lnversion tillage with straw incorporation affects the patterns of soil microbial co-occurrence and multi-nutrient cycling in a Hapli-Udic Cambisol
2023-05-08CHENXuHANXiaozengWANGXiaohuiGUOZhenxiYANJunLUXinchunZOUWenxiu
CHEN Xu, HAN Xiao-zeng, WANG Xiao-hui, GUO Zhen-xi, YAN Jun, LU Xin-chun, ZOU Wen-xiu#
1 Key Laboratory of Mollisols Agroecology, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Harbin 150081, P.R.China
2 Agricultural Technology Extension Center of Wafangdian City, Wafangdian 116300, P.R.China
3 Modern Agricultural Development Service Center of Tieling County, Tieling 112600, P.R.China
Abstract Inversion tillage with straw amendment is widely applied in northeastern China, and it can substantially increase the storage of carbon and improve multiple subsoil functions.Soil microorganisms are believed to be the key to this process, but research into their role in subsoil amelioration is limited.Therefore, a field experiment was conducted in 2018 in a region in northeastern China with Hapli-Udic Cambisol using four treatments: conventional tillage (CT, tillage to a depth of 15 cm with no straw incorporation), straw incorporation with conventional tillage (SCT, tillage to a depth of 15 cm), inversion tillage (IT, tillage to a depth of 35 cm) and straw incorporation with inversion tillage (SIT, tillage to a depth of 35 cm).The soils were managed by inversion to a depth of 15 or 35 cm every year after harvest.The results indicated that SIT improved soil multi-nutrient cycling variables and increased the availability of key nutrients such as soil organic carbon, total nitrogen, available nitrogen, available phosphorus and available potassium in both the topsoil and subsoil.In contrast to CT and SCT, SIT created a looser microbial network structure but with highly centralized clusters by reducing the topological properties of average connectivity and node number, and by increasing the average path length and the modularity.A Random Forest analysis found that the average path length and the clustering coefficient were the main determinants of soil multi-nutrient cycling.These findings suggested that SIT can be an effective option for improving soil multi-nutrient cycling and the structure of microbial networks, and they provide crucial information about the microbial strategies that drive the decomposition of straw in Hapli-Udic Cambisol.
Keywords: soil microbiome, microbial co-occurrence networks, straw amendment, soil nutrient
1.Introduction
Fungi and bacteria are the main drivers of soil biochemical processes and functions.Rather of living in isolation, fungi and bacteria develop a complex system of interspecific interactions (Freilichet al.2010).Thousands of soil microorganisms may be linked by these ecological networks, which can be used to determine the patterns of microbial co-occurrence (Chaffronet al.2010).Therefore, our understanding of microbial communities should not only focus on the characteristics of individual/species levels, such as species richness and abundance, but also more importantly on the interspecific characteristics of complex microbial communities.Interactions among species in microbial communities are important for the turnover of soil organic matter (SOM) (Zhenget al.2018).The analysis of ecological networks is an effective method for identifying potential species interactions and patterns of co-occurrence that cannot be observed directly (Fathet al.2007), which can serve as an important starting point for exploring the potential interactions in complex soil microbiomes by identifying potential biotic interactions that are worthy of further experimental validation (Carret al.2019).
Numerous insights into the effects of the function and dynamics of terrestrial ecosystems on the structure and networks of soil microbial communities have been obtained.Water availability, soil nutrients, vegetation density and soil pH can all influence negative and positive microbial interactions (Creameret al.2016; Hernandezet al.2021).Changes in the structure and networks of soil microbial communities are often attributed to variations in the levels of soil nutrients, such as carbon (C) and nitrogen (N).For example, the abundance of microbial-network hubs has been strongly linked to multiple ecological processes in agricultural ecosystems, such as the cycling of C, N, phosphorus (P) and sulfur (S) (Shiet al.2020).The key taxa in co-occurrence networks have also been correlated with soil P levels and pH (Banerjeeet al.2019).The structure and networks of microbial communities are critical for simultaneously supporting multiple ecosystem functions and services, including nutrient cycling, primary production and litter decomposition (van der Heijdenet al.2008; Wagget al.2014).Ecosystems are also multifunctional, i.e., different ecological processes occur concurrently rather than individually.Thus, multi-nutrient cycling is the most essential process of terrestrial ecosystems for understanding and predicting the services provided by soils and ecosystems (Delgado-Baquerizoet al.2016) and how such services react to agricultural management practices.Our understanding of the mechanisms of microbial networks in different agricultural management practices, however, remains limited.Additional experimental data are needed to determine how soil multinutrient cycling and microbial interactions may be affected in managed agricultural soils.
Crop straw is an important source of organic carbon in agroecosystems and has great potential for sequestering carbon in arable soil (Choudhuryet al.2014), but crop straw is typically incorporated into the topsoil by conventional tillage.Soil carbon concentrations generally decrease with depth (Jobbagy and Jackson 2000), implying that subsoils may hold more carbon than surface soils.Combining deep plowing with straw incorporation into both the topsoil and subsoil may contribute to improvements in the fertility and resilience of sandy soil, but this practice is not common due to the lack of scientific data.Most studies of soil fertility have focused only on the topsoil, because subsoil carbon and microorganisms have been assumed to be relatively stable and unresponsive to agricultural management practices, but this narrow focus has limited the complete adoption of applying straw in agricultural production.The differences in the biochemistry of SOM and mechanisms of stabilization between topsoil and subsoil, and the scarcity of information on the dynamics of the subsoil OM and microbial community, hinder the prediction of whether and how subsoil carbon stocks and microorganisms might respond to agricultural management practices, especially when different practices (e.g., tillage and straw amendments) are combined.Traditional management systems lead to the loss of carbon, mainly due to losses from subsurface soil layers (Samsonet al.2021), even after the adoption of conservation practices (Steinmannet al.2016).Additionally, with straw incorporation, the structures of microbial networks may converge at various soil depths depending on their nutritional preferences, and then they can separate once again when the supply of easily decomposable material runs out.The arrangement of microorganisms into trophic groups and functionally distinct niches enables their coexistence (Schimel and Schaeffer 2012).Learning more about the exchange of carbon compounds, competition for nutritional sources and predation or parasitism among microbes during tillage and straw amendments would be very helpful, especially in subsoils.Most studies have focused solely on topsoils, so our knowledge about microbial interactions and functions in deeper layers remains limited.
Generally, conventional tillage uses a 20-cm rototiller blade, resulting in a tillage depth of about 15 cm.Due to the long-term adoption of shallow rototilling operations, the plough layer is becoming thinner and the plow sole is becoming thicker, which reduces soil and air permeability and limits plant root development.In addition, it is common practice to rototill crushed above-ground straw immediately following the harvest, which is then ridged for the next year’s seedlings, although this system may easily result in poor crop emergence.The dense clayey soils in northeastern China are typically managed by the inversion of the soils (soil “flipping”) to a depth of 35 cm after harvest.A proper depth allows the straw to be sufficiently buried into the soil to eliminate the impact on the next year’s seedlings and to reduce straw burning.Field experiments on clayey soils have demonstrated that inversion tillage with straw amendment can greatly improve the SOC concentration, bulk density, root growth and plow-layer (Zhanget al.2021), but the impact on sandy soils is uncertain.
