淤胆通方对α-萘异硫氰酸酯诱导的胆汁淤积小鼠肠道菌群和肠道屏障功能的影响
2023-04-29吴晓明何强侯林毅胡艳甄小芳郝静盛燕
吴晓明 何强 侯林毅 胡艳 甄小芳 郝静 盛燕
摘要:目的 观察淤胆通方对α-萘异硫氰酸酯(ANIT)诱导的胆汁淤积小鼠的治疗作用,并基于肠道菌群和肠道屏障功能探讨其作用靶点和机制。方法 将24只C57BL/6小鼠随机分为对照组、模型组、淤胆通方组、熊去氧胆酸(UDCA)组,每组6只。模型组、淤胆通方组、UDCA组小鼠分别于第1天、第4天、第7天、第10天、第13天予ANIT 35 mg·kg-1·d-1灌胃,淤胆通方组、UDCA组小鼠每天分别予淤胆通方、UDCA灌胃,连续15 d,第16天取材。观察肝脏组织病理学,检测肝功能指标;免疫组化法检测肝caspase-1、IL-1β、FXR的蛋白表达,流式细胞术检测肝脏CD11b+、CD86+、CD45+免疫细胞的比例;对粪便微生物进行16S rDNA测序及信息分析;免疫组化法检测肠FXR/NLRP3通路的蛋白表达,免疫熒光法检测肠E-cadherin、Occludin的蛋白表达。当计量资料满足方差齐性,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验;当资料不满足方差齐性,采用Welch检验,进一步两两比较采用Games-Howell检验。结果 HE染色显示,模型组小鼠的部分肝细胞脂肪变性,肝小叶内肝细胞大面积坏死,肝小叶结构破坏,伴有大量炎性细胞浸润,淤胆通方组和UDCA组小鼠的肝细胞脂肪变性减轻,肝小叶内肝细胞坏死不明显,炎性细胞减少;模型组小鼠的血清ALT、AST、GGT、ALP、TBil、DBil、TBA水平较对照组明显升高(P值均<0.05);与模型组相比,淤胆通方组的血清ALT、AST、GGT、ALP、TBil、DBil、TBA水平显著降低(P值均<0.05),UDCA组的血清GGT、TBil、DBil、TBA水平显著下降(P值均<0.05)。与对照组比较,模型组肝脏caspase-1、IL-1β水平明显升高、肝FXR的表达明显下降(P值均<0.05),与模型组比较,淤胆通方组肝脏caspase-1、IL-1β水平及UDCA组肝脏IL-1β水平显著下降,淤胆通方组和UDCA组的肝FXR表达水平显著升高(P值均<0.05)。模型组的肠道菌群组成较对照组发生显著变化(P<0.05);淤胆通方组的肠道菌群结构与模型组存在统计学差异(P<0.05);UDCA组肠道菌群构成与对照组、模型组均有统计学差异(P值均<0.05);相对于对照组,模型组的肠道嗜黏蛋白阿克曼菌丰度明显升高,约氏乳杆菌丰度明显下降(P值均<0.05);与模型组比较,淤胆通方组、UDCA组嗜黏蛋白阿克曼菌的丰度均显著下降,淤胆通方组的鼠乳杆菌、UDCA组的鼠乳杆菌和假长双歧杆菌的丰度均明显升高(P值均<0.05)。与对照组比较,模型组的肠FXR蛋白表达明显降低,肠NLRP3蛋白表达明显升高,肠E-cadherin和Occludin表达均明显下降(P值均<0.05);与模型组相比,淤胆通方组和UDCA组的肠FXR表达显著上调,肠NLRP3表达显著下降,肠E-cadherin和Occludin的蛋白表达均显著升高(P值均<0.05)。结论 淤胆通方可减轻ANIT诱导的胆汁淤积小鼠的肝损伤,改善肠道菌群和增强肠壁屏障功能可能是其作用靶点和机制之一。
关键词:胆汁淤积; 胃肠道微生物组; 淤胆通方; 小鼠
基金项目:国家自然科学基金资助项目(82104926)
Effect of Yudantong decoction on intestinal flora and intestinal barrier function in mice with cholestasis induced by α-naphthyl isothiocyanate
WU Xiaoming, HE Qiang, HOU Linyi, HU Yan, ZHEN Xiaofang, HAO Jing, SHENG Yan. (Department of Traditional Chinese Medicine, Beijing Childrens Hospital, Capital Medical University, National Center for Childrens Health, Beijing 100045, China)
Corresponding author:
HOU Linyi, houlinyii@sohu.com (ORCID:0000-0001-6510-5194)
Abstract:
Objective To investigate the therapeutic effect of Yudantong decoction in mice with α-naphthyl isothiocyanate (ANIT)-induced cholestasis, as well as its targets and mechanism based on intestinal flora and intestinal barrier function. Methods A total of 24 C57BL/6 mice were randomly divided into control group, model group, Yudantong decoction group (YDTF group), and ursodeoxycholic acid (UDCA) group, with 6 mice in each group. The mice in the model group, the YDTF group, and the UDCA group were given ANIT 35 mg/kg/day by gavage on days 1, 4, 7, 10, and 13, and those in the YDTF group and the UDCA group were given Yudantong decoction or UDCA by gavage for 15 consecutive days; related samples were collected on day 16. Liver histopathology was observed, and liver function parameters were measured; immunohistochemistry was used to measure the protein expression levels of caspase-1, interleukin-1β (IL-1β), and FXR in the liver, and flow cytometry was used to measure the percentages of CD11b+, CD86+, and CD45+ immune cells in the liver; 16S rDNA sequencing and information analysis were performed for fecal microorganisms; immunohistochemistry was used to measure the protein expression of the intestinal FXR/NLRP3 pathway, and immunofluorescence assay was used to measure the protein expression of intestinal E-cadherin and occludin. A one-way analysis of variance was used for comparison of continuous data with homogeneity of variance between multiple groups, and the least significant difference t-test was used for further comparison between two groups; the Welch test was used for comparison of data with heterogeneity of variance between multiple groups, and the Games-Howell test was used for further comparison between two groups. Results HE staining showed that the model group had partial hepatocyte fatty degeneration, massive necrosis of hepatocytes in hepatic lobules, damage of lobular structure, and massive inflammatory cell infiltration, and the YDTF group and the UDCA group had alleviation of hepatocyte fatty degeneration and hepatocyte necrosis in hepatic lobules, with a reduction in inflammatory cells. Compared with the control group, the model group had significantly higher serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin (TBil), direct bilirubin (DBil), and total bile acid (TBA) (all P<0.05); compared with the model group, the YDTF group