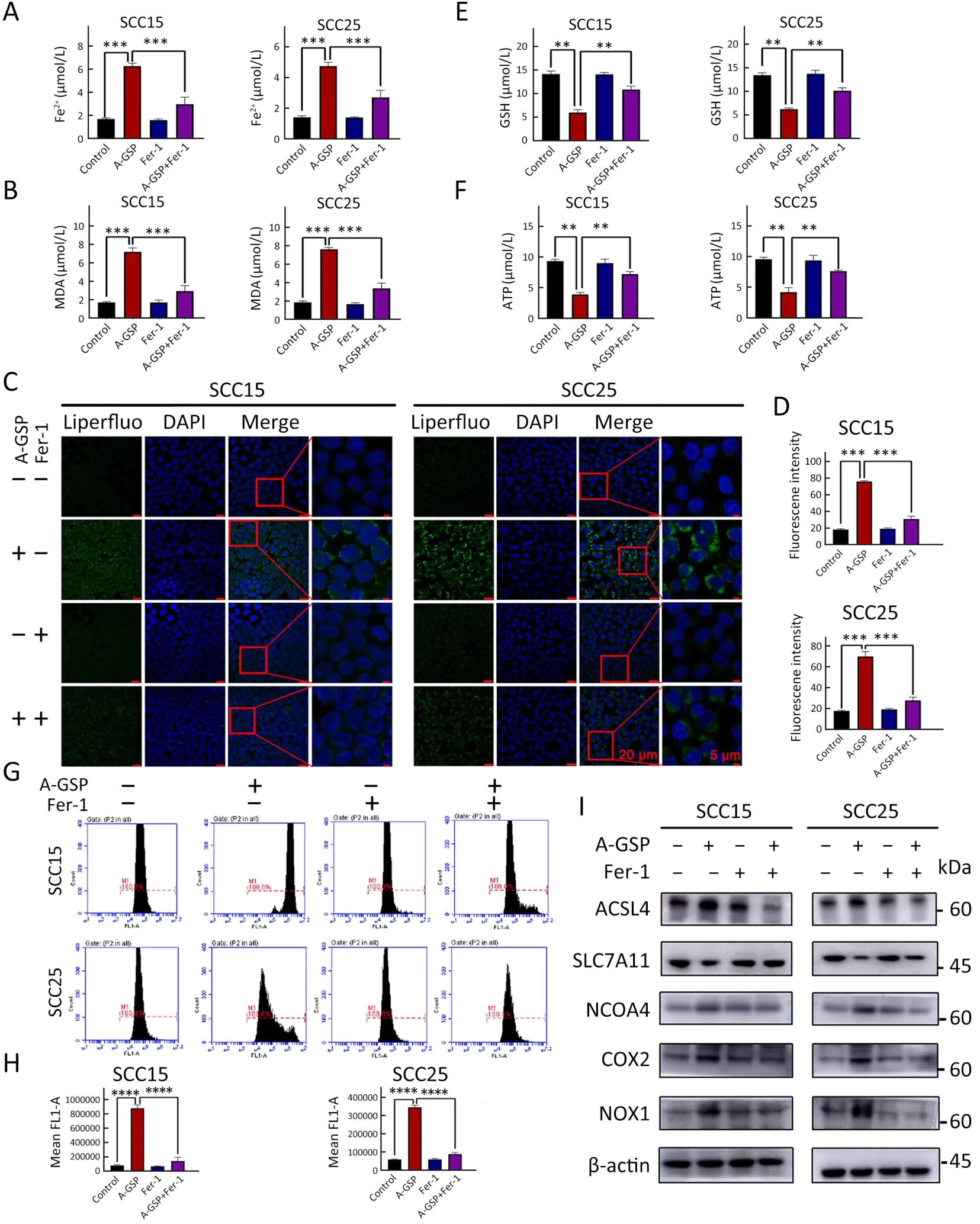
Figure 4 Numerous ferroptosis events induced by A-GSP can be reversed by ferrostatin-1.(A) Ferrostatin-1 and A-GSP were mixed with concentrations of 10 μmol/L and 1,000 μg/mL,respectively.Cells were treated for 72 h (All related experiments with ferrostatin-1 added in this figure have the same treatment conditions).Changes in Fe2+ content in SCC15 and SCC25 cells were detected;(B) Ferrostatin-1 reverses the increase in intracellular MDA content caused by A-GSP;(C) In the Liperfluo experiment,A-GSP-induced increase in intracellular lipid peroxides can be alleviated by ferrostatin-1 (Scale bars are 20 μ m and 5 μ m,respectively);(D) Statistical analysis of Liperfluo experimental results;(E) A-GSP-induced decrease in intracellular GSH content can be reversed by ferrostatin-1;(F) Changes in ATP content of tumor cells induced by A-GSP were reversed by ferrostatin-1;(G) A-GSP-induced massive accumulation of intracellular ROS can be alleviated by ferrostatin-1;(H) Statistical results of ROS fluorescence intensity;(I) Changes in A-GSP-induced ferroptosis gene can be blocked by ferrostatin-1.A-GSP,aqueous-soluble sporoderm-removed G.lucidum spore powder;MDA,malondialdehyde;ROS,reactive oxygen species.**,P<0.01;***,P<0.001;****,P<0.0001.