Hapli-Udic Cambisol is widely distributed in northeastern China and has a long history of reclamation.Some Hapli-Udic Cambisols have a coarse texture and are primarily sandy loam.Inappropriate practices, however, have destroyed the ecological balance, and soil erosion is increasingly aggravated, causing the Hapli-Udic Cambisol to lose its fertile humus layer and generally exposing the subsoil layer.Therefore, the proper management of Hapli-Udic Cambisol has been encouraged to prevent these phenomena, such as fertilization using organic manure and the return of crop residues to fields.Long-term conventional tillage only disturbs the topsoil, so the structures of microbial communities differ dramatically between the topsoil and subsoil.The composition of the microbial community in each soil layer also tends to be stable, leading to considerable stratification.Mixing the topsoil and subsoil can widen the plow layer and provide additional storage space for accumulating SOC, but little is known about the features of microorganism redistribution.A field experiment was conducted in a Hapli-Udic Cambisol in northeastern China to explore the cross-kingdom networks among bacteria and fungi under the continuous cropping of maize with different strategies of tillage and straw management.The diversity of microbes and their ecological networks play critical roles in soil multi-nutrient cycling, such as the decomposition of organic matter (OM) and the transformation of available C and N.We consequently hypothesized that: 1) inversion tillage with straw incorporation would influence the networks of cooccurring microbes more than other treatments, and 2) the interactions of co-occurring microbes would become the essential drivers of soil multi-nutrient cycling in agroecosystems.
2.Materials and methods
2.1.Site description and experimental design
This field experiment was established in Wafangdian County, Liaoning Province, northeastern China (39.33°N, 122.03°E) in 2018.The soil type in this area is a Hapli-Udic Cambisol (FAO classification).The percentages of sand (0.02–2 mm), silt (0.002–0.02 mm) and clay (<0.002 mm), based on the international system of particle-size fractions (ISSS 1930), are 66.0, 18.9 and 14.7% in the 0–15 cm layer, and 64.7, 21.2 and 14.1% in the 15–35 cm layer, respectively, so the soil is dominated by a coarse sandy loam texture.The experimental area has a typical mild temperate continental monsoon climate, with hot summers and cold winters.The mean annual temperature and precipitation are 9.6°C and 510 mm, respectively.The mean monthly temperature is the highest in July (24°C) and the lowest in January (–7.1°C).Precipitation is mainly concentrated from July to September.The frost-free period is 165–185 days.

Table 1 The soil physicochemical characteristics in the 0–35 cm profile before the experiment
2.2.Soil sampling and chemical analyses
Soil samples were collected with a 5-cm diameter soil drill in July 2020 from the 0–15 and 15–35 cm layers in each plot at three randomly selected points, and then mixed into composite samples.These composite samples were packed in sterile bags and transported to the laboratory.All visible roots and fresh plant litter wereremoved and the remaining soil was sieved to 2 mm.In total, 24 samples were collected.Each sample was then divided into two subsamples for analysis.One subsample was air-dried and used for determining the chemical properties: pH and the concentrations of available N (AN), available P, available potassium (AK), SOC and total N (TN).The amount of available N was measured using alkaline diffusion (Bremner and Keeney 1965), available P was measured using NaHCO3extraction (Lu 2000) and available K was measured using NH4OAc (Jackson 1973).Soil pH was determined from a 1:2.5 (w/v) soil:water suspension using a standard pH meter (Pansu and Gautheyrou 2006).Carbonates were washed from the soil using hydrochloric acid, and the SOC and total N concentrations were then determined using a VarioEL CHN elemental analyzer (Heraeus Elementar Vario EL, Hanau, Germany).The other subsample was passed through a 2-mm sieve and stored at –80°C for DNA extraction and high-throughput sequencing.
2.3.DNA extraction and high-throughput sequencing
Metagenomic DNA was extracted from 0.5 g of soil using a FastDNA SPIN Kit for Soil (MP Biomedicals, Cleveland, USA) following the manufacturer’s instructions.The extracted DNA was quantified using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, USA).The 16S rRNA gene (V3–V4 region) and the fungal internal transcribed spacer (ITS1 region) were amplified by PCR using the universal primers 515F/907R (Yusoffet al.2013) and ITS1/ITS2 (Ghannoumet al.2010), respectively.The primers were tagged with unique barcodes for each sample.PCR reactions (Qiagen, Valencia, USA) were performed in a 50-μL mixture containing 0.25 μL of TaKaRaExTaqHS (5 U mL–1), 4 μL of dNTPs (2.5 mmol L–1each), 5 μL of 10×ExTaqbuffer (Mg2+Plus), 1 μL of each primer (10 μmol L–1) and 1 μL of template DNA (50 ng).The thermal program was: 94°C for 5.0 min; 30 cycles of 94°C for 45 s, 55°C for 45 s and 72°C for 1 min; 72°C for 10 min and holding at 4°C.Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina Inc., San Diego, USA) following the standard protocols of Majorbio Bio-Pharm Technology Co., Ltd.(Shanghai, China).Raw pyrosequencing reads were processed using the Quantitative Insights Into Microbial Ecology Pipeline ver.1.9.1 (http://qiime.org/tutorials/tutorial.html).Sequences were clustered to operational taxonomic units (OTUs) using a similarity threshold of 97%.The bacterial and fungal OTUs were taxonomically annotated using the Silva taxonomy database (Release138, http://www.arb-silva.de) and Unite (Release 8.0, http://unite.ut.ee/index.php), respectively.The raw sequences have been deposited to the NCBI Sequence Read Archive with accession numbers PRJNA822479 (bacterial raw sequences) and PRJNA822491 (fungal raw sequences).
2.4.Real-time PCR
By measuring the copies of the 16S rRNA gene and the fungal internal transcribed spacer using the universal 515F-907R and ITS1/ITS2 primer pair, the sizes of the bacterial and fungal communities could be quantified.Real-time quantitative PCR was performed using an ABI 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, United States).Each PCR reaction contained 16.5 μL of AceQ SYBR Green qPCR Master Mix (2×), 0.8 μL of 5 μmol L–1forward and reverse primers (each) and 2.0 μL of template DNA.The 16S rRNA gene PCR reactions were run at 95°C for 5 min, then 40 cycles of 95°C for 30 s of denaturation, 50°C for 30 s of annealing, and 72°C for 60 s of elongation.The fungal ITS gene PCR samples were subsequently incubated at 95°C for 5 min, followed by 40 cycles of 5 s at 95°C, 30 s for annealing at 50°C, and 40 s for elongation at 72°C.Instead of soil DNA, the negative controls consisted of all of the reagents with sterilized water.Three replicate samples were used to average the threshold cycles.The known number of copies in the standard curves was converted to the cycle threshold value using a regression equation, and the copy number was then calculated.