had significant reductions in the serum levels of ALT, AST, GGT, ALP, TBil, DBil, and TBA (all P<0.05), and the UDCA group had significant reductions in the serum levels of GGT, TBil, DBil, and TBA (all P<0.05). Compared with the control group, the model group had significant increases in the levels of caspase-1 and IL-1β and a significant reduction in the expression of FXR in the liver (all P<0.05); compared with the model group, the YDTF group had significant reductions in the levels of caspase-1 and IL-1β in the liver and the UDCA group had a significant reduction in the level of IL-1β in the liver, and both the YDTF group and the UDCA group had a significant increase in the expression level of FXR in the liver (all P<0.05). The model group had a significant change in the composition of intestinal flora compared with the control group (P<0.05); there was a significant difference in the structure of intestinal flora between the YDTF group and the model group (P<0.05), and there was also a significant difference in the composition of intestinal flora between the UDCA group and the control/model groups (P<0.05). Compared with the control group, the model group had a significant increase in the abundance of intestinal Akkermansia muciniphila and a significant reduction in the abundance of Lactobacillus johnsonii (both P<0.05); compared with the model group, both the YDTF group and the UDCA group had a significant reduction in the abundance of intestinal Akkermansia muciniphila, and the YDTF group had a significant increase in the abundance of Lactobacillus murinus, while the UDCA group had significant increases in the abundance of Lactobacillus murinus and Bifidobacterium pseudolongum (all P<0.05). Compared with the control group, the model group had a significant reduction in the protein expression of intestinal FXR, a significant increase in the protein expression of intestinal NLRP3, and significant reductions in the expression of intestinal E-cadherin and occludin (all P<0.05); compared with the model group, both the YDTF group and the UDCA group had a significant increase in the protein expression of intestinal FXR, a significant reduction in the protein expression of intestinal NLRP3, and significant increases in the expression of intestinal E-cadherin and occludin (all P<0.05). Conclusion Yudantong decoction can alleviate liver injury in mice with ANIT-induced cholestasis, possibly by improving intestinal flora and enhancing intestinal barrier function.