We also hypothesized that that ferroptosis inhibitors can reverse upregulation of ferroptosis-related gene and protein expression in A-GSP group.Western blotting was performed on the master proteins of the ferroptosis pathway.As shown inFigure 4I,the protein level of SLC7A11 was increased using the ferroptosis inhibitor,while the protein levels of ACSL4,COX2,NOX1,and NCOA4 were decreased by the ferroptosis inhibitor when compared with the group treated with A-GSP alone,indicating that ferrostatin-1 could block changes of key proteins in A-GSP-induced ferroptosis.In summary,the ferroptosis inhibitor ferrostatin-1 can reverse A-GSPtriggered ferroptosis in OSCC cells.
A-GSP suppresses tumor growth via ferroptosis induction in vivo
To verify the tumor suppression effect of A-GSPin vivo,two A-GSP administration groups of mice: a low dose (500 mg/kg) and a high dose (1,000 mg/kg) group,and one control group were set up.Xenograft tumor growth rate was significantly slower in the A-GSP-administered groups compared with control group,even low doses of A-GSP can produce a marked tumor inhibitory effect (Figure 5A-C).The results were consistent within vitroexperiment in SCC15 and SCC25 cells.Meanwhile,tumor weights were accordingly reduced in A-GSP-treated mice (Figure 5D).To evaluate the safety of A-GSP,we recorded body weight of mice changes during treatment and collected the serum at experiment end to detect multiple biochemical indicators.Neither body weight nor serum biochemical indicators changed significantly between groups (Figure 5E,F,Supplementary Figure S2),suggesting that A-GSP exhibits low toxicity in mice.In addition,hematoxylin and eosin (H&E) staining of hepatic and kidney tissue showed no significant difference among the groups (Figure 5G).Thus,thein vivodata indicate that A-GSP has a remarkable tumor-suppressing effect with no detectable toxic side effects in mouse models.
To further confirm ferroptosis occurrence,we performed IHC to detect six markers of ferroptosis and one tumor-growth marker.Ki67 was down-regulated in the tumors of the A-GSP group (Figure 6A),along with the decreased anti-ferroptosis proteins (GPX4,FTH1,SLC7A11,and NRF2).Correspondingly,the expression of ferroptosis-promoting proteins (ACSL4 and NCOA4) was considerably increased (Figure 6A,Supplementary Figure S3).H&E staining of tumors in xenograft mice (N=6 in each group) are showed inFigure 6B.Taken together,we demonstrated that A-GSP exerts a tumor-suppressing effect via effectively inducing ferroptosisin vivo.

Figure 5 A-GSP inhibits tumor cell growth in vivo.(A,B) Tumors of SCC15 cells (A) and SCC25 cells (B) were subcutaneously isolated from mice,measured and photographed;(C) Collected tumor growth data of xenograft mice and their growth curves;(D) Tumor weight comparison;(E,F) After administering A-GSP,changes in body weight of mice with SCC15 cells (E) and SCC25 cells (F);(G) Mice injected with SCC25 cell line were subjected to H&E staining of liver and kidney (Scale bar=100 μm).A-GSP,aqueous-soluble sporoderm-removed G.lucidum spore powder;H&E,hematoxylin and eosin.ns,P>0.05;**,P<0.01.