2.5.Network construction
Theigraphpackage in R software was used to construct the co-occurrence networks based on Pearson correlations of log-transformed OTU abundances as described previously (Yuanet al.2021), followed by an approach based on random matrix theory that establishes the correlation cut-off threshold (|r|>0.6,P<0.05).Only OTUs with relative abundances >0.01% were included.Theigraphpackage was also used to obtain network characteristics.The network topological features of each sample were implemented in the subgraph function using theigraphpackage (Maet al.2016).Node number (the number of OTUs), edge number (the number of connections between all nodes), average connectivity (the strength of the correlation between nodes), the clustering coefficient (the degree of clustering between adjacent nodes) and the average path length (the average distance between two nodes) were the parameters used in this study to describe the topological characteristics of a network.
2.6.Statistical analysis
The two-way ANOVA was used to determine the effects of straw amendments and tillage on microbial diversity, richness, abundance and soil physicochemical properties by the car R package.Principal coordinate analysis (PCoA) was used to visualize the variations in the microbial-community structures of the treatments.Permutational multivariate analysis of variance (PERMANOVA) based on a Bray-Curtis matrix was used to test the effects of the various treatments on microbialcommunity composition.The Mantel test was performed to measure the effects of the soil properties on microbialcommunity structure using the vegan R package.The importance of the topological properties of microbial cooccurrence networks to soil multi-nutrient cycling and properties was identified using a classification Random-Forest (RF) analysis.The percentage increases in the mean squared error (MSE) of the variables were used to estimate the importance of these indices, where a high MSE% implies a high importance of the variables (Breiman 2001).
Soil multi-nutrient cycling (MNC) was quantified by creating an index using a widely used method involving a synthetic variable obtained from the standardized transformation of the concentrations of SOC, TN, AN, AP and AK (Jiaoet al.2018).In summary, the data were checked for normal distribution using the Shapiro-Wilk test before analysis.To bring non-normally distributed data closer to a normal distribution, logarithm or squareroot transformations were used.Each variable was then normalized to a scale of 0 to 1, and the average of these converted values was used to obtain the multi-nutrient cycling values for each plot (Faninet al.2018).
3.Results
3.1.Soil physicochemical properties
Straw amendments and tillage had significant main effects on the soil nutrients (Table 2).The soil properties (SOC, TN, AN, AP and AK concentrations) were significantly higher in the 0–15 cm layer than the 15–35 cm layer in the different treatments, whereas bulk density (BD) and pH had the opposite trend.Soil multi-nutrient cycling variables were significantly higher in the 0–15 cm layer in SCT and SIT, and higher in the 15–35 cm layer in SIT and IT than the other treatments.The concentrations of SOC, TN, AN, AP and AK followed a similar trend.The SOC concentration ranged from 6.2 to 10.2 g kg–1, and it was higher in the 0–15 cm layer in SCT and SIT thanthe other treatments.Soil pH in the 0–15 cm layer was significantly higher than in CT and SCT by 8.1 and 8.6% in SIT, and by 7.1 and 7.6% in IT, respectively, but the pH differed little among the treatments in the 15–35 cm layer.BD was significantly lower than in CT in the two layers by averages of 10.0 in IT and 12.4% in SIT.The soil properties were similar in CT and SCT in the 15–35 cm layer, because that layer was not plowed.
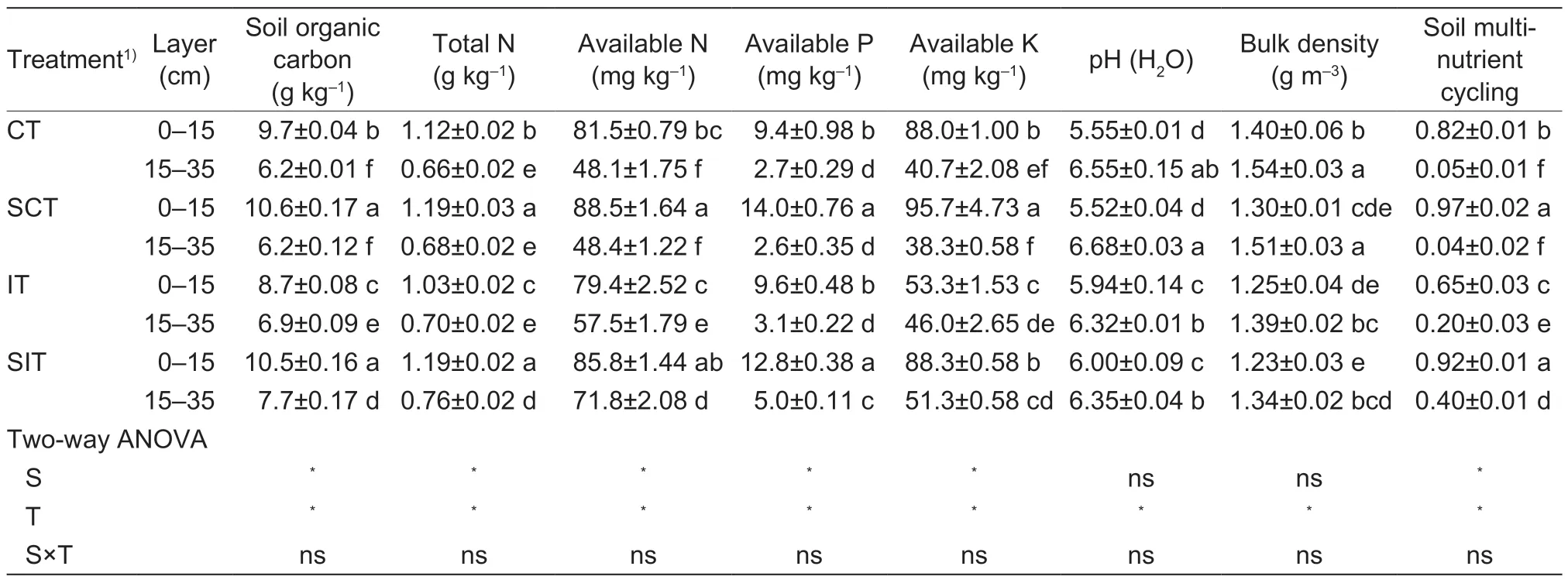
Table 2 Effects of tillage and straw management practices on soil properties
3.2.Characterization of the bacterial and fungal communities
Bacterial and fungal abundances, measured using gene abundances, were significantly influenced by straw amendments and tillage.Both were significantly higher in SIT than the other treatments in both layers, except fungal abundance in the 0–15 cm layer was the highest in SCT (Fig.1).Microbial abundance in the 15–35 cm layer did not differ significantly between CT and SCT, but was significantly lower than in IT and SIT.Bacterial abundance in the 0–15 cm layer was significantly lower than in IT by 33.4% in CT, but significantly higher by 66.8% in SCT.Fungal abundance in the 0–15 cm layer was significantly higher than in IT by 55.5% in CT and 584.4% in SIT.Bacterial diversity in the treatments, estimated using the Shannon index and Chao1 richness, were lower in the 0–15 cm layer than in the 15–35 cm layer, but fungal diversity was not.Bacterial diversity and richness varied little among the treatments.Fungal diversity and richness averaged 30.7 and 13.6% lower in SIT than in the other treatments in both layers.Fungal diversity in the 0–15 cm layer was also significantly lower by 13.3 and 15.3% in SCT than in CT and IT, respectively.