Key words:
Cholestasis; Gastrointestinal Microbiome; Yudantong Decoction; Mice
Research funding:
National Natural Science Foundation of China (82104926)
小儿胆汁淤积性肝病(childhood cholestatic liver disease, cCLD)是指肝内外各种原因造成胆汁形成、分泌、排泄障碍,胆汁流无法正常进入十二指肠,导致胆汁酸在肝内蓄积,从而引发肝脏病变的临床综合征,目前该病已成为小儿肝病就诊及住院的首位原因[1]。该病若胆汁淤积持续不缓解,可加速肝细胞损害,随病程进展而发展为肝纤维化和肝硬化,严重危及患儿的健康和生命[2]。cCLD的病因非常复杂,已知病因包括近百种遗传代谢病、多种病原体感染、胆道发育畸形等。目前临床治疗cCLD的药物非常有限,常用药物为熊去氧胆酸(ursodeoxycholic acid, UDCA),但UDCA对大约1/3的cCLD疗效不佳[3],因此越来越多的研究开始寻找cCLD新的治疗靶点并研发新型药物。最新研究[4]显示,肠道微生态失衡是cCLD发生发展的重要机制,其涉及肠道菌群紊乱、胆汁酸代谢障碍、肠道屏障破坏、内毒素易位等相互关联的病理过程,最终加重肝脏炎症反应和胆汁淤积。改善肠道菌群,稳定肠壁屏障功能,抑制肝脏炎症和纤维化进展,是当前治疗cCLD的重要策略[5-6]。
cCLD属于中医学“黄疸”的范畴,因中医疗效显著,现已成为临床中医的优势病种[3]。本院首都名医裴学义教授多年从事儿童肝胆疾病的临床及理论研究,裴老强调cCLD的病位虽在肝胆,但其本源于脾,脾失健运、湿邪内生为根本原因,治疗重在健脾祛湿、清热利胆,并创制了淤胆通方,该方在缓解胆汁淤积、保护肝功能方面具有非常突出的作用和优势[7-9],但其作用机制尚不明确。本研究以α-萘异硫氰酸酯(alpha-naphthyl isothiocyanate,ANIT)诱导的胆汁淤积小鼠为研究对象,以肠道菌群、肠壁屏障功能为切入点,探讨淤胆通方治疗cCLD的效果及作用靶点和机制。
1 材料与方法
1.1 材料
1.1.1 动物 雄性C57BL/6小鼠,清洁级,20~22 g,购自北京维通利华实验动物技术有限公司,实验动物生产许可证号:SCXK(京)2021-0006,实验动物使用许可证号:SYXK(京)2020-0050。小鼠饲养于SPF级实验动物房,分笼饲养,环境温度为20~25 ℃,相对湿度60%,采用明暗各12 h交替的动物照明光循环照明,自由饮水和进食。
1.1.2 药物 淤胆通方的基本组成为:生麦芽10 g、茯苓10 g、白术4 g、茵陈12 g、金钱草10 g、通草3 g、丹参10 g、泽兰10 g、黄柏3 g、青黛0.3 g、血竭0.3 g、琥珀0.3 g、明矾0.3 g,中药由北京儿童医院提供,并由院内制剂室水煎煮制备成含生药0.366 g/mL的药液200 mL。UDCA胶囊(优思弗)购自北京儿童医院,称取适量胶囊内容物加水配置成有效成分含量为5 mg/mL的混悬液,灌胃时充分混匀。
1.1.3 试剂 ANIT购自上海麦克林生化科技有限公司,ANIT溶液的配制:将1 g ANIT溶于50 mL色拉油,搅拌均匀,备用。核苷酸结合寡聚化结构域样受体蛋白3(nod-like receptor protein 3,NLRP3)、半胱氨酸天冬氨酸特异性蛋白酶-1(caspase-1)、IL-1β、E-cadherin、Occludin、法尼醇X受体(farnesoid X receptor, FXR)抗体购自Abcam公司,CD86、CD45、CD11b抗体购自Miltenyi biotec公司。
1.2 分组与干预 24只小鼠使用简单随机化分组法分为对照组、模型组、淤胆通方组、UDCA组,每组6只。参考相关文献[10-11]及预实验结果,模型组、淤胆通方组、UDCA组的小鼠分别于第1天、第4天、第7天、第10天、第13天予ANIT溶液35 mg·kg-1·次-1,1 次/d灌胃,对照组小鼠在相同时间灌胃等体积色拉油。淤胆通方组小鼠每天予中药18 mL·kg-1·次-1,1 次/d灌胃,连续15 d,对照组和模型组给予等体积蒸馏水灌胃,1 次/d,连续15 d。UDCA组每天给予UDCA混悬液100 mg/kg,1 次/d灌胃,连续15 d。第16天,予戊巴比妥钠麻醉后下腔静脉取血,留取肝脏、回肠、粪便等进行后续相关检测。