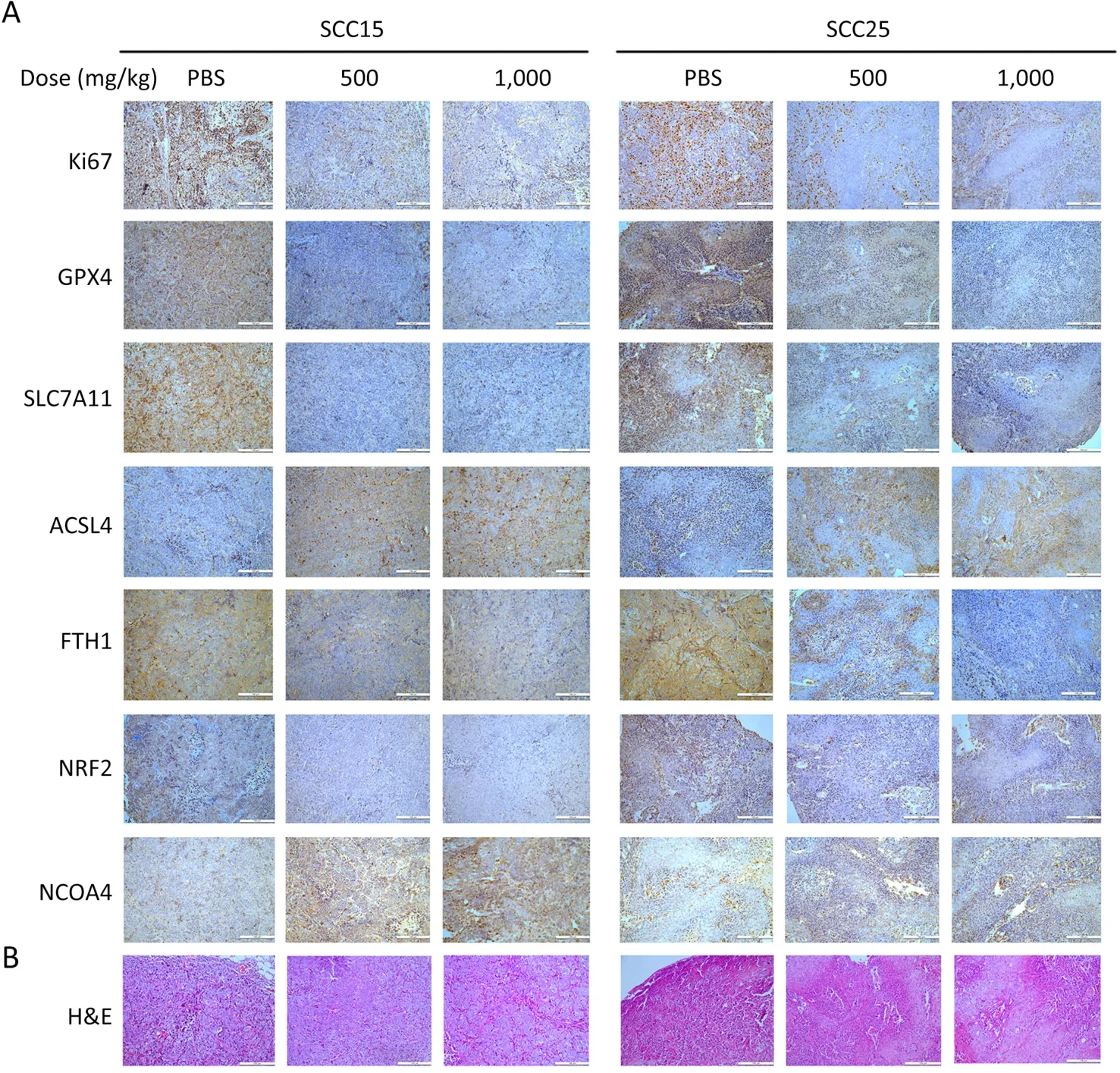
Figure 6 Tumor tissues from xenografted mice is immunohistochemically stained.(A) Immunohistochemical staining results of one tumor growth indicator and six ferroptosis molecules (Scale bar=100 μm);(B) H&E staining of tumors in xenograft mice (Scale bar=100 μm).H&E,hematoxylin and eosin.