I shut off the recorder and Rebekah sighed deeply. Thank you, Nan, she said with a weak smile. You ll give this one to them, won t you? she murmured as she slid into sleep.
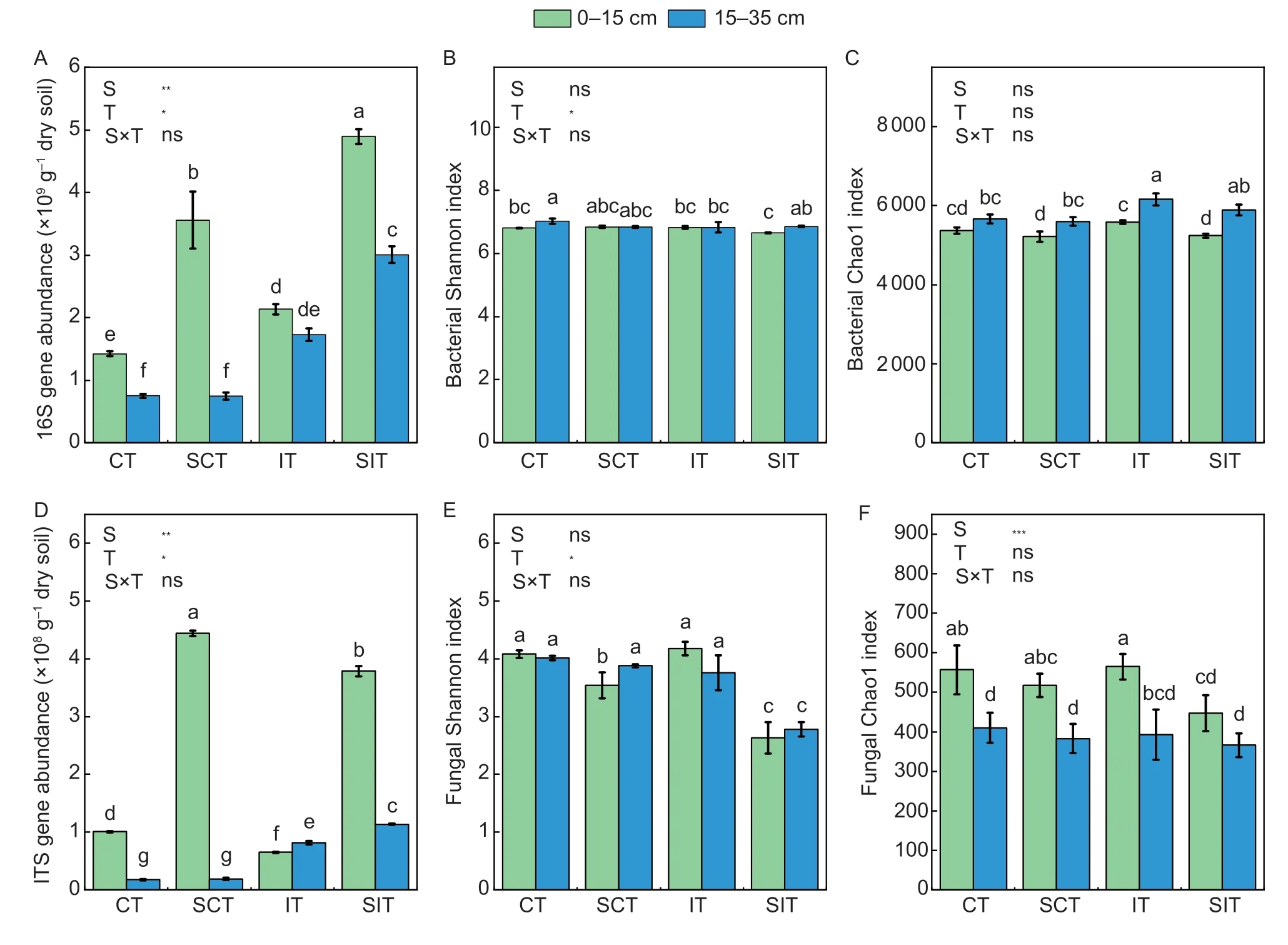
Fig.1 Responses of bacterial (A–C) and fungal (D–F) abundance and diversity in the 0–15 and 15–35 cm layers among the treatments.CT, conventional tillage; SCT, straw incorporation with conventional tillage; IT, inversion tillage; SIT, straw incorporation with inversion tillage.ITS, internal transcribed spacer; S, straw amendment; T, tillage; S×T indicate the individual and interactive effects of straw amendment and tillage.Data are mean±SD (n=3).Different letters indicate significant differences among the treatments (P<0.05).ns, no significance; *, P<0.05; **, P<0.01; ***, P<0.001.
Microbial-community compositions were strongly affected by subsoil tillage and straw incorporation, and the concurrent changes in soil properties (Appendices A and B).The bacterial communities were primarily comprised of members of the phyla Proteobacteria (26.4%), Actinobacteriota (23.1%), Acidobacteriota (13.4%) and Chloroflexi (9.2%) (Appendix C).The phylum Proteobacteria was enriched in the 15–35 cm layer in IT and SIT.The abundance of the phylum Acidobacteriota was the lowest in SIT in both layers.The majority of the fungal sequences belonged to the phyla Ascomycota (66.0%), Mortierellomycota (22.1%) and Basidiomycota (10.2%).The relative abundances of the phylum Ascomycota in the 0–15 cm layer and the phylum Mortierellomycota in the 15–35 cm layer were higher in SIT than in the other treatments.The comparison of the fungal-community compositions in the PCoA identified a significant (P<0.01) separation between the effects of subsoil tillage and straw amendment (PERMANOVA,R2=0.401,P=0.001).
3.3.Microbial networks
The microbial networks integrating bacterial and fungal communities were constructed for the treatments with straw amendment and inversion tillage (Fig.2).Multiple topological properties of the patterns of microbial cooccurrence, i.e., the numbers of nodes and edges, the clustering coefficient, average connectivity and path length, generally varied greatly among the networks in the treatments (Fig.3; Appendix D).SCT generally had the highest node number, edge number and average connectivity, whereas SIT had the opposite trend.The trend of the clustering coefficient was SCT>SIT>CT>IT.The average path length was significantly longer for IT and SIT than for CT and SCT.