1.3 研究方法
1.3.1 肝功能检测 血液样本于室温静置3 h,3 000 r/min离心10 min,取血清,检测小鼠血清ALT、AST、GGT、ALP、TBil、DBil、总胆汁酸(total bile acid,TBA)水平。
1.3.2 肝组织病理学HE染色 取多聚甲醛固定后的肝组织进行石蜡包埋、切片、HE染色,并在放大200倍的显微镜下进行观察。
1.3.3 免疫組化 脱蜡、水化组织切片;滴加过氧化物酶灭活试剂孵育5 min,蒸馏水冲洗,PBS洗2 min×2次;柠檬酸钠缓冲液中微波修复抗原,PBS溶液洗3 min×2次;滴入5%羊血清后置于室温下10~30 min;加入已稀释的一抗,4 ℃过夜;PBS洗5 min×5次,加入已稀释的二抗后37 ℃恒温烤箱中30 min,PBS洗5 min×5次;DBA工作液显色5~10 min,PBS洗3 min×3次后,用双蒸水洗5 min,苏木素染液20 s,自来水冲洗,双蒸水洗5 min,PBS返蓝5 min;脱水,透明,中性树胶封片,显微镜下观察、拍照。
1.3.4 免疫荧光染色 组织切片脱蜡入水;抗原微波修复10~15 min,自然冷却至室温;羊血清封闭,37 ℃,60 min;滴加一抗,4 ℃过夜,PBS冲洗5 min3次;滴加荧光素标记的二抗,避光,37 ℃,60 min,PBS冲洗5 min 3次,封片,4 ℃避光保存,荧光显微镜观察拍照。
1.3.5 流式细胞术 取各组小鼠的新鲜肝脏组织,研磨,过滤,收集适量细胞;加入抗体试剂,37 ℃避光孵育30 min,离心去上清液,PBS洗涤3次;加入1 mL 4 ℃预冷的PBS完全重悬细胞,1 500 r/min离心5 min,弃上清液,沉淀用200 μL的PBS重悬;上机检测。
1.3.6 粪便微生物16 S rDNA测序及信息分析 本实验由北京诺禾致源科技股份有限公司完成。采用SDS方法提取样本的基因组DNA,之后利用琼脂糖凝胶电泳检测DNA的纯度和浓度,取适量的样本DNA于离心管中,使用无菌水稀释样本至1 ng/μL。以稀释后的基因组DNA为模板,根据测序区域的选择,使用带Barcode的特异引物,New England Biolabs公司的Phusion High-Fidelity PCR Master Mix with GC Buffer,和高效高保真酶进行PCR,确保扩增效率和准确性。PCR产物使用2%浓度的琼脂糖凝胶进行电泳检测,根据PCR产物浓度进行等量混样,充分混匀后使用2%的琼脂糖凝胶电泳检测PCR产物,对目的条带使用qiagen公司提供的胶回收试剂盒回收产物。使用TruSeq DNA PCR-Free Sample Preparation Kit建库试剂盒进行文库构建,构建好的文库经过Qubit和Q-PCR定量,文库合格后,使用NovaSeq6000进行上机测序。
1.4 统计学方法 使用SPSS 28.0统计软件进行数据分析。计量资料以x±s表示。当资料满足方差齐性,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验;当资料不满足方差齐性,采用Welch检验,进一步两两比较采用Games-Howell检验。P<0.05为差异有统计学意义。
2 结果
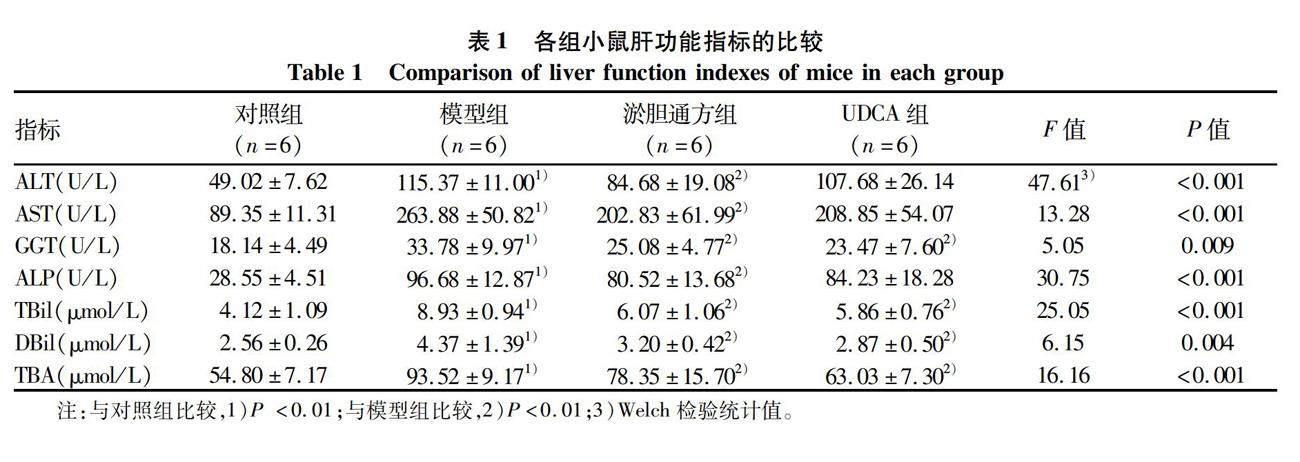
2.1 淤胆通方对胆汁淤积小鼠的肝功能及肝脏病理的影响 模型组小鼠的血清ALT、AST、GGT、ALP、TBil、DBil、TBA水平较对照组明显升高(P值均<0.05);与模型组相比,淤胆通方组的血清ALT、AST、GGT、ALP、TBil、DBil、TBA水平均显著降低,UDCA组的血清GGT、TBil、DBil、TBA水平显著下降(P值均<0.05)(表1)。
HE染色结果显示,模型组小鼠的部分肝细胞脂肪变性,细胞内可见形态规则的圆形或椭圆形空泡,肝小叶内肝细胞大面积坏死,肝小叶结构破坏,伴有大量炎性细胞浸润,淤胆通方组和UDCA组小鼠的肝细胞脂肪变性减轻,肝小叶内肝细胞坏死不明显,炎性细胞减少(图1)。提示淤胆通方对胆汁淤积小鼠的肝脏具有保护作用。
2.2 淤胆通方对胆汁淤积小鼠的肝脏炎症及肝脏FXR的影响 免疫组
化检测结果显示:模型组小鼠的肝caspase-1、IL-1β水平较对照组明显升高(P值均<0.05);与模型组比较,淤胆通方组的肝caspase-1、IL-1β水平以及UDCA组的肝IL-1β水平均显著降低(P值均<0.05)(图2,表2)。流式细胞术结果显示:模型组CD11b+细胞、CD86+细胞、CD45+细胞的比例明显高于对照组(P值均<0.05),而淤胆通方、UDCA均可显著下调上述肝脏免疫细胞的比例(P值均<0.05)(图3,表3)。免疫组化结果表明:模型组小鼠的肝FXR表达量较对照组明显下降(P<0.05),淤胆通方组和UDCA组的肝FXR表达水平较模型组显著升高(P值均<0.05)(图2,表2)。提示淤胆通方可抑制胆汁淤积小鼠的肝脏炎症,激活肝FXR。