Discussion
Recently,increasing attention is attracted on ferroptosis owing to its unique cell death characteristics.Ferroptosis has been reported to participate in cancer progression and displays broad therapeutic prospects,including reversing chemotherapy and radiotherapy resistance (30).However,targeting ferroptosis clinically faces two major problems that require urgent solutions.Firstly,clinically applicable drugs targeting ferroptosis are in shortage.Although erastin can inhibit system Xc-activity and induce ferroptosis,its poor water solubility and metabolic instabilityin vivomake it unsuitable for treatment.Therefore,further research is needed to improve its specificity and adjust its dosage (31).Additionally,most GPX4 inhibitors,such as ML210,RSL3 and ML162,exhibit poor pharmacological properties in animal models,which limit their clinical translation potential (32).
Secondly,the toxicity of chemotherapeutic drugs has long been not been neglected.For example,ferroptosisinducing sorafenib has been widely used in clinical practice,but its therapeutic effect is short-lived,and almost all patients develop drug resistance within a few months of treatment by sorafenib (33).Moreover,treatment is often accompanied by a series of side effects,such as abdominal distension,diarrhea,rash,loss of appetite,hair loss and infection.Long-term use can even cause more serious complications,such as bone marrow suppression,myocardial infarction,hypertension,and bleeding.These toxic side effects may further aggravate the deterioration of the disease in some cases and are even more detrimental to the prognosis of patients.Other commonly used chemotherapeutic drugs,such as cisplatin,paclitaxel,cetuximab,5-fluorouracil,and immune checkpoint inhibitors,have common adverse reactions as well.Faced with these clinical dilemmas,the development of safe and efficient anticancer drugs is crucial.A-GSP can be used as a dietary supplement to provide the body with nutritional elements and has the advantage of being safer than existing chemotherapeutic drugs.Fortunately,we revealed that AGSP can exert tumor-killing effects bothin vitroandin vivoby targeting ferroptosis
A-GSP-induced transcriptional alterations showed that the ferroptosis pathway was highly activated.Ferroptosis changes mitochondrial structure and causes hyperpolarization of the mitochondrial membrane (34,35),distinguishing it from other forms of cell death.Interestingly,after A-GSP treatment,the microscopic mitochondrial characteristics pointed to ferroptosis in SCC15 and SCC25 cells.Mechanistically,the occurrence of A-GSP-induced ferroptosis is triggered by multiple links.The accumulation of intracellular Fe2+is the primary link in triggering ferroptosis.NCOA4 binds to the ferritin phagocytosis geneFTH1and transports it to the lysosome where it releases iron ions,mediating the autophagic degradation of ferritin (36).These results suggest that AGSP caused excessive accumulation of Fe2+in the cytoplasm by upregulatingNCOA4and downregulatingFTH1.Thus,while the induction of Fe2+accumulation is proposed as a new way of inhibiting tumors,the tolerance of normal tissues needs to be considered.The nuclear transcription factor NRF2 has a central role in maintaining the body’s redox homeostasis (37).SLC7A11andGPX4,as downstream target genes ofNRF2,encode key proteins regulating ferroptosis (38).System Xc-is a heterodimer formed by two subunits,SLC7A11 and SLC3A2,and is mainly responsible for cysteine transport for GSH synthesis.As a reducing agent of GPX4,GSH is an essential cofactor for GPX4 to exert its antioxidant effect.Inhibition of system Xc-activity will result in GPX4 inactivation and oxidative damage (35).In this study,NRF2,SLC7A11,and GPX4 were all down-regulated and GSH was considerably depleted after A-GSP treatment.Hence,A-GSP was speculated to have effectively inhibited the signaling of the NRF2-system Xc--GSH-GPX4 axis.GPX4has long been considered a marker gene of ferroptosis,showing great potential as a tumor therapeutic target.