Fig.2 The microbial co-occurrence networks in conventional tillage (CT), straw incorporation with conventional tillage (SCT), inversion tillage (IT) and straw incorporation with inversion tillage (SIT).Large modules with ≥5 nodes are shown in various colors, and smaller modules are shown in gray.Details of the network topological attributes are listed in Appendices D and E.
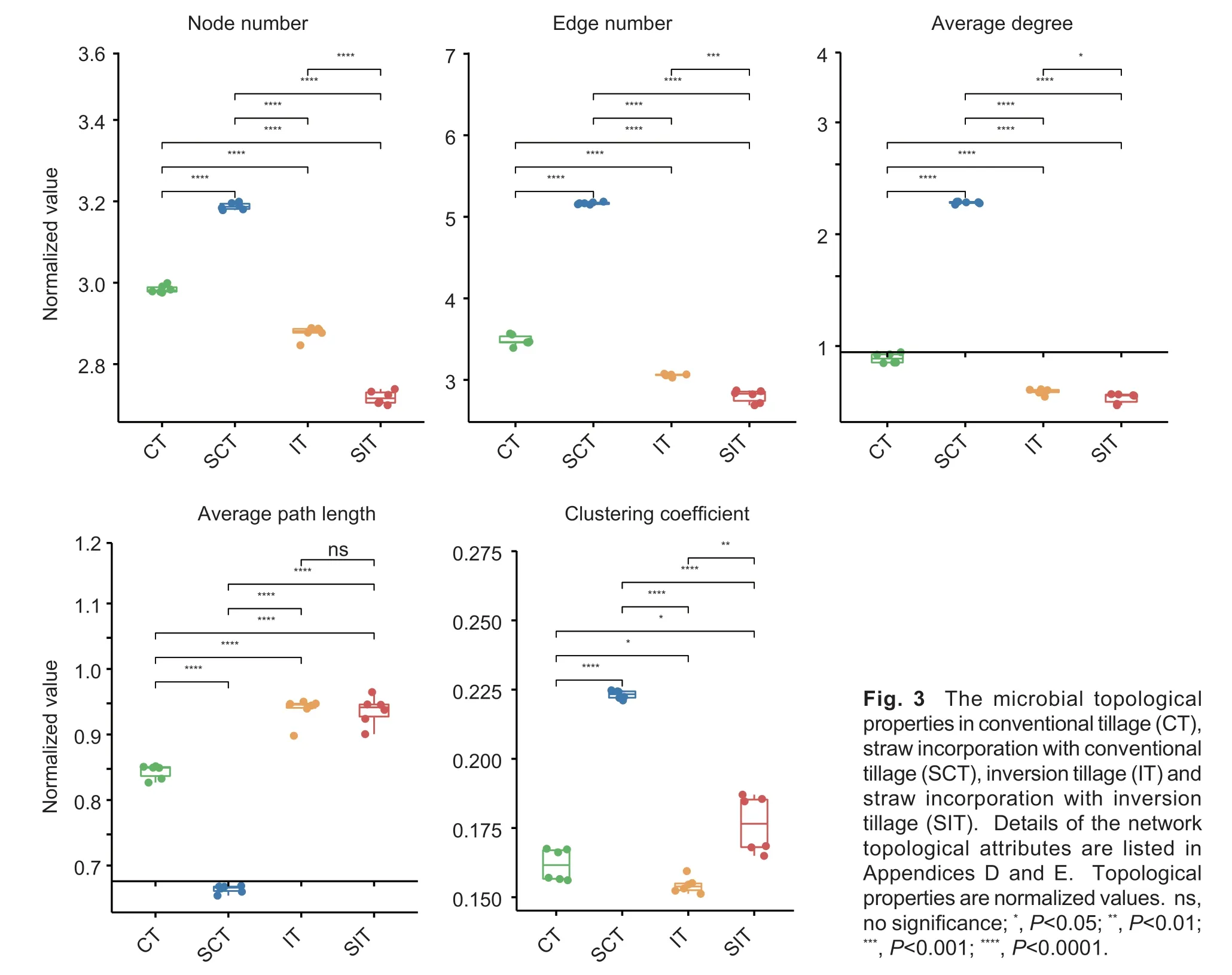
Fig.3 The microbial topological properties in conventional tillage (CT), straw incorporation with conventional tillage (SCT), inversion tillage (IT) and straw incorporation with inversion tillage (SIT).Details of the network topological attributes are listed in Appendices D and E.Topological properties are normalized values.ns, no significance; *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001.
The number of nodes in each treatment was calculated, and the results indicated that the proportion of fungal nodes was higher in SCT, and similar proportions of fungal and bacterial nodes were detected between IT and SIT (Fig.4).The node proportion forAscomycotawas the highest in SCT, followed by SIT and IT.SIT had higherBasidiomycotaand lower Mortierellomycota node proportions than the other treatments.The node proportions for Acidobacteriota and Actinobacteriota were notably lower in SCT than in the other treatments.SIT had the highest proportions of Actinobacteriota and Proteobacteria, and IT had the lowest proportion of Proteobacteria.The node proportion for Chloroflexi was lower in SIT and higher in CT than in the other treatments.IT had a higher proportion and SIT had a lower proportion of bacterial–fungal interactions than the other treatments (Fig.4; Appendix E).The proportion of negative bacterialfungal links was higher in SCT than CT, and higher in SIT than IT.