2.3 淤胆通方对胆汁淤积小鼠肠道菌群的影响
采用16S rDNA高通量测序技术分析各组小鼠的肠道菌群,结果如下。
2.3.1 肠道菌群α多样性分析 不同组别的α多样性稀释曲线均接近平缓,说明测序深度已基本覆盖到样品中所有的物种,可以真实反映肠道菌群构成(图4)。另外,α多样性曲线可以间接反映不同样品中物种的丰富程度,4组小鼠的肠道菌群α多样性分析指数(shannon、simpson、chao1、ACE、goods_coverage、PD_whole_tree)比较,差异均无统计学意义(P值均>0.05),说明4组小鼠的肠道菌群物种丰富程度无明显差异。
2.3.2 肠道菌群β多样性分析 基于OUT(operational taxonomic units)水平的NMDS分析及ANOSIM分析结果表明:模型组小鼠的肠道菌群组成较对照组发生显著变化(P<0.05);淤胆通方组的肠道菌群结构与对照组无明显差异(P>0.05),而与模型组存在显著差异(P<0.05);UDCA组的肠道菌群构成与对照组、模型组均有明显差异(P值均<0.05)(图5,表4),提示胆汁淤积小鼠的肠道菌群失调,淤胆通方可恢复胆汁淤积小鼠的肠道菌群结构。
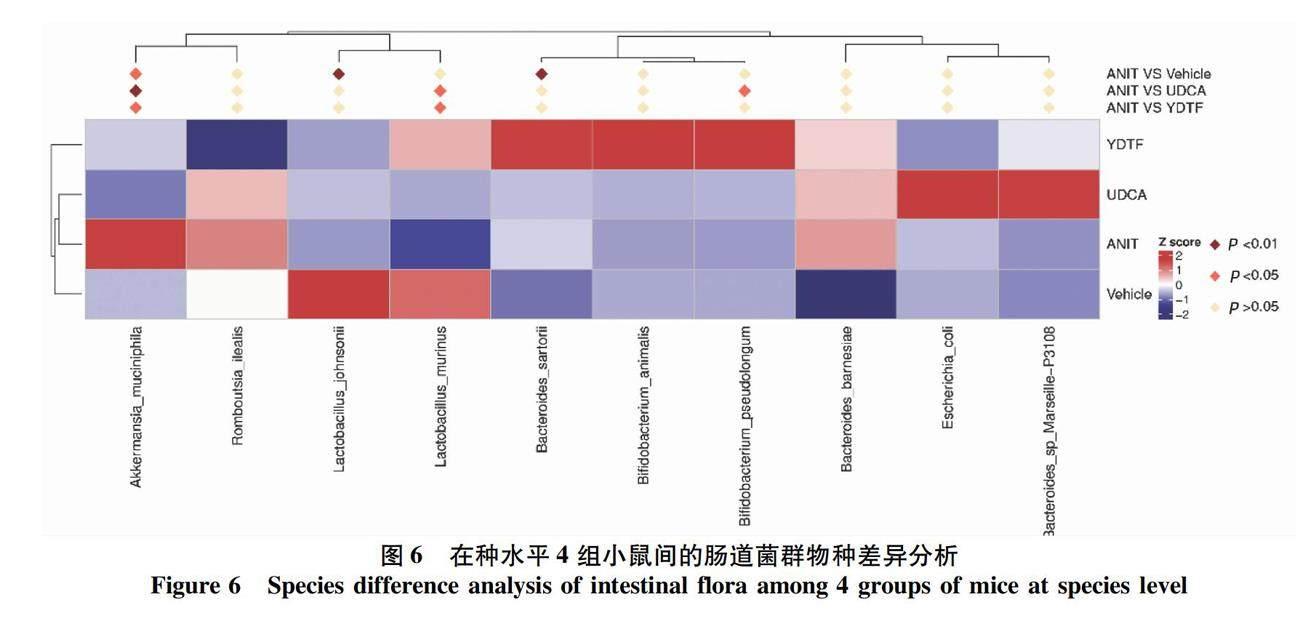
2.3.3 组间差异物种分析 在种水平上,相对于对照组,模型组小鼠的Akkermansia muciniphila(嗜黏蛋
白阿克曼菌)、Bacteroides sartorii(擬杆菌)丰度明显升高,Lactobacillus johnsonii(约氏乳杆菌)丰度显著下降(P值均<0.05);与模型组比较,淤胆通方组和UDCA组的
Akkermansia muciniphila丰度均显著下降,淤胆通方组的Lactobacillus murinus(鼠乳杆菌)丰度明显升高,UDCA组的Lactobacillus murinus、Bifidobacterium pseudolongum(假长双歧杆菌)丰度显著上升(P值均<0.05)(图6)。
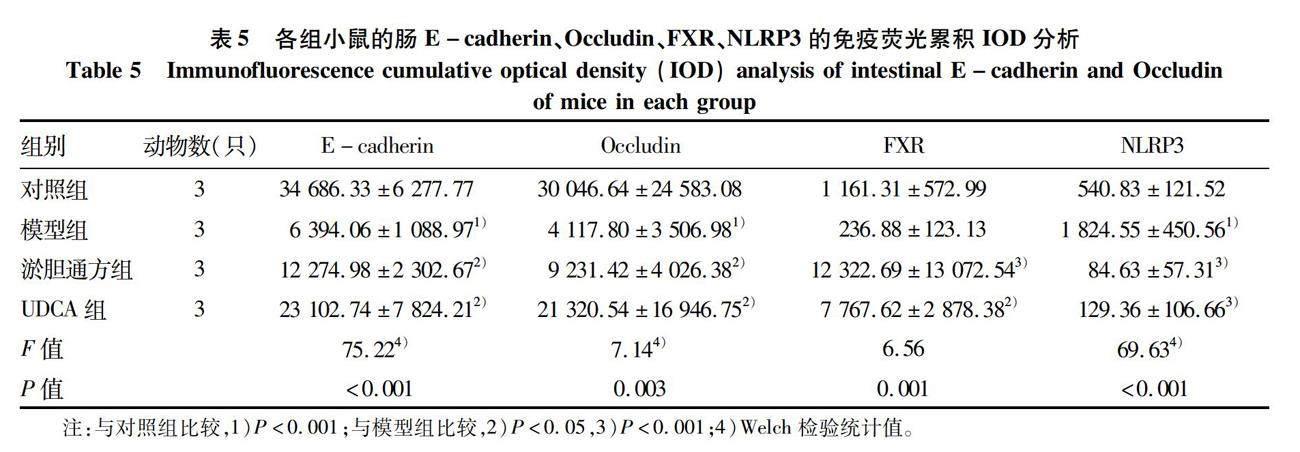
2.4 淤胆通方对胆汁淤积小鼠肠道黏膜屏障功能及FXR/NLRP3通路的影响 免疫荧光结果显示:相对于对照组,模型组小鼠的肠E-cadherin与Occludin的表达均明显下降(P值均<0.05);与模型组比较,淤胆通方组和UDCA组小鼠的肠E-cadherin和Occludin的蛋白表达均显著升高(P值均<0.05),提示淤胆通方可保护胆汁淤积小鼠的肠道屏障功能(图7,表5)。免疫组化结果显示:相对于对照组,模型组小鼠的肠FXR蛋白表达明显降低,肠NLRP3蛋白表达明显升高(P值均<0.05);与模型组比较,淤胆通方组和UDCA组小鼠的肠FXR表达均显著上调,肠NLRP3表达均显著下降(P值均<0.05),提示淤胆通方可调控胆汁淤积小鼠的肠FXR/NLRP3通路,保护肠道屏障功能(图7,表5)。
3 讨论
从脾论治是中医治疗肝病的经典方法,疗效显著。中医认为肝、脾同处中焦,在结构、生理、病理上紧密联系,肝脾升降如轴运轮转,脾为轴、肝为轮,论治肝病,当先健运脾胃以轴运带轮转。淤胆通方的基本组成为生麦芽、茯苓、白术、茵陈、金钱草、通草、丹参、泽兰、黄柏、青黛、血竭、明矾、琥珀。方中以大剂量的生麦芽生发脾胃之气、消食化滞、疏肝解郁;茯苓、白术健脾祛湿,固护中焦之气;茵陈、金钱草祛湿解热、利胆退黄;通草、黄柏清热利尿,引肝胆湿热下行从小便而出;丹参、泽兰活血行滞、疏通肝脉、利胆退黄。此外,酌情使用少量青黛、琥珀、明矾、血竭4味药冲服,以加强祛湿浊、化瘀滞之功,防止胆汁淤积日久转生癥瘕、積聚等。本课题组前期临床研究[8-9]发现,淤胆通方可明显促进患儿黄疸消退,显著改善肝功能,降低肝硬化风险,临床总有效率优于UDCA等西药,并具有依从性好、用量少、见效快、副作用少等优势,但其作用机制尚不明确。研究[12]表明,肠道微环境是从脾论治肝病的重要生物学基础。
胆汁淤积时,肝脏NLRP3炎症小体活化,pro-caspase-1自剪切成有活性的caspase-1,caspase-1可诱导促炎因子IL-1β的惰性前体成熟并分泌至细胞外,促进炎症级联反应[13-14]。炎症因子可进一步诱导相关免疫细胞聚集,如中性粒细胞、巨噬细胞等,CD11b、CD86主要表达于巨噬细胞,CD45是白细胞的共同抗原。FXR是胆汁酸代谢的核心因子和抗炎的关键因素,肝FXR激活有利于减轻肝脏炎症反应,缓解肝内胆汁淤积[15]。胆汁淤积时,肠道NLRP3炎症小体明显活化,继而下调肠黏膜黏着连接蛋白E-cadherin和紧密连接蛋白Occludin的表达,导致肠黏膜通透性增加[16],FXR可与NLRP3或caspase-1直接相互作用,对NLRP3进行负调控,从而发挥抗炎作用[17]。