An excessive increase in lipid peroxides levels also contributes to the induction of ferroptosis.As key regulatory genes driving lipid peroxidation,ACSL4andCOX2have emerged as important markers of ferroptosis(39).In the OSCC cell lines SCC15 and SCC25,A-GSP increased the expression ofACSL4andCOX2and then rapidly induced the production of lipid peroxides,favoring the onset of ferroptosis.Activation of NOX1 is reported to be a key step in regulating ROS generation,exacerbating the process of ferroptosis (40).Consistently,we detected increase of NOX1 expression and ROS production,suggesting that A-GSP may synergistically induce intracellular accumulation of ROS by upregulating NOX1 and disrupting iron homeostasis.Interestingly,ferroptosis inhibitors restored cell viability by reversing these series of changes,further proving the targeting effect of A-GSP on ferroptosis.Although the changes in Fe2+,ROS,lipid peroxides and related genes are involved in the ferroptosis process,their internal connection remains a mystery and requires further exploration.Answers to these questions will provide new insights into therapeutic strategies targeting ferroptosis.
At present,harmful effects caused by chemotherapy drugs remain a major problem that cannot be ignored in clinical practice.Reducing the side effects of drugs is a common goal of doctors and patients today.In this study,our research data strongly demonstrated that A-GSP produced no detectable toxic side effects on the mice’s normal tissues,highlighting its safety.
Nevertheless,additional challenges remain when targeting ferroptosis with A-GSP.The relationship between various malignant properties of tumor cells and ferroptosis remains to be clarified.There may be differences in the sensitivity of patients with different disease stages and tumor cells of different tissue origins to ferroptotic drugs.Hence,to address these issues,it is necessary to elucidate the new mechanism of ferroptosis in the future to provide a better theoretical basis for the development of these drugs.Furthermore,considering that A-GSP is a mixture of active ingredients and the specific components that induce ferroptosis remain to be identified,further exploration on extraction and purification methods as well as relevant clinical trials may also be required to verify the mechanism of action of A-GSP.
All together,we provide herein vitroandin vivoevidence that A-GSP promotes ferroptosis in OSCC cells,which may bring new therapeutic hope to patients with oral cancer (Figure 7).Considering the anticancer effects of other reports,we can reasonably expect that A-GSP may provide new treatment opportunities for other refractory cancers in the future.
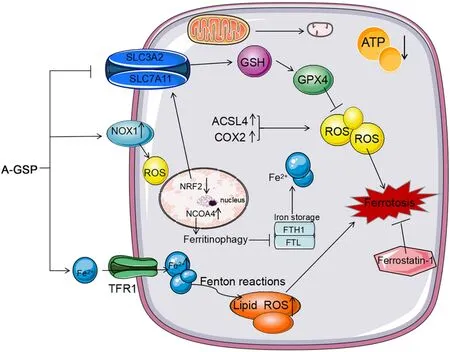
Figure 7 A-GSP exerts a tumor-suppressive effect by inducing ferroptosis without detectable toxic and side effects in vitro and in vivo.AGSP,aqueous-soluble sporoderm-removed G.lucidum spore powder;GSH,glutathione;ROS,reactive oxygen species.
Conclusions
This study revealed the anticancer mechanism of A-GSPin vitroandinvivo.A-GSP can induce Fe2+influx,accumulation of lipid peroxides and ROS,and inhibition of active transmission of NRF2-system Xc--GSH-GPX4 axis,resulting in severe depletion of GSH and inactivation of GPX4.Furthermore,reduction in mitochondrial volume and mitochondrial ridges was observed in presence with AGSP,resulting in impaired mitochondrial function and triggerring ferroptosis.Additionally,the ferroptosis inhibitor ferrostatin-1 was able to reverse the tumor inhibitory effect induced by A-GSP.
Acknowledgements
This study was supported by grants from the Pilot Project(4th Round) to Reform Public Development of Beijing Municipal Medical Research Institute (No.2021-1) and the Science Foundation of Peking University Cancer Hospital(No.17-01).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Adjuvant therapy for hormone receptor-positive breast cancer:Perspective from a survey on breast cancer physicians’acceptance of practice-changing data
- Exploration and optimization of surgical techniques for laparoscopic transhiatal lower mediastinal lymph node dissection for adenocarcinoma of esophagogastric junction: A prospective IDEAL 2a study with qualitative design
- Variant rs8400 enhances ALKBH5 expression through disrupting miR-186 binding and promotes neuroblastoma progression
- Integrated strategies for chemotherapy cycles in nasopharyngeal carcinoma patients: Real-world data from two epidemic centers guiding decision-making
- Transforming cancer cells for long-term living with cancer:An inspiring new approach
- When immunotherapy meets liver transplantation for hepatocellular carcinoma: A bumpy but promising road
