Elucidation of the structure, antioxidant, and interfacial properties of flaxseed proteins tailored by microwave treatment
2023-05-08YUXiaoDUANZiqiangQINXiaopengZHUYingyingHUANGFenghongPENGDengfengBAIYanhongDENGQianchun
YU Xiao , DUAN Zi-qiang, QIN Xiao-peng, ZHU Ying-ying, HUANG Feng-hong PENG Deng-feng BAI Yan-hong#, DENG Qian-chun#
1 Oil Crops Research Institute, Chinese Academy of Agricultural Sciences/Hubei Key Laboratory of Lipid Chemistry and Nutrition/Key Laboratory of Oilseeds Processing, Ministry of Agriculture and Rural Affairs, Wuhan 430062, P.R.China
2 College of Food and Bioengineering, Zhengzhou University of Light Industry/Henan Key Laboratory of Cold Chain Food Quality and Safety Control, Zhengzhou 450002, P.R.China
Abstract The microwave treatment is commonly applied to flaxseed to release nutrients, inactivate enzymes, remove cyanogens, and intensify flavors.The current study aimed to explore the influences of microwave exposure on the antioxidant and interfacial properties of flaxseed protein isolates (FPI), focusing on the altering composition and molecular structure.The results showed that after microwave exposure (700 W, 1–5 min), more compact assembly of storage proteins and subsequent permeation by membrane fragments of oil bodies occurred for cold-pressing flaxseed flours.Moreover, the particle sizes of FPI was progressively reduced with the decrement ranged from 37.84 to 60.66% (P<0.05), whereas the zeta potential values initially decreased and then substantially recovered during 1–5 min of microwave exposure.The conformation unfolding, chain cross-linking, and depolymerization were sequentially induced for FPI based on the analysis of fluorescence emission spectra, secondary structure, and protein subunit profiles, thereby affecting the dispersion or aggregation properties between albumin and globulin fractions in FPI.Microwave exposure retained specific phenolic acids and superior in vitro antioxidant activities of FPI.The inferior gas–water interface absorption and the loose/porous assembly structure were observed for the foams prepared by FPI, concurrent with obviously shrinking foaming properties upon microwave exposure.Improving oil–water interface activities of FPI produced the emulsion droplets with descending sizes and dense interface coating, which were then mildly destabilized due to the lipid leakage and weakened rheological behavior with microwave exposure extended to 5 min.Our findings elucidated that microwave treatment could tailor the application functionality of protein fractions in flaxseed based on their in situ structural remodeling.
Keywords: microwave exposure, flaxseed protein isolates, antioxidant activities, interfacial properties, composition structure
1.Introduction
Flaxseed is typically consumed either as whole flaxseed, ground flaxseed, partially defatted flaxseed flours, flaxseed oil preparation, or plant milk alternative due to the excellent health benefits (Zhanget al.2022).Due to the increasing dietary preference for plant-based food, multiple health benefits have been gradually explored for flaxseed proteins and protein-derived peptides, as reviewed in recent publications (Wuet al.2019; Sridharet al.2022).As for the techno-functionality, lower water-holding capacity and reluctantly acceptable interfacial property had been observed for flaxseed proteins.Therefore, flaxseed proteins produced the emulsion with relatively smaller particle size but inferior physical stability (Karacaet al.2011; Alu’dattet al.2014).Besides the intrinsic composition structure, traditional high-temperature roasting and high-pressure hot-pressing have been generally implicated in flaxseed oil preparation in order to obtain appreciable oil yield and flavor (Zenget al.2022).Thus, the aforementioned process definitely led to the excessive thermal denaturation and irreversible aggregation of proteins in defatted flaxseed flours (Waszkowiaket al.2018).Indeed, the techno-functionality, particularly for the foaming and emulsifying properties of flaxseed proteins, heavily depends on the naturally occurring gum polysaccharides, owing to the increased solubility and hydrodynamic properties (Wanget al.2010; Kaushiket al.2015).Thus, it was still imperative to seek a preferable treatment method for flaxseed to concurrently achieve the promotion of oil quality and tailoring of techno-functionality for proteins based on theinsitustructural modifications (Rabetafikaet al.2011).
Microwave exposure has been gradually applied in the high-quality preparation of flaxseed oil, resulting in improved flavor and oxidative stability (Waszkowiaket al.2018; Suriet al.2020; Chenget al.2022).Early studies only demonstrated that the roasting of flaxseed (180–200°C, 8 min) led to cross-linking and depolymerization, especially for 13–19 kDa of protein subunits (Waszkowiaket al.2020).Structurally, the protein fractions in flaxseed mainly existed as protein bodies (PBs) in cotyledon cells, which contained approximately 80% of salt-soluble globulin (252–298 kDa) and 20% of water-soluble albumin (16–18 kDa) (Rabetafikaet al.2011).The neutral triacylglycerols in flaxseed primarily existed in the preemulsified lipid droplets defined as oil bodies (OBs) varying from 0.5 to 2.0 μm, which maintained individuality and presented intimate spatio–temporal relationships with PBs within cotyledon cells (Şenet al.2022).Indeed, the involvement of storage proteins in the interface remodeling of OBs directly revealed their promotion of interfacial activities following high-speed shearing of flaxseed exposed to microwaves (Yuet al.2022a).However, it was still undefined how the interfacial properties, as evidenced by foaming and emulsifying properties, changed for flaxseed proteins based on thein-situmulti-scale structural modifications upon microwave exposure.
Flaxseed was a rich source of plant lignans secoisolariciresinol (SECO, ~2 mg g–1), along with nonlignan phenolic acids (Denget al.2018).Increasing evidence has revealed that the free gallic, protocatechuic,p-hydroxybenzoic, caffeic, syringic, sinapic, ferulic andp-coumaric acids, were naturally migrated into flaxseed proteins, particularly for albumin fraction (Alu’dattet al.2014; Qinet al.2022).Although there is no direct evidence, the above free phenolic acids might be mainly localized in the interface of OBs and/or PBs in flaxseed based on the noncovalent interactions with membraneanchored proteins (Whiteet al.2006; Alu’dattet al.2013).An initial decrease and subsequent increase in total phenolic contents were observed for flaxseed oil when flaxseed was subjected to microwave exposure (180–540 W; 5–10 min), indicating the varying retention in defatted flaxseed flours (Suriet al.2020).More directly, upon microwave exposure, the favorable retention of phenolic compounds in storage proteins-embedded PBs occurred, which undoubtedly affected the specific techno-functionality, particularly for the antioxidant activities of flaxseed proteins (Yuet al.2022a).Indeed, when flaxseed was employed in thermal treatment under a microwave field, the potential changes in spatial conformation and subunit patterns might affect the interaction with specific free phenolic acids and migration behavior into flaxseed proteins during the extraction process (Kauret al.2017).
Based on this, the current study aimed to comparatively explore the antioxidant and the emulsifying and foaming properties of flaxseed proteins, focusing on their phenolic acid retention and gas– and oil–water interface behavior following microwave exposure.Then, the changing composition and molecular structure of protein fractions were elucidated upon microwave treatment in order to provide the theoretical basis for broadening its application in health food.
2.Materials and methods
2.1.Materials
Flaxseed was provided by Gansu Academy of Agricultural Sciences, China.Protein carbonyl assay kit, Folin-Ciocalteu reagent, 2,4,6-tris(2-pyridyl)-striazine (TPTZ, 99%), 2,2´-diphenyl-1-picrylhydrazyl radical (DPPH, 95%), gallic acid and rutin were purchased from Solarbio Life Sciences (Beijing, China).Sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) gel preparation kit was acquired from Beyotime Biotechnology (Shanghai, China).Vanillin, coumarin,p-coumaric, gallic, protocatechuic, vanillic, caffeic, syringic, sinapic, ferulic, sallcylic, and cinnamic acids were provided by Shanghai Yuanye Biological Technology Co., Ltd.(Shanghai, China).Other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd.(Beijing, China).