最近研究[5]发现,肠道菌群紊乱通过“肠-肝轴”在cCLD肝脏炎症、胆汁淤积、肝损伤、肝纤维化、肝硬化的发生发展中起至关重要的作用。正常情况下,肠道微生物产生的胆汁盐水解酶(bile salt hydrolase, BSH)可将结合型胆汁酸水解为游离型胆汁酸,结合型胆汁酸对胆汁酸核受体FXR具有明显的拮抗作用,而游离型胆汁酸激活FXR的能力更强[18]。胆汁淤积时肠道分泌BSH的有益菌数量下降,肠道BSH活性降低,导致肠道中的结合型胆汁酸无法水解为游离型胆汁酸,进而抑制肠FXR信号通路[19]。FXR作为抗炎的关键因子,在调控肠壁通透性中起核心作用[20]。胆汁淤积时肠FXR表达降低,肠黏膜屏障受损,细菌和内毒素经门静脉入血造成肝损伤,激活肠FXR可抑制肠道急性炎症反应,减少肠黏膜中促炎因子产生,改善肠道屏障功能[21],其机制可能是FXR与NLRP3或caspase-1直接相互作用,阻止NLRP3炎症小体组装,从而抑制下游促炎基因表达,发挥抗炎作用[17]。因此,调节肠道菌群、稳定肠壁屏障功能,进而抑制肝脏炎症和纤维化进展,是治疗cCLD的重要方法。本研究从改善肠道菌群、加强肠壁屏障功能的角度入手,探讨该方从脾论治cCLD的部分科学内涵。 为探讨淤胆通方减轻cCLD肝损伤的机制,本研究利用16S rDNA测序技术检测小鼠的肠道菌群。文献[22]报道,cCLD婴儿肠道细菌的丰富度较健康婴儿增加,但未相应导致微生物群功能多样化,本研究亦发现在物种多样性方面存在模型组>对照组>淤胆通方组的趋势,但差异无统计学意义。肠道菌群β多
样性分析显示淤胆通方能改善胆汁淤积小鼠的肠道菌群失调,进一步采用MetaStat方法筛选组间具有显著性差异的物种:Lactobacillus murinus、Lactobacillus johnsonii属于乳杆菌属,具有抗炎特性,均可有效抑制肠道炎症,增强肠壁屏障功能,降低血清内毒素水平,改善系统性炎症[23-24]; Bifidobacterium pseudolongum和Bifidobacterium animalis(动物双歧杆菌)均属于双歧杆菌属,Bifidobacterium animalis可通过上调紧密连接蛋白改善肠壁屏障功能,降低血清内毒素水平,进而促进肝脏免疫稳态,减轻肝损伤[25],口服Bifidobacterium pseudolongum可使肠壁黏液厚度大幅度增加,从而增强肠道的黏液屏障功能[26];Akkermansia muciniphila是定植于肠道黏液层的细菌,依靠降解肠黏液层的黏蛋白生存,其在某些情况下若增殖异常,可能导致肠道屏障损伤,诱发肠道炎症,使内毒素进入血液增加[27]。本研究中,胆汁淤积小鼠的肠道Lactobacillus johnsonii、Lactobacillus murinus、Bifidobacterium animalis、Bifidobacterium pseudolongum的丰度较正常小鼠均有下降,而Akkermansia muciniphila的丰度明显升高,这可能是促进胆汁淤积小鼠肠道炎症和肠壁通透性增高的重要原因,而淤胆通方和UDCA能不同程度地上调Lactobacillus johnsonii、Lactobacillus murinus、Bifidobacterium animalis、Bifidobacterium pseudolongum的丰度,显著下调Akkermansia muciniphila的丰度,这可能是其减轻肠道炎症、加强肠壁屏障功能的关键机制。
综上所述,本研究发现淤胆通方可调节胆汁淤积小鼠的肠道菌群,抑制肠道炎症,加强肠壁屏障功能,减轻肝损伤,初步揭示了淤胆通方从脾论治cCLD的作用机制。然而,淤胆通方是否通过增加肠道益生菌的丰度,提高肠道BSH活性,促进肠道胆汁酸代谢,进而激活肠FXR信号通路,保护肠道屏障功能和减轻肝损伤,有待进一步探索。
伦理学声明:本研究方案经由北京迈德康纳生物技术有限公司实验动物伦理委员会审批,批号:MDKN-2022-007,符合实验室动物管理与使用准则。
利益冲突声明:本研究不存在研究者、伦理委员会成员以及与公开研究成果有关的利益冲突。
作者贡献声明:吴晓明负责课题设计、实验操作、撰写论文;何强、侯林毅负责课题思路和实验设计指导;胡艳、甄小芳、郝静负责指导撰写文章和修改论文;盛燕负责修改论文和最后定稿。
参考文献:
[1]
YU RH, WANG YZ, ZHANG T. Clinical characteristics of infantile liver disease[J]. J Clin Pediatr, 2021, 39(1): 1-5. DOI: 10.3969/j.issn.1000-3606.2021.01.001.
余荣华, 王怡仲, 张婷. 婴儿期肝病临床特点分析[J]. 临床儿科杂志, 2021, 39(1): 1-5. DOI: 10.3969/j.issn.1000-3606.2021.01.001.
[2]JIN M. Progress of cholestatic liver disease and intestinal flora in children[J]. Int J Pediatr, 2020, 47(8): 548-551. DOI: 10.3760/cma.j.issn.1673-4408.2020.08.008.
