The Influence of CL-20 Particle Size on the Thermal Decomposition and Combustion Performances of 3D Printed Photocurable Propellants
2023-05-12LIManmanWURuohengGAOYuchenYANGWeitaoHURuiWANGQionglin
LI Man-man, WU Ruo-heng, GAO Yu-chen, YANG Wei-tao, HU Rui, WANG Qiong-lin
(1.Xi′an Modern Chemistry Research Institute, Xi′an 710065, China;2.School of Electronic Information, Northwestern Polytechnical University, Xi′an 710119, China)
Abstract:In order to clarify the influence of CL-20 particle size on the thermal decomposition and combustion behaviors of 3D printed gun propellants, the photocurable gun propellants containing 24, 56 and 152μm CL-20 particles were fabricated by extrusion 3D printing, and the thermal behavior of 3D-printed propellants was studied by the non-isothermal DSC (differential scanning calorimetry) method. The decomposition activation energy was obtained by the modified Kissinger-Akahira-Sunose (KAS) method. It was found that all the CL-20-based propellants presented a two-step decomposition process. The first decomposition step is considered an accelerated process resulting from reducing the crystal quality of CL-20 affected by the photocurable binder. The critical temperatures of thermal explosion (Tpe) of the CL-20/PUA (polyurethane acrylate) composites was decreased about 27℃ compared with pure CL-20. Regarding the influence of particle size, the experimental results indicated that the fine CL-20 was affected more by the binder than the other particle sizes due to the greater surface area. Propellant samples containing finer CL-20 particles presented lower activation energy and critical temperature. The combustion results also indicated that the propellant containing fine CL-20 presented higher burning rate and lower pressure exponent. It is recommended to use fine CL-20 to construct the photocurable propellants for 3D printing.
Keywords:physical chemistry; CL-20; HNIW; photocurable propellant; 3D printing; particle size; thermal decomposition
Introduction
Gun propellants are a kind of chemical substance used in producing energy or pressurized gas that is subsequently used to generate propulsion of projectiles or other objects. The performance of a propellant charge depends on the gas generation rate, which is mainly controlled by the propellant geometry and burn rate[1]. In recent years, 3D printing has been vigorously studied to promote 3D-printed gun propellants into practice[2]. Sun and Zhou[3-4]studied the 3D extrusion printing of a nitrocellulose-based solution. Schaller studied the GAP(Glycidyl azide polymer)-based EPTE (energetic thermoplastic elastomers) filaments which can be printed by the fused deposition modeling (FDM) 3D printer[5]. TNO scientists studied the 3D printing of epoxy/RDX propellants using the DLP (digital light processing) printer and extrusion 3D printer[6].
The energetic content is improved by using 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20 or HNIW) in propellant formulations, making it an attractive candidate[7-9]. CL-20 based energetic inks for 3D printing, including HTPB/CL-20 based[10], polyurethane acrylate (PUA)/CL-20 explosive ink[11-13], epoxy acrylate (EA)/nitrocellulose (NC)/CL-20 explosive ink[14], PUA/EA/NC/CL-20 based explosive ink[15]and PVA/EC/CL-20[16]have been studied. In our previous study, photocurable PUA/CL-20 and APNIMMO/CL-20 ink were printed by the SLA (Stereolithography) printer and 3D extrusion printer to fabricate gun propellant[17-18].
Thermal decomposition and combustion performance of these formulations play a vital role in the thermal responses and interior ballistic behavior. Previous work has demonstrated that the decomposition of CL-20 started with a loss of a NO2group of CL-20[19-20]. Unlike RDX or HMX, no experimental evidence was reported that a melting layer emerged from CL-20, revealing a solid-state decomposition process of CL-20[21]. The thermal decomposition mechanism of CL-20 has been studied and published in previous work[22-24].
By far, there is very scarce data on the influence of the particle size of CL-20 on the decomposition and combustion behavior of 3D-printed propellants. Therefore, it is essential to understand the relationship between the particle and their performance. This paper studied the decomposition and combustion performances of 3D-printed gun propellant composed of CL-20 with different particle sizes and photocurable acrylate polymer.
1 Experimental
1.1 Materials
The ε-CL-20 used was supplied by Qingyang Chemical Industry Corporation, China North Industries Group. TheD50of CL-20-1, CL-20-2 and CL-20-3 is 24, 56 and 152μm, respectively. The PUA-based binder matrix is a photocurable resin including 67% polyurethane acrylate (PUA), 30% acrylic diluent and 3% diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide as photoinitiator. All propellant compositions consisted of 70% CL-20 and 30% PUA resin. The predicted thermal-chemical properties including force constant and flame temperature of the designed propellants are calculated by Ematrix code. The calculated force constant, flame temperature, average gas molecular weight and ratio of specific heats is 1061kJ/kg, 2776K, 21.8g/mol and 1.28, respectively.
1.2 CL-20/PUA ink preparation and 3D printing
The schematic illustration of the extrusion 3D printing process of CL-20/PUA ink was shown in Fig.1. The composite propellant slurry (ink) containing PUA resin and CL-20 particles was prepared in an electric stirrer. The slurries were extruded by a piston injector with a 1mm nozzle and deposited on the moving platform. The deposited slurry is cured under exposure of 405nm UV light. The exposure time is about 20s for each layer. At last, the cured propellant slices were taken away from the platform. The density of printed samples was 1.68—1.70g/cm3.