2.2.Microwave exposure and extraction of flaxseed protein isolates
As described by Yuet al.(2022b), the final water content of flaxseed was adjusted to 30–35% (w/w) and kept at constant temperature and relative humidity ((25±2)°C; (75±5)%) for 24 h.Then, 150 g of flaxseed was placed in eight Petri dishes (9.5 cm diameter) and exposed to microwave for 0, 1, 3, and 5 min at a fixed power of 700 W (CEM Mars-6, North Carolina, USA), respectively.After cold pressing by a screw expeller Komet CA59G (Komet, Germany), the partially defatted flaxseed flours were added inton-hexane at 1:5 (m/v), stirred for 2 h at room temperature, and centrifuged at 5 000×g for 10 min at 4°C.After the solvent evaporation, the absolutely defatted flaxseed flours were added into deionized water at 1:15 (w/v), adjusted the pH value of 8.5 with 1 mol L–1NaOH solution, and stirred at room temperature for 2 h.After centrifugation at 10 000×g for 15 min at 4°C, the supernatant was adjusted to the pH value of 4.0–4.2 with 0.5 mol L–1HCl solution to precipitate protein fractions.Then, the protein precipitation was dispersed into deionized water and adjusted the pH value of 6.8–7.0.After the freeze-drying, flaxseed protein isolates (FPI) were ground into powder and stored at 4°C for further analysis.
2.3.Analysis of the morphology of protein fractions in cold-pressing flaxseed and FPl
Block of cold-pressing flaxseed cake was fixed with 2.5% glutaraldehyde in cacodylate buffer (0.1 mol L–1, pH 7.2), washed three times with phosphate buffer solution, and post-fixed with 1% osmium tetroxide OsO4in phosphate buffer.The sample was rinsed twice into 0.1 mol L–1PBS (pH 7.2), dehydrated in progressive ethanol solutions (30, 50, 70, 90, and 100%; v/v), and then embedded in Spurr’s resin.Then, the embedded sample was cut using an ultra-microtome and stained with uranium acetate and lead citrate.Theinsitumorphology of protein fractions was obtained using the Hitachi H-7650 transmission electron microscope operated at 100 kV (Tokyo, Japan).The freeze-dried powder of FPI was fixed on the sample table with conductive adhesive and subjected to gold spraying at 10 mA for 60 s.The surface morphology of proteins was observed using the Hitachi Regulus 8100 scanning electron microscope (Tokyo, Japan) at 3 kV.
2.4.Analysis of physicochemical properties and composition structure of FPl
The mean particle size, size distribution, and zeta potential valuesThe freeze-dried powder of FPI was mixed into 50 mmol L–1phosphate buffer solution (1.0 mg mL–1, pH 6.8) and stirred at room temperature for 2 h.After dilution with distilled water at a ratio of 1:100 (w/w),the mean particle size, size distribution, and zeta potential values of FPI dispersion were measured using the Zetasizer Nano-ZS90 (Malvern Instruments Ltd., Worcestershire, UK) at 25°C with the refractive index of water and particles of 1.33 and 1.47, respectively (Perreaultet al.2017).
The endogenous fluorescence analysisThe spatial conformation of FPI was assessed by the changes in the endogenous fluorescence intensity.In brief, FPI solution (0.1 mg mL–1) was performed on a RF-7000 fluorescence spectrophotometer (Schimadzu, Kyoto, Japan) with the excitation wavelength and scanning emission spectrum range at 280 and 300–500 nm, respectively.
The SDS-PAGE analysisAfter the quantification using a BCA protein assay kit, equal amounts of protein extracts were subjected to heat denaturation in loading buffer (4:1, v/v) and then separated by 10% SDS-PAGE.The gels were stained with 0.1% (w/v) of Coomassie brilliant blue solution under gentle shaking, washed with destaining solution, and imaged on a ChemiDoc XRS+System (Bio-Rad, Hercules, CA).
The Fourier transform-infrared (FT-lR)and circular dichroism (CD) spectraThe freeze-dried powder sample of FPI was fully mixed into a potassium bromide pellet and recorded using FT-IR (Vertex 70, Bruker, Germany) in the wavelength range 400–4 000 cm–1.The CD spectra of FPI solution (0.1 mg mL–1) were collected from a Chirascan-plus spectrophotometer (Applied Photophysics Ltd., Surrey, UK) in the far-UV spectra between 180 and 260 nm at 25°C.The contents of α-helix, β-strand, β-turns, and random coil were estimated using the Deconvolution program (CDNN, version 2.1).
The free sulfhydryl group (–SH) and disulfide bonds (S–S) contentsAs described by Yu Xet al.(2020), the freeze-dried powder samples of FPI (30 mg) were dissolved into 10 mL of Tris-Gly buffer.Then, 0.1 mL of 5,5´-dithio-bis-2-nitrobenzoic acid in Tris-Gly buffer (4 mg mL–1) was added and violently mixed for 30 s.After reaction for 1 h in the dark at 25°C, the mixture was centrifuged at 5 000×g for 10 min.The absorbance was measured at 412 nm against the Tris-Gly buffer as a blank.As for the contents of total sulfhydryl group (–SH) contents, 30 mg of FPI samples were suspended in 10 mL of Tris-Gly with 10 mol L–1urea.Then, 0.03 mL of mercaptoethanol was added into 0.5 mL of this solution and incubated for 1 h at 25°C.Finally, three 5 mL of 12% (v/v) trichloroacetic acid was added, stranded for 1 h, and centrifuged at 5 000×g for 10 min.The residues were mixed into 3 mL of Tris-Gly with 8 mol L–1urea and 0.04 mL of DTNB and centrifuged at 5 000×g for 10 min after incubation for 30 min in dark.The absorbance was determined at 412 nm against reagent blank.The –SH and S–S contents were calculated according to the following equation:
Where A412was the absorbance at 412 nm, D was the dilution factor, and C was the protein concentration (mg mL–1).
2.5.Analysis of phenolic compounds, antioxidant capacities, and oxidative status of FPl
As previously described by Nieet al.(2022), FPI was extracted with 3 mL of methanol aqueous solution (80%, v/v) by vortex mixing and subsequent ultrasonic bath and centrifugated at 5 000×g for 10 min at 4°C.The contents of total phenolic acids and flavonoids in the extracts were determined using the Folin-Ciocalteau and aluminium nitrate assays, respectively.The results were expressed as mg gallic acid and rutin equivalents (RE) per 100 g protein samples, respectively.The free phenolic acids in the extracts were identified on an Agilent 1290 ultrahigh performance liquid chromatography (UPLC) with a photo-diode array detector (PDA)(California, USA) and ACQUITY UPLC BEH Shield RP18 column (2.1 mm ×100 mm, 1.7 μm, Waters, Milford, USA).The mobile phase consisted of the aqueous solutions of 2% acetic acid (A) and methanol (B), respectively.The gradient elution was modified as follows: 0–7.4 min, 95–75% A; 7.4–10.07 min, 75–71% A; 10.07–16.73 min, 71–64% A; 16.73–23.4 min, 64–55% A; 23.4–25.4 min, 55–35% A; 25.4–27.4 min, 35–95% A; 27.4–30.4 min, 95% A; 30.4−35 min, 95% A.The flow rate was 0.21 mL min–1, and the injection volume was 3 μL.The quantitative analysis of free phenolic acids was achieved based on the external standard method.
The antioxidant activities of FPI were performed by ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) methods.For the FRAP assay, 700 μL of freshly prepared FRAP reagent was incubated to 37°C for 10 min, and then absolutely mixed with 100 μL of the extracts.After incubation for 20 min at 37°C, the absorbance was recorded at 590 nm against a reagent blank, and the results were presented as mmol FeSO4·7H2O per 100 g.For the DPPH assay, 200 μL of the extracts were added to 800 μL of DPPH in ethanol solution (0.5 mmol L–1).After mixing vigorously for 10 s, the mixture was transferred into a quartz capillary tube, and the spin adduct was measured on an ER 200D-SRC ESR spectrometer (Bruker, Germany) upon a 0–2 min reaction time.The experimental parameters were set as follows: magnetic field, 3 487 Gs; power, 5.00 mW; modulation frequency, 86 kHz; amplitude, 1.80 Gs; and sweep time, 20.97 s×4 times.The ascorbic acid was used as a standard positive control.The DPPH radical scavenging activity (RSA)=100×(AC–AS)/AS, in which theACandASwere the double integral values of the ESR spectrogram for samples with and without protein fractions, respectively.
The contents of protein carbonyls in FPI were determined using the 2,4-dinitrophenylhydrazine (DNPH)-based spectrophotometric assay according to the manufacturer’s instructions (Solarbio Life Science, China).Briefly, 120 μL of DNPH solution was added to 60 μL of FPI solution (0.25%, w/v).After 1 h of incubation in the dark at 37°C, 150 μL of the trichloroacetic acid solution was added and then incubated at 4°C for 5 min.After centrifugation at 12 000×g for 15 min, the protein pellets were collected and washed three times with 300 μL of ethanol–ethyl acetate mixture (1:1, v/v).The final pellets were dissolved into 300 μL of guanidine hydrochloride (6.0 mol L–1), and the absorbance was recorded at 370 nm using a UV–Vis spectrophotometer (Yu Xet al.2020).