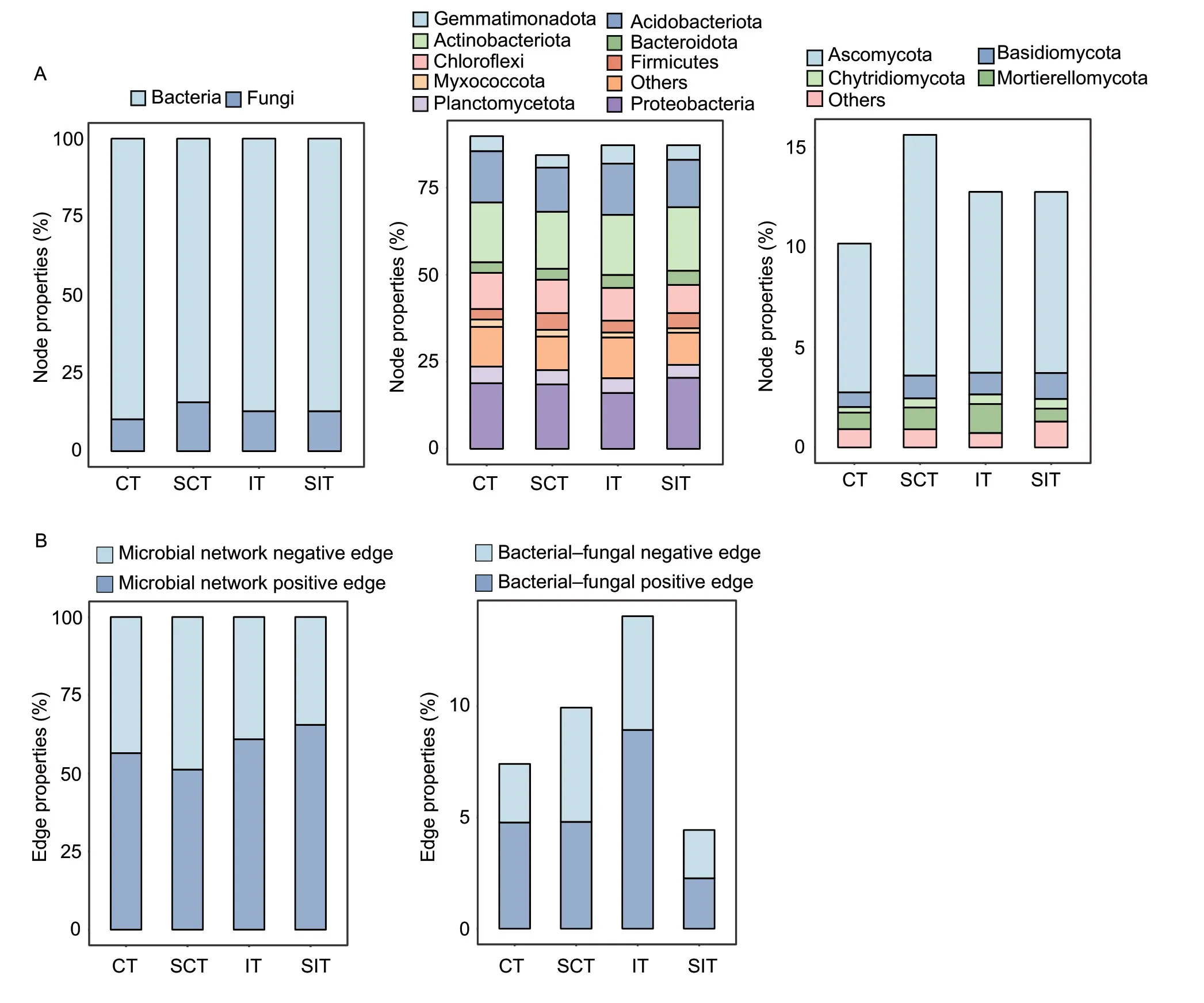
Fig.4 Node (A) and edge (B) properties of the microbial networks in conventional tillage (CT), straw incorporation with conventional tillage (SCT), inversion tillage (IT) and straw incorporation with inversion tillage (SIT).Node properties represent the percentage of dominant taxonomic nodes in the network.Edge properties represent the percentage of edges among species in the network.
3.4.Relationships between soil properties and microbial-community characteristics
The main microbial co-occurrence assemblies were determined for soil multi-nutrient cycling using RF analysis to identify the potential main drivers of the soil multi-nutrient cycling variables in the treatments (Fig.5).Average path length was the most important variable for predicting soil multi-nutrient cycling, followed by the clustering coefficient and node number.The biological contributions of the microbial co-occurrence assemblies to the soil properties were also evaluated.Not all microbial co-occurrence assemblies contributed equally to the various soil variables.For example, the clustering coefficient was the most important variable for predicting most soil properties, including the SOC, AN and AK concentrations (P<0.01).Other important variables forpredicting the soil properties were the node number for SOC and AK concentrations (P<0.01), and the average path length for pH and the SOC, TN, AN, AP and AK concentrations (P<0.05).
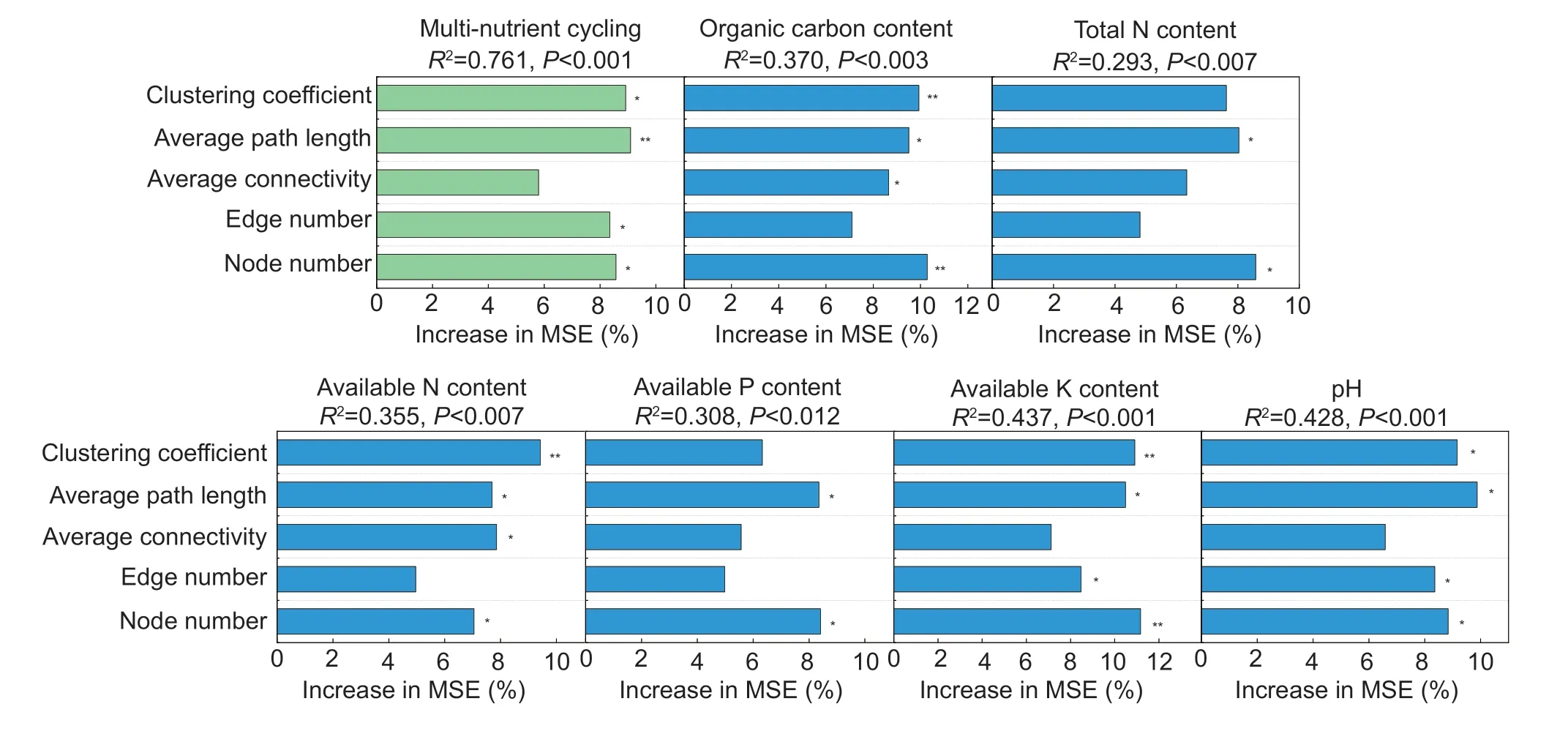
Fig.5 Potential drivers of variation in the soil multi-nutrient cycling and properties in the treatments.Percentage increases in the mean squared error (MSE) of the variables were used to estimate the importance of these predictors, and higher MSE values imply more important predictors.*, P<0.05; **, P<0.01.