Fig.1 Schematic illustration of 3D printing process of CL-20/PUA ink
1.3 SEM and XRD tests
The 3D-printed sample was fractured in liquid nitrogen, and the inner morphology of composite was observed with a QUANTA FEG 250 scanning electron microscope (SEM).
The polymorph of 3D-printed propellant was detected by X-ray diffract meter (XRD, Malvern Panalytical, Empyrean instrument) with a tube voltage of 40kV, a tube current of 30mA, and diffraction angle of 5° to 90°.
1.4 Thermal decomposition
Six samples CL-20-1, CL-20-2, CL-20-3, CL-20-1/PUA, CL-20-2/PUA, CL-20-3/PUA, are involved in these experiments. The samples were tested by a Mettler-Toledo HP DSC 827e. Temperature range: 50—300℃. Heating rate: 2, 5, 7 and 10℃/min. Sample mass: about 1mg. Dynamic nitrogen atmosphere: 50mL/min.
Activation energy (Ea) with the conversion rate was obtained by the modified Kissinger-Akahire-Sunose (KAS) method (Eq.1)[25-26].
(1)
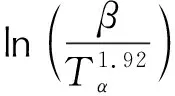
1.5 Closed bomb tests
The combustion properties of printed CL-20/PUA composites sheet were investigated by a 102mL closed vessel test with a loading density of 0.12g/cm3. The propellant sheets were 4mm×10mm×100mm. The ignition powder is 1.1g nitrocellulose with 12.6% nitrogen content. The pressure history was recorded, and the burn rate was calculated by the form function of geometry.
2 Results and Discussion
2.1 SEM and XRD results
The internal morphology of each formula was observed first. Differences were observed between the morphology of each propellant formulation. Fig.2 presents the SEM photos of three samples containing different CL-20 particles. It is clear to find the interface between CL-20 particles and polymer binder and CL-20 particles protruding from the formulation. The 24μm CL-20 particles are much more uniform in size and distributed evenly in the binder. In contrast, 152μm CL-20 consists of agglomerated particles with irregular shapes, resulting in a less homogeneous distribution of CL-20 particles.

Fig.2 Inner stucutre of CL-20/PUA propellants
Fig.3 presented the XRD spectra of printed CL-20 composites compared with pure CL-20. It can be seen that the main diffraction peaks of the sample are consistent with that of pure ε-CL-20 reported in the literature[3]. The XRD results present strong characteristic peaks at 12.52°, 25.73° and 30.28°, coinciding with pure ε-CL-20. The intensity of XRD was weakened resulting from the binder. The results indicate that the 3D printing did not cause the crystalline phase transition of CL-20.

Fig.3 XRD spectra of CL-20/PUA propellants and pure CL-20 with various particle sizes
2.2 Thermal decomposition
To study the influence of particle size on the thermal behavior of the formulations, Fig.4 presents the DSC curves of CL-20/PUA composites in comparison with the pure CL-20. Table 1 summarizes the thermal behavior characteristics of each exothermic peak, including decomposition enthalpies (ΔH) and peak temperature (Tp). According to the decomposition curves of pure CL-20 in Fig.4(a)—(c), there was only one exothermic peak of 230—250℃ at the heating rate of 2 to 10℃/min. Fig.4(d)—(f )present the DSC curves of CL-20/PUA composites with decreased heat release due to the inert binder. It is noticeable to find two exothermic peaks in the decomposition of CL-20/PUA composites, indicating the accelerated decomposition of some CL-20. The second exothermic peak temperature of CL-20/PUA composites is near to that of pure CL-20 with same particle size, while the first exothermic peak is about 24—31℃ lower.