2.6.Analysis of foaming properties for FPl focused on the gas–water interfacial behavior
The foaming ability and stabilityIn brief, 20 mL of FPI dispersion (1.0%, w/v) was accurately added into a graduated container and homogenized at 10 000 r min–1for 2 min.The foaming capacity (FC, %)=100×(V0/VL), and foaming stability (FS, %)=100×(V1/VL).Where, VLwas the volume of non-shearing protein dispersion, 20 mL; V0was the volume of foams immediately after shearing; V1was the volume of foams 30 min after shearing.
The morphological structure of foamsApproximately 20 μL of foams were deposited on a glass slide with a groove and coverslip and observed using an optical microscope (Scope A1, Carl Zeiss, Germany) equipped with a CCD camera (Jenoptik C14).The high-resolution field emission scanning electron microscope (FE-SEM) (Regulus 8100, Hitachi, Tokyo, Japan), equipped with a cryo preparation system (PP3010T, Quorum, UK) was used to study the micromorphology of foams.In brief, 2.0 μL of foams were placed on rivets mounted on a stub, frozen in liquid nitrogen slush, and then transferred into the cryo-preparation chamber.The frozen samples were fractured with a blade and carefully etched at −80°C and 1.3×10−6mbar for 8 min, followed by coating with platinum for 60 s at 5 mA.The samples were observed using FE-SEM at an accelerated voltage of 1 kV with a magnification of 1 000×.
The gas–water interfacial activities and absorption capacities of FPIThe time-dependent air–water interfacial pressure (π) of FPI was determined with a K100 tensiometer (Kruss, Germany) using the Wilhelmy plate technique.In brief, the platinum plate was immersed in 40 mL of protein dispersion (1.0%, w/v)to a depth of 2 mm.The time-dependent π values were continuously recorded for 3 600 s at 25°C.Then, the percentage of adsorbed proteins at the air–water interface was determined for FPI dispersion.Upon highspeed shearing, the subnatant was carefully collected using a syringe.Then, the protein contents in initial dispersion (C0) and subnatant (CS) were determined using the BCA method, respectively.The adsorbed proteins (AP)=100×(C0−CS)/C0.
2.7.Analysis of emulsifying properties of FPl focused on the oil–water interfacial behavior
Preparation of oil–water emulsionsThe FPI dispersion (1.5%, w/v) was prepared with 50 mmol L–1of phosphate buffer solution (pH 6.8) by continuous stirring for 2 h at 25°C and placed in 50 mL centrifuge tubes.The course emulsions were prepared by mixing 10% (w/w) of flaxseed oil into protein dispersion using a high-speed dispenser at 10 000 r min–1for 2 min.
The physicochemical properties and physical stability of emulsionsThe mean particle sizes (μm) of emulsions were evaluated by dynamic light scattering (DLS) using a particle size analyzer (LS 230, Beckman Coulter, USA).The zeta potential values were assessed by electrophoretic light scattering (ELS)viaa ZetaSizer Nano-ZS90 (Malvern Instruments Ltd., Worcestershire, UK).The emulsions were dispersed into 5 mmol L–1PBS (pH 7.0) at a dilution ratio of 1:100 and performed at 25°C.The refractive indices of flaxseed oil and dispersant used were set at 1.476 and 1.330, respectively.The physical stability of emulsions was determined by the multiple light scattering (MLS) measurement (Turbiscan LAB, Formulaction, France).In brief, the freshly prepared emulsions were transferred to a cylindrical glass cell.Then, the curves of transmitted and backscattered light intensity versus the scanning height over a whole length of 40 mm were scanned at 25°C for 30 min.The delta backscattering (ΔBS) was developed to determine the emulsion stability index (ESI) by backscattering light detection.Turbiscan stability index (TSI) was calculated from the BS of near-infrared light as a function of height.
The rheological behavior and interfacial microstructure of emulsionsThe rheological behavior of emulsions was measured using a Discovery Series Hybrid Rheometer (Discovery HR-1, TA, USA).The complex viscosity measurements of the emulsions were carried out with different shear rates ranging from 0.01 to 10 Hz at 25°C.The data of viscosity, storage modulus (G´), and loss modulus (G´´) were obtained directly from the instrumental software.For the analysis of the microstructure of emulsions, a drop of the sample was frozen in liquid nitrogen, transferred into the chamber, cut into the cross section, and then sublimated at –80°C and 1.3×10–6mbar for 8 min using the PP3010T Cyro-SEM Preparation System (Quorum, UK).The morphological structure of emulsions was observed using FE-SEM (Regulus 8100, Hitachi, Tokyo, Japan) at an accelerated voltage of 1 kV with the magnification of 2 000× and 6 000×, respectively.The oil–water interfacial activities of FPlThe oil–water interface tension (π) of FPI (1.5%, w/v) was measured by a K100 surface tensiometer (Kruss, Germany) using the Wilhelmy plate technique.In brief, the platinum plate (19.9 mm×10 mm×0.2 mm) was immersed in 14 g of FPI dispersion to a depth of 3 mm.Then, an interface between the aqueous phase and oil phase was created under static loading by carefully pipetting 40 g of flaxseed oil.The test was conducted over 3 600 s, and the temperature was maintained at 25°C throughout the duration of the test.
2.8.Statistical analysis
All experiments were conducted in triplicate.The data were presented as mean±standard deviation (SD).The One-way analysis of variance (ANOVA) and Duncan’s multiple range test were performed using SPSS Software (version 21.0 for Windows, IBM Inc., Chicago, IL, USA).Significant differences were determined at the 0.05 level (P<0.05).
3.Results and discussion
3.1.Effects of microwave exposure on the aggregate morphology of protein fractions and physiochemical properties of FPl
As previously described, the protein fractions were directly assembled within PBs in fully mature flaxseed (Zienkiewiczet al.2014).As shown in Fig.1-A, the protein fractions were loosely accumulated in cotyledon cells of native flaxseed due to the incomplete structure destruction for PBs following the cold-pressing process.Moreover, the membrane fragments of the oleosin–phospholipid complex from OBs were definitely unable to penetrate through the plasmodesmata (pores) in cotyledon cell walls, thereby mainly locating around the inner walls of cotyledon cells in native flaxseed.In particular, obvious incompatibility between the membrane fragments and storage proteins was observed for the untreated defatted flaxseed flours.Following the microwave exposure, the assembly of storage proteins in defatted flaxseed flours tended to be more compact, which could be explained by theinsitustructural collapse of PBs (Yuet al.2022b).Notably, the partial permeation of lipophilic OBs-associated oleosins into storage proteins occurred, indicating their preferentialinsituspatial contacts in flaxseed.Additionally, more serious deformation of cotyledon cell walls was induced when flaxseed was subjected to microwave exposure, including mild breakage, uneven thickness, and roughness.These results were comparable to the findings reported by Yu G Wet al.(2020).The weakly fragmented cotyledon cell walls further supported the improved mechanical pressing performance and subsequent higher yields of flaxseed oil (Suriet al.2020).Similarly, the loss of sub-cellular OBs and PBs due to the cytoplasmic network disruption was also observed for hot air and infrared processing of hazelnuts (Lambertiet al.2021).Undoubtedly, the altering cell wall architecture and discrepant accumulation of protein components in flaxseed were related to the explosive gasification and extracellular leakage of water molecules upon microwave thermal mass transfer (Koubaaet al.2016).