4.Discussion
4.1.Inversion tillage and straw management alter the multi-nutrient cycling of the soil
This examination of four treatments with different tillage and straw management identified significant changes in soil multi-nutrient cycling in both the topsoil and subsoil.Inversion tillage and straw incorporation can improve soil structure and increase the availability of nutrients and soil functionality.The SOC concentration in the 0–15 cm soil layer was lower in IT than CT, as expected (Table 2).Inversion tillage mixes SOC-poor subsoil material into the topsoil, which initially decreases topsoil fertility by diluting the SOC (Alcantaraet al.2016).This practice could potentially allow the accumulation of SOC in the new topsoil and preserve buried topsoil carbon in the subsoil.The formation of a new SOC pool in the newly exposed topsoil after inversion tillage was faster than the loss of the buried pool.However, tillage with straw incorporation, such as the SIT treatment in this study, can favor the provision of fresh carbon and nutrients to the soil (Angers and Eriksen-Hamel 2008).Straw incorporation in this study increased the SOC concentration in the topsoil, with a larger increase in the plowed soil in SIT, confirming that incorporating straw can help reduce the loss of carbon in plowed soil.Inversion tillage also involves the incorporation of straw into the subsoil and can substantially improve the nutrient supply and the physical properties of the subsoil, as was found in this study.
The details of plowing, especially the depth of inversion, may be critically important for determining the persistence of buried carbon.For example, a study of Cambisol in Germany reported that traditional shallow plowing to a depth of <30 cm did not lead to the accumulation of buried carbon (Voset al.2019).This finding indicated that carbon accumulation at depth is not ubiquitous andunderscores the need to develop a better understanding of the mechanisms that could possibly protect SOC at depth.The results presented here also confirmed this, as the SOC concentration did not differ significantly between SCT and SIT, suggesting that greater straw incorporation inhibited SOC sequestration in the 0–15 cm layer in SCT due to the slow decomposition of residues when they were present in high quantity and with a very high C/N ratio.In this case, more time is required for C sequestration.Straw incorporation in the topsoil is also not conducive to deepening the plow layer (Liuet al.2021).
Other soil properties also were sensitive to specific combinations of the management practices tested here (tillage with straw incorporation) in the sandy loam.The concentrations of TN, AN, AP and AK followed a trend similar to the SOC concentration, i.e., inversion tillage with straw incorporation can facilitate the transport of soil nutrients to subsoil layers, as found in this study.Inversion tillage with straw incorporation may also improve other soil properties, such as pH and BD.Conventional tillage can compact the subsoil (Dimassiet al.2013).For example, Schjønning and Thomsen (2013) found that shallow tillage increased BD by 15% compared with plowing to depths of 14–18 cm, which is consistent with the higher BD in CT than in IT in both the 0–15 and 15–35 cm layers observed in this study.Straw residues can clog soil pores in sandy loam, leading to a large reduction in microporosity, which in turn can reduce soil compaction (Feizieneet al.2018).Soil pH and BD also trended similarly across the treatments in this study.An initial decrease in pH by soil dilution in the newly formed subsoil is to be expected.These subsoils may alleviate physical constraints and increase crop-rooting depth, thus stabilizing the soil environment.The development of appropriate agronomic practices is therefore critical for maximizing the potential benefits of inversion tillage and minimizing the potential risks.
4.2.Tillage and straw management influence microbial-network structure and its construction
The amendments reduced fungal-community diversity in the 0–15 and 15–35 cm layers in SCT and SIT relative to the corresponding unamended treatments (Fig.1).Such decreases in the indices after straw addition may have been due to the stimulation of the subset of the total community represented by copiotrophic populations and the strengthening role of the dominant species (Tardyet al.2015).The characteristics of fungal communities are more responsive than those of bacterial communities, and their contribution to the microbial communities is stimulated by the input of fresh organic matter, particularly in subsoil (Sanaullahet al.2016).The degradation of recalcitrant organic materials such as maize straw is known to favor fungi over bacteria (de Boeret al.2005).Recalcitrant soil organic matter is decomposed by fungi, while bacteria are more important in the mineralization of the labile fractions, such as humic acid (Marschneret al.2011).For greater access to well-protected organic matter than bacteria, fungi have the ability to search for heterogeneously distributed nutritional resources due to their fungal hyphae (de Boeret al.2005).Inversion tillage and straw amendment also stimulated microbial abundance.Bacterial and fungal abundances in the 15–35 cm layer were retained more in IT than in CT, probably due to the increase in the availability of nutrients in the subsoil and the greater ability of fungi to degrade buried topsoil carbon, whereas some bacteria may use the products released from organic substrates by fungal exoenzymes during decomposition (Boeret al.2005; Ballhausen and de Boer 2016).The positive effect of straw amendment on microbial abundance occurred regardless of whether the straw was applied to the soil surface or was incorporated into the subsoil.Therefore, incorporating crop straw, an important source of organic carbon, is an effective way to increase fungal abundance.
The primary changes in the topology and modular features of the co-occurrence networks can be used to map the differences in the structures of the microbial communities (Chenet al.2019).The numbers of network nodes and edges were significantly lower in IT than in CT (Fig.3).This finding was not due to the absence of some microbes, because CT and IT had similar microbial diversities in both layers.Microbial networks representing microbial interactions are more important than taxonomic diversity (Woodet al.2017).Previous studies have found that networks with more nodes and links have higher microbial complexities (Herren and Mcmahon 2018), and this decreasing trend of network complexity may be disrupted by agricultural disturbance (Banerjeeet al.2019).The results of this study thus indicated a possible decrease in microbial complexity after inversion tillage in the sandy loam soil.Favorable soil environments can also alleviate both competition and cooperation among microbial species (Maet al.2018), as in this study where the number of interactions among species was lower in SIT than in IT and the other treatments.However, the opposite was observed in SCT, where a large amount of incorporated straw in the topsoil led to unfavorable soil environments, e.g., a decrease in soil–water content and the further increase in porosity in the sandy soil.
Agricultural disturbances generally alter soil properties, which can affect the structure of microbial communities.Both IT and SIT decreased network complexity, fragmenting the networks into less-well connected or isolated clusters.Ecologically, these highly concentrated (lower average path length and higher average connectivity) but lesswell connected clusters (lower clustering coefficient) mean that ambient disturbances may be transmitted to the entire network more quickly (Barabasi and Oltvai 2004).The results presented here also indicated that average connectivity was significantly higher in the CT and SCT networks than in the IT and SIT networks, indicating efficient mutual effects among taxa and a less stable network structure induced by either CT or SCT.