Table 1 DSC parameters for (a)—(f) samples

Fig.4 DSC curves of CL-20 and composites with various particle sizes
A two-phase decomposition process of CL-20 composites was observed in previous works when polyisobutylene plasticized by dioctyl sebacate and oily material[23-24], GAP[27-28]and HTPB[29]was adopted. It was considered the two-step decomposition was connected with the crystal lattice quality. On the contrary, a single decomposition process was also found when using CAB plasticized by BDNPF/A[30], silicone rubber[31]and Formex matrix[24]. In regard of the influence of particle size, experimental results show that the CL-20 with different sizes had varied influences on the thermal decomposition of the composite samples. The fine CL-20 was affected more by the binder than the other particle sizes due to the greater surface area. Thus, the portion of the first peak heat release becomes bigger during the whole heat release process.
2.3 Kinetic parameters
The modified KAS method was used to obtain the dependence of activation energy on the degree of conversion. The activation energies at the conversion rates from 0.1 to 0.9 were determined. The dependence of the extent of conversion on the temperature with the linear fitting curves at different conversion rates (0.1—0.9) was presented in Fig.5. It is obviously observed from Fig.5 that there are overlapped steps on the curves of CL-20/PUA at the conversion range of 0.4—0.6, indicating a strong mutual interaction between the two decomposiiton steps. The dependence of activation energy of studied CL-20 and its composites on the extent of conversion is represented in Fig.6.


Fig.5 The α—T curves of different CL-20 and CL-20/PUA samples at different heating rates

Fig.6 The dependence of activation energy on conversion rate for thermal decomposition of CL-20/PUA compared with pure CL-20
Based on theEa—αcurves, it can be seen that the pure CL-20 used in this study has almost constant activation energy with extent of conversion with an average value of 175.28, 181.49 and 190.52kJ/mol for CL-20-1, CL-20-2 and CL-20-3. As to the effect of particle size, Lee[31]reportedEaof 160.88kJ/mol for CL-20 with small particle size (under 44μm) andEaof 226.83kJ/mol with large particle size (under 246μm) and the influence of particle size are coincident with the results in this study.
As for CL-20/PUA composites, the mean activation energy atαof 0.1—0.3 is 150.45, 161.12 and 174.40kJ/mol, while the mean activation energy atαof 0.7—0.9 increased to 218.98, 199.62 and 181.92kJ/mol for CL-20-1/PUA, CL-20-2/PUA and CL-20-3/PUA composites. The lower activation energy values in the initial decomposition stage are indicative of its inferior thermal stability. The reuslts indicated that the CL-20-1/PUA composite presents the lowest thermal decomposition energy barrier. The high activation energy in the second step also infers the thermal stability of CL-20 residues because of the formation of nitrogen heterocyclic compounds after the N—NO2homolysis[32].
3 Influence of CL-20 Particle Size on Combustion Performance
The combustion performances of propellants with different CL-20 particle sizes were investigated by closed bomb test. Fig.7 presents thep—t,u—pand dynamic vivacity (L—B) curves of the CL-20/PUA samples.

Fig.7 p—t, u—p and L—B curves of CL-20/PUA composites with various CL-20 particle sizes
Table 2 presents the combustion parameters of three samples. It shows clearly that the combustion behaviors were also affected by the particle size of CL-20. With the decrease of CL-20 particle size, the burning time decreased from 50 to 22ms, and the maximum pressure increased from 112 to 121MPa. The maximum pressure varied because the heat loss reduced as the burn times decreased. Meanwhile, the CL-20-1/PUA propellant presents higher burning rate compared with other propellants. As mentioned above, the propellant with lower activation energy during the first decomposition step is more easily ignited by heat. As regards to the burning rate coefficient and pressure exponent, the burning rate coefficient increased with the increase of CL-20 particle size from 24 to 152μm. The pressure exponent of propellants containing 24μm CL-20 particles is 1.03, which is acceptable. While, the propellants containing 152μm CL-20 presents a pressure exponent value of 2.37, which is unacceptable for a gun propellant. It is speculated that the increased temperature in the gas phase and the com-bustion surface area of the propellant grains increased pressure exponent. With the increase of pressure, CL-20 particles with larger particle size are difficult to completely decompose in the condensed phase, and then escaped to the gas phase. Meanwhile, the CL-20 particle size also influenced the dynamic vivacity of propellants. Propellants with smaller CL-20 presented higher maximum dynamic vivacity value. While, the propellants with larger CL-20 presented smaller maximum dynamic vivacity value and flatL—Bcurve for the very low burning rate coefficient. According to the combustion results, fine CL-20 particle is recommended.

Table 2 Combustion parameters
4 Conclusions
(1) The thermal decomposition process of ε-CL-20 and its composites with various particle sizes were evaluated based on non-isothermal DSC techniques. The thermal decompositions of CL-20 and CL-20/PUA present a two-step decomposition process, and the initial step is partially controlled by the physical morphology of CL-20, which was mainly affected by the acrylate binder.
(2) The particle size has little effect on the critical temperatures of thermal explosion (Tpe). It indicates that the PUA acrylate binder could promote decomposition of CL-20 about 27℃ lower.
(3) The activation energy of propellant containing fine CL-20 is smaller and more easily ignited. Fine CL-20 particle is also recommended for lower pressure exponent.