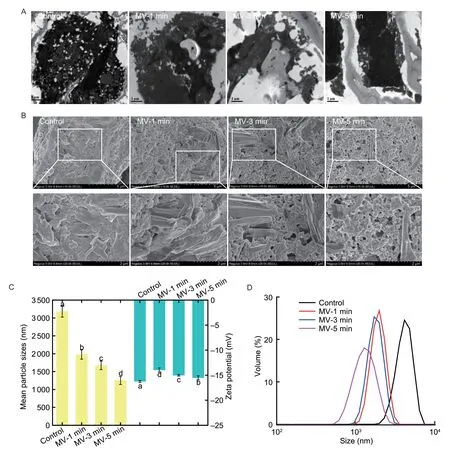
Fig.1 Changes in the morphological structure and physicochemical properties of protein fractions following microwave exposure. A, cold-pressing flaxseed flour as examined by transmission electron microscope (TEM) imaging; scale bars=1 μm.B, morphology of freeze-dried flaxseed protein isolates (FPI) as examined by scanning electron microscope (SEM) imaging, scale bars=5 and 2 μm, respectively.C, mean particle sizes and zeta potential values in aqueous solution.Data are mean±SD (n=3).Different letters in same index indicated significant differences at the P<0.05 level.D, particle size distribution aqueous solution.MV-1 min, MV-3 min and MV-5 min, indicate microwave exposure for 1, 3 and 5 min, respectively.
As depicted in Fig.1-B, the native FPI was mainly composed of aggregates of globulin with an almost spherical shape, which coexisted with the relatively lower amounts of albumin with a lamellar structure.This was almost comparable to those prepared from dehulled flaxseed (Qinet al.2022) but particularly different from the irregular lamellar and/or long tubular structure of aqueous protein-gum polysaccharide extracts from defatted flaxseed (Nieet al.2022).Upon microwave exposure, the albumin fraction in FPI was inclined to the aggregative distribution with a thin and/or curling lamellar structure, whereas the globulin fraction displayed a more explicit spherical shape.In particular, the lamellar structure of albumin fraction in FPI was fractured into extremely differing dimensions and randomly scattered in loosely packed globulin particles with microwave exposure time extended to 5 min.Accompanying by the morphological deformation of PBs in flaxseed upon microwave exposure, the chain stretching and depolymerization and wrecks of multi-subunit cross-linking might be progressively induced for albumin and globulin fraction, respectively, thus leading to different aggregates following alkali extraction and acid precipitation from flaxseed (Waszkowiaket al.2018).
The influences of microwave exposure on the physicochemical properties of FPI were assessed by the average particle diameter and size distribution.As shown in Fig.1-C and D, the native FPI possessed a unimodal particle distribution ranging from 1 990.12 to 6 438.51 nm, with an average particle size of 3 183.33 nm.With microwave exposure increased from 1 to 3 min, FPI initially experienced a great reduction in particle sizes (–15.31%;P<0.05), which then maintained the major peak at 1 675.67 nm.When microwave exposure was further extended to 5 min, the protein particles of FPI were partially broken down with a wider range (396.06–955.41 nm).Accordingly, the smaller sizes and more heterogenous particle distribution could be explained by the changed protein solubility and aggregation in the aqueous solution.The zeta potential values were intimately related to the net surface charge of protein particles in the aqueous phase (Kaushiket al.2015).The absolute zeta potential of FPI obviously decreased and then fully recovered to a level similar to untreated samples during 1–5 min of microwave exposure.Indeed, relatively higher levels of negatively charged amino acids were observed for albumin in comparison to that of globulin fraction in native flaxseed proteins (Nwachukwu and Aluko 2018).Upon microwave exposure, the changed dispersion or aggregation properties of FPI in the aqueous solution might result in the rearrangement of charged amino acid residues at the surface of albumin and globulin fractions accompanied by their varying intra- and inter-molecular interactions.This could be elucidated by theinsitustructural modifications of protein inclusions within PBs in flaxseed when subjected to microwave exposure (Yuet al.2022b).
3.2.Effects of microwave exposure on the multi-level composition structure of FPl
The altering spatial conformation of FPI was first evaluated by the intrinsic fluorescence emission spectra dependent on the environments of aromatic amino acids, giving information about the tertiary structure of protein molecules.As depicted in Fig.2-A, the native FPI possessed the maximum fluorescence emission (λmax) at the wavelength greater than 330 nm (λmax=338.4 nm), revealing the location of tryptophan (Trp) in the relatively polar environment.On the contrary, the maximum fluorescence intensity (FImax) was gradually quenched, with no obvious changes in λmaxas the microwave exposure extended from 1 to 3 min.Finally, an explosive collapse of FImaxwas observed for FPI concomitant with a 1.6-nm of blue-shift of λmaxwhen the microwave exposure time was extended to 5 min.Indeed, the gradually unfolded spatial conformation of FPI could lead to the exposure of Trp initially buried inside the hydrophobic pockets to the FPI surface in close proximity to water (Yu Xet al.2020).Moreover, the higher tendency for migration of gum polysaccharides and phenolic compounds might largely embed the Trp residues exposed to the protein surface due to the stronger interactions between them following microwave exposure (Nieet al.2022).Then, SDS-PAGE analysis was further conducted for FPI to check if the changes in intrinsic fluorescence properties could be related to the fracture of protein subunits except for the protein unfolding.As illustrated in Fig.2-B, the FPI contained a high intensity of bands at MW of 17, 20, and 33 kDa, followed by the light bands at MW of 25 and 43 kDa, respectively.No obvious change in protein subunit profiles was achieved following 1–3 min of microwave exposure.However, the intensity of the protein band at MW of 43 kDa was mildly decreased with the concomitant elevation of bands at low MW (<25 kDa) after 5 min of microwave treatment of flaxseed.As previously reported, prominent and linear decreases in band intensity at 40–60 and 22–30 kDa were identified for protein fractions from whole flaxseed flours upon microwave heating at 860 W for 4–8 min (Houet al.2021).These findings indicated that the mild decrease in intrinsic fluorescence of FPI might be merely contributed by the conformation deformation of proteins due to the heatinduced dissociation of intermolecular disulfide bonds.Meanwhile, the subunit depolymerization and subsequent conformation remodeling were also devoted to the sharp decline of intrinsic fluorescence intensities of FPI.
As expected, the nonlinear changes in proteinconformation were definitely accompanied by the altering intermolecular interactions and secondary structure backbone of FPI.As shown in Fig.2-C, the free –SH contents in FPI was 15.69 μmol g–1, which linearly decreased with the microwave exposure extended from 1 to 5 min (–11.82, –34.95, and –46.17%;P<0.05).As possibly caused by the –SH oxidation, the S–S contents of FPI successively increased by 5.53, 13.32, and 41.62% (P<0.05), respectively.Similar findings were also observed for thermal induced soybean protein fractions (90°C for 30 min) (Juet al.2023).It was found that the albumin fraction contained more hydrophilic amino acids located on the molecular surface, which might result in more easily oxidized sulfhydryl groups and the formation of S–S between protein molecules upon microwaveinduced thermal structural changes of PBs in flaxseed (Qinet al.2022; Yuet al.2022b).As shown in Fig.2-D, a detectable absorption band at 3 384.9 cm–1(OH band stretching) in the FTIR spectra of native FPI was revealed as an amide I band that occurred due to the C=O bond stretching vibrations.The native FPI possessed the amide I and II bands, as labeled between the ranges of 1 712–1 580 cm–1and 1 580–1 482 cm–1, respectively, possibly because of the carbonyl group (C=O) stretching and N–H bending vibrations.This was almost consistent with the previous study as reported by Khanet al.(2015).Then, the amide II band of FPI shifted to smaller wavelengths with peak spectra of 1 537.2 cm–1when the microwave exposure ranged from 1 to 5 min, which could be explained due to the breakage of amino groups of protein fractions.As obtained from the CD spectra in Fig.2-E, the untreated FPI had 22.92% of α-helix, 26.34% of β-sheet, 17.72% of β-turn, and 33.02% of the random coil.This data were inconsistent with the previous findings, which could be explained by the discrepant flaxseed variety, sample matrix, and extraction methods (Kaushiket al.2015).With the extending microwave exposure from 1 to 3 min, the content of α-helix in FPI was obviously decreased (–20.44%;P<0.05), which was parallel to the ascending contents of β-sheet (+7.96%;P<0.05) and random coil (+4.34%).These implied that the molecular structure of FPI was transformed from an ordered to a disordered state, which could partially explain the loosely packed morphological structure as evidenced by SEM imaging.However, when microwave exposure was further prolonged for 5 min, the content of α-helix in FPI displayed a slight increase (+9.71%;P<0.05), indicating the partial reversion of ordered structure for protein backbone structure.In comparison, no apparent alteration in the content of β-turn was achieved for FPI during the microwave exposure.As previously demonstrated, the secondary structure of proteins was mainly stabilized by hydrogen bonding and electrostatic interactions (Choet al.2008).Consequently, the microwave treatment of flaxseed definitely caused the disruption of these interactions, leading to changes in the secondary structure of FPI.