Fungal–fungal interactions were much less common than bacterial–bacterial interactions in the network, possibly due to the low fungal richness in the soil.The number of interactions among species also varied greatly among the treatments, so the relative number of bacterial–fungal interactions was used to more accurately represent the changes among treatments.In contrast to CT, IT increased both the positive and negative bacterial–fungal interactions (Fig.4).Inversion tillage can increase physiological stress in fungi, such as the destruction of hyphae by tillage, thereby increasing the competition between some fungi and bacteria for organic substrates and nutrients.This possibility was supported by the reduction of positive and negative bacterial–fungal interactions in SIT with straw amendment.The relative number of bacterial–fungal interactions was higher in SCT than in CT, which was primarily indicated by negative correlations.Large amounts of straw in the topsoil would induce competition between bacteria and fungi for space, nutrients and water in response to habitat degradation.
The number of nodes used for network construction was significantly lower in SIT than in the other treatments, but the clustering coefficient was significantly higher than in CT and IT, suggesting that the microbial taxa could associate more closely with each other in centralized clusters.SCT had the highest clustering coefficient but the lowest degree of modularity, suggesting that the microbial communities tended to be functionally homogeneous.Specifically, higher proportions of nodes belonging to Acidobacteriota, Actinobacteriota, Proteobacteria and Chloroflexi were detected in different networks.The node proportions for Acidobacteriota and Actinobacteriota were notably lower in SCT than in the other treatments.Acidobacteriota are favored under oligotrophic conditions with low carbon availability (Jianget al.2017), and Actinobacteria are ecologically important in the degradation of recalcitrant polymers, such as cellulose and chitin (Renet al.2020).SIT had the highest proportion of Proteobacteria, and IT had the lowest proportion.Many Proteobacteria are considered copiotrophic, having relatively fast rates of growth and the ability to use various substrates (Zhanget al.2016).The node proportion for Chloroflexi was lower in SIT and higher in CT than in the other treatments.Chloroflexi can tolerate extreme soil environments (Neilsonet al.2012) and may compete with other organisms for labile carbon.The node proportion for Ascomycota was the highest in SCT, and SIT had the highest Mortierellomycota node proportion in the networks.Healthy soil harbors more diverse fungal communities, with more Mortierellomycota and fewer Ascomycota compared to highly diseased soil (Yuanet al.2020).The application of straw with inversion tillage may thus help to reduce the incidence of soil diseases and facilitate the construction and development of soil microbial communities.
4.3.The properties of microbial-network structure drive soil multi-nutrient cycling
Microbial communities are well-built complex ecological networks in which microbes collaborate and interact to maximize their ecological functions (Banerjeeet al.2019), which may help to regulate soil multi-nutrient cycling.The RF analysis in this study indicated that the average path length and the clustering coefficient were the main determinants of soil multi-nutrient cycling in the microbial networks (Fig.5).Increases in the average path length and the clustering coefficient of the microbial communities indicated a more discrete network structure and a higher modularity.The microbial networks were fragmented into concentrated clusters that were less-well connected or even isolated from each other, instead of remaining widely connected.Inversion tillage with straw amendment especially fragmented the widely connected microbial networks into highly centralized clusters.Either or both of two potential causes may account for these results.First, reduced OM complexity could be followed by the dominance of an opportunistic microbial population (Xueet al.2022).Straw amendment would simplify the composition of SOM as inputs, which would then decrease the diversity of soil organic compounds but increase the abundances of certain organic compounds.Microbial cooperation in decomposing organic compounds would thus decrease, leading to the isolation of the microbial networks.Second, inversion tillage tends to decrease soil heterogeneity, so the abundance of common taxa would increase in IT and SIT, thus forming highly connected modules.The numbers of connections within the clusters may increase, but the relationship between the clusters may decrease or even disappear.Karimiet al.(2019) hypothesized that these isolations indicate the self-sufficiency or independence of microbial communities from their neighbors, and some of these network clusters may even become associated with more than one functional group for improving soil multi-nutrient cycling (Cremeret al.2019).
Soil tillage with straw amendment could simply create a more active community of decomposers that break down recalcitrant compounds using specific enzymes to obtain required nutrients, and then the roots and decomposers could release exudates into the soil.If sufficient exudates are released, they could modify multiple ecosystem processes such as the cycling of C, N, P and K in agricultural ecosystems.In addition to the clustering coefficient and average path length, the number of nodes was another important variable for predicting soil properties (SOC, AN and AK concentrations) in this study.The higher numbers of nodes in the networks may have been due to the higher microbial diversity, which could enhance the rapid breakdown of straw, increasing the SOM concentration and fostering the activity of the microbial communities (Yeet al.2021).Similarly, OM needs to be degraded from complex and recalcitrant polymers into simpler and more labile monomers, a process requiring the cooperation of a large and diverse group of microorganisms.Soil nutrients are released by microbes during this process.Soils with high microbial diversities and stable ecological networks in agricultural ecosystems also have higher plant productivities and can decompose more organic matter (Fanet al.2021).
5.Conclusion
SIT is an effective option for improving the soil structure and multi-nutrient cycling in Hapli-Udic Cambisol, which would alleviate physical constraints, thus stabilizing the soil environment.The favorable habitat in SIT alleviated both competitive and mutualistic processes among microbial species and induced a more stable microbial network.In contrast, inversion tillage only reduced the physiological stress on microbes but enhanced the bacterial–fungal interactions competing for organic substrates and nutrients.The topological network properties of average path length and the clustering coefficient were the main determinants of soil multi-nutrient cycling.SIT formed a looser network structure but had highly centralized clusters due to its higher average path length and clustering coefficient for improving soil multi-nutrient cycling.In comparison with conventional tillage performed by a rototiller, the adoption of SIT is a ‘win–win’ strategy for sustainable and improved agricultural production and also for improving the physicochemical and biological properties of sandy loamy soil in northeastern China and similar agroecological systems elsewhere.
Acknowledgements
The study was funded by the National Key Research and Development Program of China (2022YFD1500100), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28070100), the National Natural Science Foundation of China (41807085) and the earmarked fund for China Agriculture Research System (CARS04).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2022.12.011
杂志排行
Journal of Integrative Agriculture的其它文章
- Herbicidal activity and biochemical characteristics of the botanical drupacine against Amaranthus retroflexus L.
- Developing a duplex ARMS-qPCR method to differentiate genotype l and ll African swine fever viruses based on their B646L genes
- The effects of maltodextrin/starch in soy protein isolate–wheat gluten on the thermal stability of high-moisture extrudates
- Elucidation of the structure, antioxidant, and interfacial properties of flaxseed proteins tailored by microwave treatment
- Effects of planting patterns plastic film mulching on soil temperature, moisture, functional bacteria and yield of winter wheat in the Loess Plateau of China
- The effects of co-utilizing green manure and rice straw on soil aggregates and soil carbon stability in a paddy soil in southern China