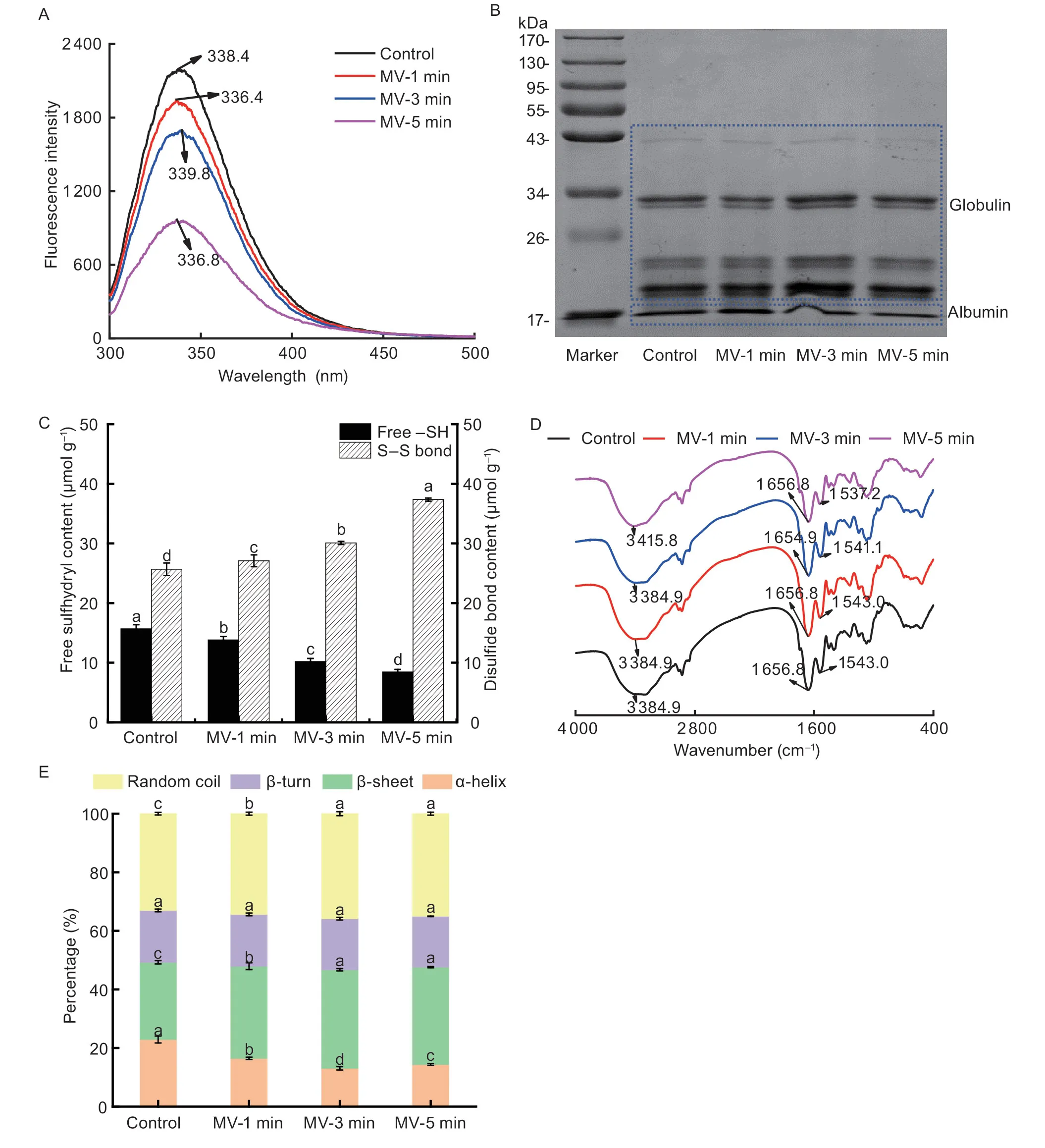
Fig.2 Changes in the composition structure of flaxseed protein isolates (FPI) following microwave exposure.A, endogenous fluorescence intensities.B, sodium dodecylsulfatepolyacrylamide gel electrophoresis (SDS-PAGE) analysis.C, free –SH and S–S contents.D, Fourier transform-infrared spectra (FT-IR).E, secondary structure drawn from circular dichroism (CD) spectra.MV-1 min, MV-3 min and MV-5 min, indicate microwave exposure for 1, 3 and 5 min, respectively.Data in Fig.2-C and E are mean±SD (n=3).Different letters in same index indicated significant differences at the P<0.05 level.
3.3.Effects of microwave exposure on the retention of phenolic compounds and antioxidant capacities and the oxidative state of FPl
As shown in Table 1, the total phenolic content in untreated FPI reached 303.85 mg 100 g–1.The microwave exposure for 1–3 min just led to a mild increase in total phenolic acids of FPI, which eventually displayed great promotion with the microwave exposure further extended to 5 min (+99.36%;P<0.05).Relatively, the accumulation of flavonoids in native FPI was extremely inferior, which could be explained by the extremely low intrinsic amount in flaxseed.Upon microwave exposure for 1–5 min, the flavonoids in FPI exhibited a similar upward trend (+78.36%;P<0.05).Indeed, the beneficial retention of phenolic compounds in FPI might be attributed to the more effective release of lignans from the sclerite layer in the seed coat, as well as the free phenolic acids from PBs or OBs in cotyledon cells following theirinsitustructural deformation (Yuet al.2022a).The FRAP values and DPPH radical scavenging capacity of FPI were 85.77 FeSO4·7H2O per 100 g and 21.23%, respectively.With the extending microwave treatment of flaxseed from 1 to 5 min, the FRAP values and DPPH radical scavenging capacity of FPI linearly increased to almost twice as high as that of the untreated samples (P<0.05).This was in agreement with the changing trends of total phenolic acids and flavonoids in FPI, particularly for the free phenolic extracts, which largely determined the antioxidant capacities of flaxseed proteins (Alu’dattet al.2016).The native FPI contained relatively low amounts of protein carbonyls with (2.11 μmol 100 g–1), which was remarkably lower when compared to the values of flaxseed proteins (Yu Xet al.2020).Following microwave exposure for 1 min, the levels of protein carbonyls in FPI apparently ascended (+27.49%;P<0.05).With the microwave exposure extended from 3 to 5 min, the generation of protein carbonyls was substantially depressed and even lower than that of the control sample (–54.98%, –52.61%;P<0.05).The relatively higher contents of sulphurcontaining amino acids in globulin than that of albumin might largely contribute to the formation of protein carbonyls in FPI (Qinet al.2022).Indeed, the shrinking protein carbonyls in FPI might be attributed to the deficiency of reactive carbonyl species derived from lipid peroxidation, which was subjected to thermal volatilizationand degradation with the prolonging microwave exposure (Yuet al.2022a).
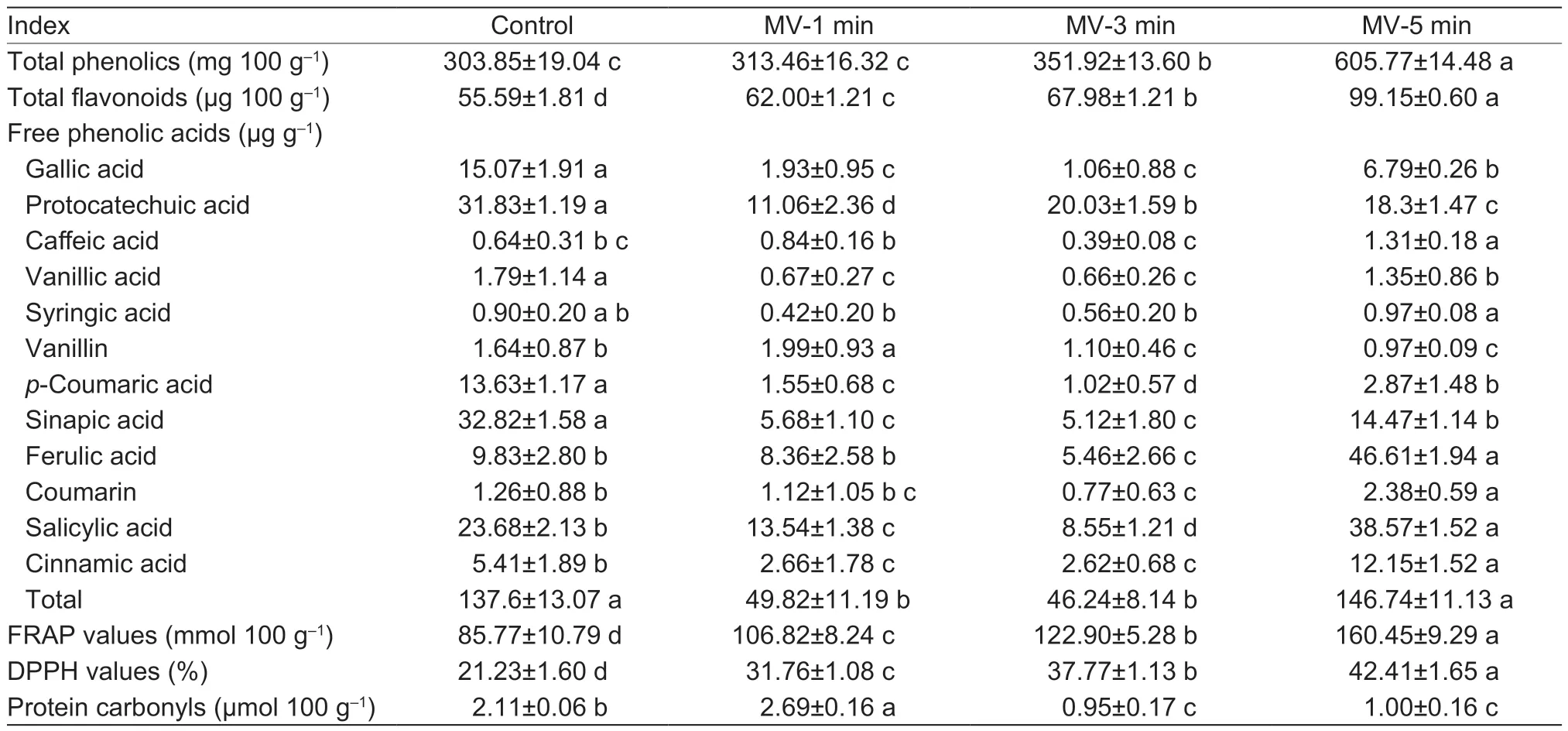
Table 1 Changes in the phenolic compounds, antioxidant capacities, and oxidative status of flaxseed protein isolates (FPI) following microwave exposure1)
The free sinapic acid, protocatechuic acid, salicylic acid, gallic acid,p-coumaric acid, and ferulic acid were mainly identified in native FPI, accounting for 23.70, 22.98, 17.10, 10.88, 9.84, and 7.10% of total amounts (138.48 mg 100 g–1), respectively.Meanwhile, small amounts of free cinnamic acid, vanillic acid, vanillin, coumarin, syringic acid, and caffeic acid were also detected, with a cumulative proportion of 7.11% in FPI.As previously reported, the free phenolic acids were identified in the supernatants remaining after extraction of protein isolates and then selectively migrated into FPI following the isoelectric point precipitation (Alu’dattet al.2016).Thus, the presence of reversible protein–phenolic interactions in protein isolates from flaxseed, usually involved in hydrogen bonding, hydrophobic bonding, and van der Waals forces, was intimately related to the type and structure of phenolic acids (Ozdalet al.2013).Indeed, a much more favorable retention of phenolic acids was observed for the albumin compared with that of globulin from flaxseed, which was attributed to the single subunit profile, lower molecular weight, and natively stretched spatial conformation and favorable noncovalent interaction between them (Nwachukwu and Aluko 2018).With microwave exposure extended from 1 to 3 min, the retention of total free phenolic acids into FPI was greatly suppressed (–65.71%;P<0.05), particularly for sinapic acid, salicylic acid, gallic acid, andp-coumaric acid.However, when microwave exposure time reached 5 min, the contents of total free phenolic acids in FPI increased to a level comparable to that of untreated sample.Most notably, intermolecular transformation or more preferential migration of free phenolic acids occurred with the substantially increased proportion of salicylic acid and ferulic acid into FPI (P<0.05) upon microwave exposure.Accordingly, the eventual chain depolymerization and concomitant tendency towards a more unfolded structure of FPI might be favorable to produce stronger noncovalent interactions of albumin/globulin fractions with free phenolic acids (Alu’dattet al.2013; Qinet al.2022).
3.4.Effects of microwave exposure on the foaming properties of FPl implication of gas–water inter- facial behavior
The foaming properties, including foaming ability, foaming stability, and foam morphological structure formulated by FPI were comparatively analyzed before and after microwave exposure.As shown in Fig.3-A–C, the native FPI dispersion exhibited relatively desirable foaming properties upon high-speed shearing, which was in line with the results described by Lanet al.(2020).Indeed, albumin possessed a relatively superior foam formation capacity than globulin fraction in FPI, but the latter could be more easily tailored (Juodeikieneet al.2020).When flaxseed was subjected to microwave exposure from 1 to 5 min, the foaming abilities of FPI were linearly weakened, as evidenced by the shrinking foam volumes (–26.63, –50.01, and –56.49%;P<0.05).Meanwhile, the foaming stability prepared by FPI also displayed a steep drop-off and then almost maintained unchanged during 1–5 min of microwave exposure.As evidenced by the microscopic imaging, the sizes of foams freshly prepared by native FPI were distributed in a relatively narrow range, further suggesting the favorable foaming properties.Instead, the foams obtained by FPI presented an obvious variation, including the increase in foam sizes but the decrease in foam amounts when microwave exposure time ranged from 1 to 3 min.Thus, the aforementioned foams might be inclined to destabilize due to the easy coalescence or collapse after standing.By comparison, after 5 min of microwave exposure, FPI dispersion primarily produced foams with extremely small sizes, which inevitably could lead to a faster drainage rate after formation.
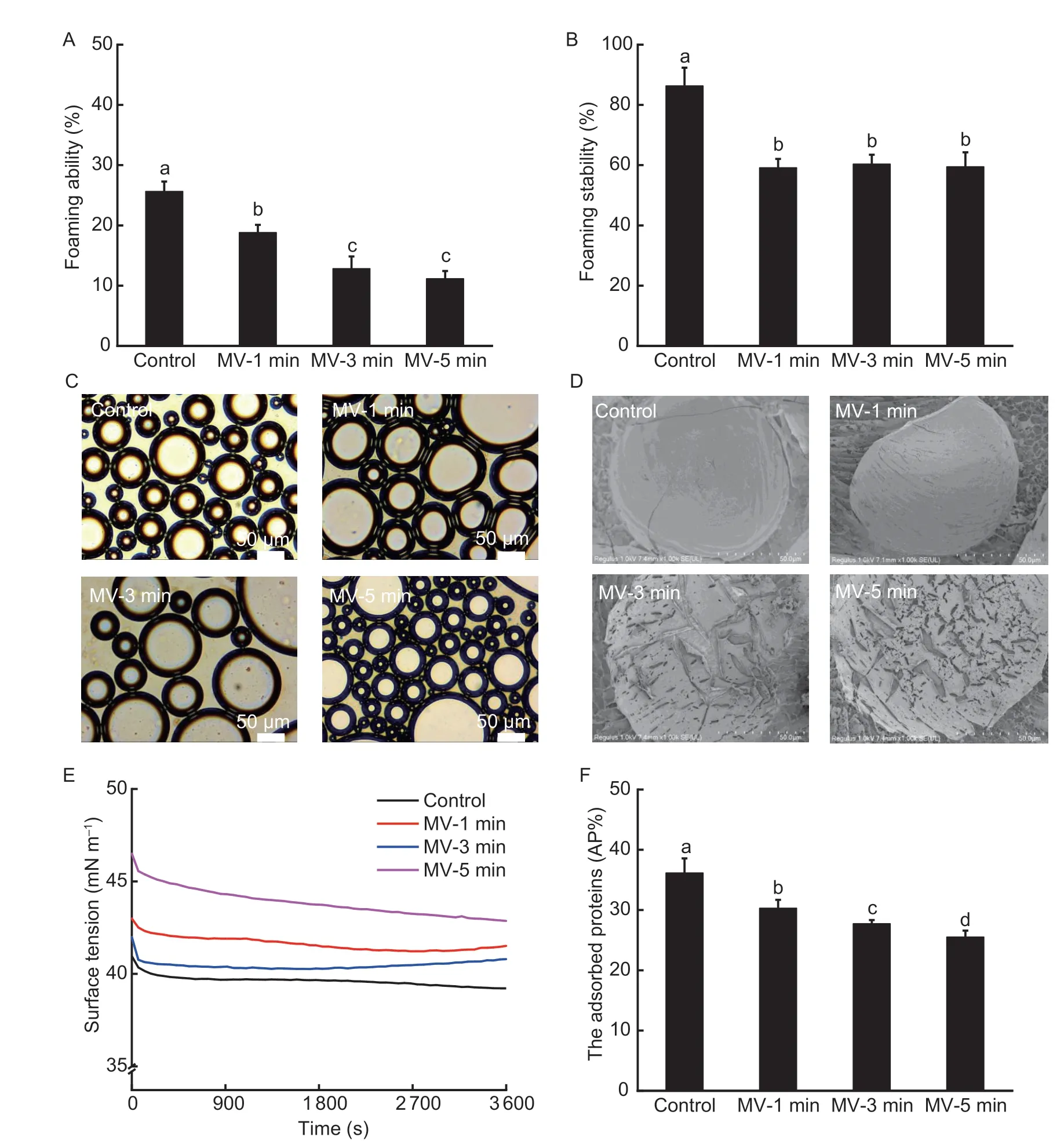
Fig.3 Changes in the foaming properties of flaxseed protein isolates (FPI) following microwave exposure.A and B, foaming ability and stability.C and D, foam morphology and interface microstructure.E, surface tension at air–water interface.F, interface protein loading.MV-1 min, MV-3 min and MV-5 min, indicate microwave exposure for 1, 3 and 5 min, respectively.Data in Fig.3-A, B and F are mean±SD (n=3).Different letters in same index indicated significant differences at the P<0.05 level.
We further explored the microstructure of foams produced by FPI using cryo-SEM imaging.As presented in Fig.3-D, the foams produced by native FPI possessed a compact and thick interface film, which was distinct from the lamellar gel network structure in spaces between foams.These further confirmed the conformation deformation and sufficient lateral intermolecular crosslinking of FPI at the gas–water interface following the migration from the bulk aqueous phase upon highspeed shearing (Yang and Sagis 2021).Nevertheless, the interface layers of foams gradually became thinner and loose, which was largely due to the substantially impaired interface strength when flaxseed was exposed to microwave exposure.Definitely, the particularly obvious pore membrane of foams prepared by FPI sufficiently supported the faster foam drainage upon standing.Then, we further explored the absorption and spreading behavior of FPI at the gas–water interface.As presented in Fig.3-E–F, the percentage of adsorbed protein (AP%) at the interface of foams reached approximately 36.19% when the native FPI was subjected to high-speed shearing.Relatively, when flaxseed was subjected to microwave exposure for 1–5 min, FPI presented inferior interface absorption capacity, as presented by the lower protein load at the gas–water interface (–29.44%;P<0.05).Moreover, the initial π value of native FPI achieved 40.95 mN m–1, which mildly decreased with the equilibrium time extended to 3 600 s.By contrast, FPI possessed a higher initial π value, exhibiting inferior interface migration capacities (+4.97, +2.52, and +13.55%;P<0.05), which then displayed progressively weaker decline with the extension in equilibrium time (–3.4, –2.8, and –7.8%) following microwave exposure for 1–5 min.The lower gas–water interface migration, the subsequently limited conformation adaptability, and lateral intermolecular interactions of FPI might be primarily responsible for the inferior foam stability due to the mild chain depolymerization and conformation unfolding when flaxseed was subjected to microwave exposure.In addition, the predominant retention of flavonoids and phenolic acids based on the superior protein–phenolic interactions could also modify the dispersion properties and successive interface activities of FPI (Santoset al.2022).
3.5.Effects of microwave exposure on the emulsi- fying properties of FPl implication of oil–water interfacial behavior
The foaming ability and stability of FPI were investigated based on the absorption properties of proteins at the oil–water interface and subsequent microstructure of lipid droplets in emulsions.As shown in Fig.4-A, the average particle sizes of emulsion droplets immediately prepared by native FPI reached 10.15 μm.When the microwave exposure ranged from 1 to 5 min, FPI generated the lipid droplets with decreasing dimensions (–16.75, –58.32, and –72.61%;P<0.05), reaching the minimum value of 2.78 μm.The zeta potential value of emulsion droplets prepared by native FPI was 22.91 mV, which displayed an initial decrease and then almost recovery upon 1–5 min of microwave exposure.These implied that the adsorbed protein layers of emulsion droplets were evidently changed upon theinsitustructural alteration of FPI induced by microwave thermal treatment.As demonstrated in Fig.4-B–C, the relatively steep TSI values as a function of measurement time (0–1 800 s) suggested that the emulsion droplets constructed by native FPI tended to be unstable.The apparent decrease and increase in ΔBS curves were observed in the bottom (0–15 mm) and upper part (33–40 mm) of the measuring cell, respectively, further confirming the occurrence of lipid droplet floating.The microwave exposure for 1–3 min exerted favorable regulation of physical stability for emulsions prepared by FPI, as indicated by the slowly increased TSI values.Accordingly, the almost unchanged ΔBS curves in the measurement cell height between 12 and 32 mm, indicating no flocculation and coalescence of lipid droplets.However, when microwave exposure extended to 5 min, the physical stability of emulsions was impaired due to the aggravating lipid droplet flotation, flocculation, and coalescence as characterized by the changing ΔBS curves.

Fig.4 Changes in the emulsifying properties of flaxseed protein isolates (FPI) following microwave exposure.A, mean particle sizes and zeta potential values.Data are mean±SD (n=3).Different letters in same index indicated significant differences at the P<0.05 level.B, turbiscan stability index (TSI).C, delta backscattering (ΔBS).MV-1 min, MV-3 min and MV-5 min, indicate microwave exposure for 1, 3 and 5 min, respectively.
The rheological behavior was further explored to comparatively illustrate the varying physical stability of emulsions.As depicted in Fig.5-A, the apparent viscosity values of native FPI-stabilized emulsions rapidly decreased over the frequency range from 0.01 to 10 Hz, exhibiting a typical shear-thinning behavior.This was consistent with the finding reported by Wanget al.(2010).The initial apparent viscosity values of FPI-stabilized emulsions depended strongly on the microwave exposure.However, FPI dispersion produced comparable apparent viscosity values of emulsions as the frequency varied from 0.01 to 10 Hz during 1–3 min of microwave exposure.When microwave exposure time reached 5 min, a substantial promotion of the apparent viscosity values was observed for FPI-stabilized emulsions independent at the shear rate (0.01–10 Hz).As depicted in Fig.5-B–C, the storage modulus (G´) values were obviously higher than loss modulus (G´´) values for native FPI-stabilized emulsions over the low-frequency range (0.01–10 Hz), exhibiting the typical gel-like properties.When flaxseed experienced microwave exposure for 1 min, the G´ values of FPI-stabilized emulsions remarkably ascended over the frequency range between 0.01 and 10 Hz, leading to a greater gap between G´ and G´´ values.Following 3 min of microwave exposure, the G´´ values of FPIstabilized emulsions increased obviously and thus reversibly narrowed the gap between G´ and G´´ values.Further extending microwave exposure time for 5 min, both the G´ and G´´ values of FPI-stabilized emulsions exhibited a synchronously ascending trend.As previously described, the existence of bound and free phenolic acids contributed to maintaining the viscoelastic properties of FPI, which might partially explain the nonlinear promotion in elastic- or viscous-like behavior of FPI-stabilized emulsions (Alu’dattet al.2014).
Then, the time-dependent interface tension (π) for FPI dispersion at static oil–water interface was recorded without or with microwave exposure.As shown in Fig.5-D, the native FPI dispersion possessed the initial π value of 16.41 mN m–1, which gradually descended with an ultimate reduction of 16.37% (P<0.05) when the equilibration time reached 3 600 s.The method of FPI production by salt extraction has shown higher interfacial activity due to greater solubility compared to those produced by isoelectric precipitation (Karacaet al.2011).However, when microwave exposure continued from 1 to 3 min, the initial π value of FPI continuously descended by 8.95–10.95% (P<0.05) but displayed a marginal decrement from 20.67 to 28.06% (P<0.05) as a function of measurement time.Upon 5 min of microwave exposure, the initial π value of FPI was conversely ascended, concomitant with no obvious alteration in the reduction range of π values up to 3 600 s.These data indicated that the stretched conformation of FPI could induce favorable migration and instant absorption at the oil–water interface but impair the subsequent deformation potential owing to the inferior lateral intermolecular interactions.These might be intimately related to the low aqueous solubility of FPI when experiencing the overstretched molecular conformation, mild subunit depolymerization, and retention of phenolic compounds (Alu’dattet al.2014; Phamet al.2019).
We further explored the microstructure of lipid droplets and bulk aqueous phase in emulsionsviacyro-SEM imaging.As shown in Fig.5-E, the relatively compact interface was tightly coated with the lipid droplets prepared by native FPI, further revealing desirable oil–water interface activities upon high-speed shearing.Concurrently, the emulsion droplets with varying particle sizes were anchored across the single, double, or multilayer lamellar but incompletely dense gel network structure in the bulk aqueous phase.Definitely, the relatively higher concentration of FPI dispersion (1.5%, w/v) was requisite for the physical stability of emulsionsviathe direct interface coating and movement restriction of emulsion droplets (Qinet al.2022).When flaxseed was subjected to microwave exposure for 1 min, FPI dispersion produced the lipid droplets with the compact but loosely packed interface, as well as denser lamellar gel structure in the bulk aqueous phase.Upon 3 min of microwave exposure, lipid droplets with smaller particle sizes were obtained due to the remarkably improved interface activities of FPI.When microwave exposure reached 5 min, smaller amounts of lipid droplets were newly formulated by FPI.In particular, the nuclear neutral lipids were partially leaked from the lipid droplets and then captured by the curly and porous lamellar gel network in the bulk aqueous phase, further explaining the physical stability of emulsions following sustained microwave exposure.
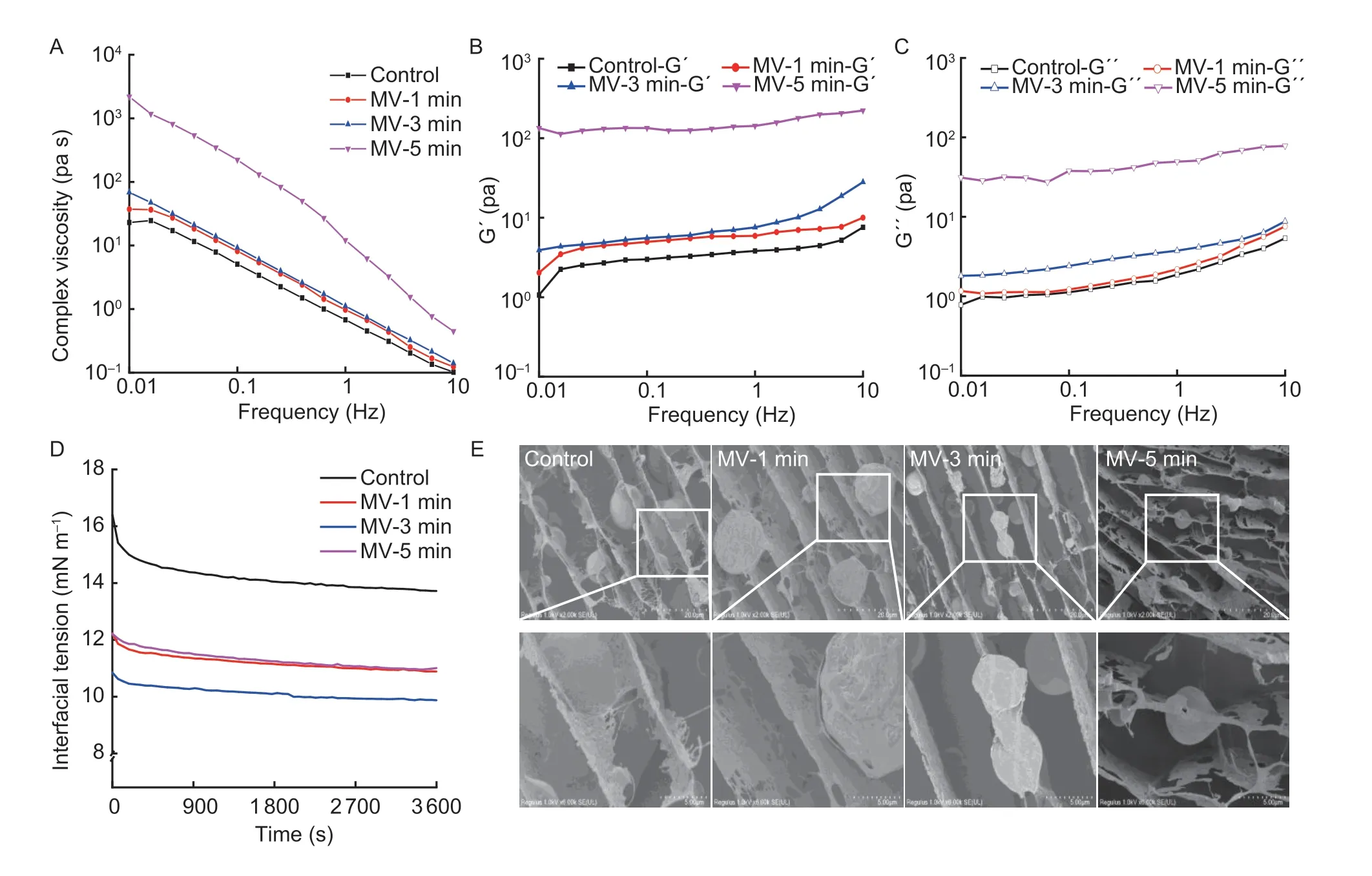
Fig.5 Changes in the rheological behavior and interfacial microstructure of emulsions stabilized by flaxseed protein isolates (FPI) following microwave exposure.A, complex viscosity.B, storage moduli (G´).C, loss moduli (G´´).D, surface tension at oil–water interface.E, micromorphology as examined by cryo-SEM.MV-1 min, MV-3 min and MV-5 min, indicate microwave exposure for 1, 3 and 5 min, respectively.
4.Conclusion
Microwave exposure resulted in a compact assembly of protein fractions and permeation by OBs membrane fragments due to the lost sub-cellular organization upon mechanical cold-pressing of flaxseed.As for the protein morphology, the albumin displayed initial aggregates with thin and curling lamellar structure and then fractured into wide ranges of particle sizes, which was randomly scattered in loosely packed and more explicit spherical globulin fraction in FPI.Moreover, the conformation unfolding, chain cross-linking, and mild depolymerization were sequentially screened for FPI, which further affected the aqueous dispersion or aggregation of protein particles owing to varying intra-/inter-molecular interactions between albumin and globulin.The microwave exposure led to the favorable retention of specific phenolic acids and flavonoids, thus largely promoting the antioxidant activities of FPI.The inferior gas–water interface absorption and subsequent loose/porous interface definitely contributed to the shrinking foaming properties of FPI when microwave exposure was prolonged from 1 to 5 min.The FPI formulated the lipid droplets with smaller sizes due to earlier and higher oil–water interface adsorption, as well as dense interface anchoring upon short-term microwave exposure.However, the mild leakage of neutral lipids and subsequent capture by FPI with curly lamellar gel network in the aqueous phase mildly weakened the physical stability of emulsions after 5 min of microwave exposure.Overall, microwave exposure could be effective to tailor the functionality and subsequent application scenarios of protein fractions in health food.Further work is needed to clarify the digestive and nutritional properties of protein fractions and better understand the underlying influences of microwave treatment on flaxseed.
Acknowledgements
Thanks to the National Natural Science Foundation of China (32072267), the Wuhan Scientific and Technical Payoffs Transformation Project, China (2019030703011505) and the Key Scientific Research Projects of Henan Province, China (2321021110139) for providing financial supports.
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Herbicidal activity and biochemical characteristics of the botanical drupacine against Amaranthus retroflexus L.
- Developing a duplex ARMS-qPCR method to differentiate genotype l and ll African swine fever viruses based on their B646L genes
- The effects of maltodextrin/starch in soy protein isolate–wheat gluten on the thermal stability of high-moisture extrudates
- Effects of planting patterns plastic film mulching on soil temperature, moisture, functional bacteria and yield of winter wheat in the Loess Plateau of China
- lnversion tillage with straw incorporation affects the patterns of soil microbial co-occurrence and multi-nutrient cycling in a Hapli-Udic Cambisol
- The effects of co-utilizing green manure and rice straw on soil aggregates and soil carbon stability in a paddy soil in southern China