金萌. 膽汁淤积性肝病与儿童肠道菌群研究进展[J]. 国际儿科学杂志, 2020, 47(8): 548-551. DOI: 10.3760/cma.j.issn.1673-4408.2020.08.008.
[3]FANG KL, ZHENG XT, XU LP, et al. Experimental research progress of traditional chinese medicine in prevention and treatment of cholestatic liver disease[J]. Chin J Integr Tradit West Med Liver Dis, 2020, 30(4): 375-377. DOI: 10.3969/j.issn.1005-0264.2020.04.027.
方凯璐, 郑秀婷, 徐丽萍, 等.传统中药防治胆汁淤积性肝病的实验研究进展[J]. 中西医结合肝病杂志, 2020, 30(4): 375-377. DOI: 10.3969/j.issn.1005-0264.2020.04.027.
[4]LARUSSO NF, TABIBIAN JH, OHARA SP. Role of the intestinal microbiome in cholestatic liver disease[J]. Dig Dis, 2017, 35(3): 166-168. DOI: 10.1159/000450906.
[5]ZHOU R, FAN X, SCHNABL B. Role of the intestinal microbiome in liver fibrosis development and new treatment strategies[J]. Transl Res, 2019, 209: 22-38. DOI: 10.1016/j.trsl.2019.02.005.
[6]GUO C. Clinical and basic study on intestinal microecology of cholestasis[D]. Shijiazhuang: Hebei Medical University, 2020.
郭城. 胆汁淤积症肠道微生态学的临床与基础研究[D]. 石家庄: 河北医科大学, 2020.
[7]HU Y, YAO Y, LIU J, et al. Pei Xueyis experience in treating infantile hepatitis syndrome[J]. Chin J Inf Tradit Chin Med, 2012, 19(2): 87. DOI: 10.3969/j.issn.1005-5304.2012.02.041.
胡艳, 幺远, 柳静, 等. 裴学义治疗婴儿肝炎综合征经验[J]. 中国中医药信息杂志, 2012, 19(2): 87. DOI: 10.3969/j.issn.1005-5304.2012.02.041.
[8]CHEN L, HU Y, YANG M, et al. Interventional effect of traditional Chinese medicine on infants with biliary atresia after operation and its long-term effect[J]. JETCM, 2016, 25(2): 353-356. DOI: 10.3969/j.issn.1004-745X.2016.02.060.
陈黎, 胡艳, 杨梦, 等. 中药对婴儿胆道闭锁术后的干预作用及其远期疗效的观察[J]. 中国中医急症, 2016, 25(2): 353-356. DOI: 10.3969/j.issn.1004-745X.2016.02.060.
[9]HU Y, CHEN L, SHU J, et al. Clinical observation on 60 cases of infant cytomegalovirus hepatitis treated with traditional Chinese medicine[J]. Chin Pediatr Integr Tradit Wset Med, 2012, 4(1): 98-99. DOI: 10.3969/j.issn.1674-3865.2012.02.002.
胡艳, 陈黎, 舒静, 等. 中药治疗婴儿巨细胞病毒性肝炎60例疗效观察[J]. 中国中西医结合儿科学, 2012, 4(1): 98-99. DOI: 10.3969/j.issn.1674-3865.2012.02.002.
[10]DU LN, YANG Y. Establishment and application of animal models of cholestasis[J]. J Clin Hepatol, 2019, 35(2): 444-447. DOI: 10.3969/j.issn.1001-5256.2019.02.046.
杜丽娜, 杨燕. 胆汁淤积动物模型的构建及应用前景[J]. 临床肝胆病杂志, 2019, 35(2): 444-447. DOI: 10.3969/j.issn.1001-5256.2019.02.046.
[11]LUO YS, ZHENG XT, ZHANG HY, et al. The cholestatic fibrosis induced by α-naphthylisothiocyanate in mice and the inflammation pathway[J]. CJAP, 2020, 36(2): 152-157. DOI: 10.12047/j.cjap.5903.2020.034.
罗怡爽, 郑秀婷, 章浩月, 等. α-荼异硫氰酸酯诱导小鼠胆汁淤积性肝纤维化及其炎症通路[J]. 中国应用生理学杂志, 2020, 36(2): 152-157. DOI: 10.12047/j.cjap.5903.2020.034.
[12]ZHANG CY, LIU TH, WANG W, et al. Discussion on intestinal microenvironment as an important biological basis for the theory of treating liver disease from the spleen[J]. CJTCMP, 2019, 34(7): 2877-2880.
张晨阳, 刘天浩, 王维, 等. 论肠道微环境是从脾论治肝病的重要生物学基础[J]. 中华中医药杂志, 2019, 34(7): 2877-2880.
[13]LIAO L, SCHNEIDER KM, GALVEZ E, et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis[J]. Gut, 2019, 68(8): 1477-1492. DOI: 10.1136/gutjnl-2018-316670.
[14]SWANSON KV, DENG M, TING JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics[J]. Nat Rev Immunol, 2019, 19(8): 477-489. DOI: 10.1038/s41577-019-0165-0.
[15]JIA SQ, DOU XG. Farnesol X receptor and its agonists and liver diseases[J]. Chin Hepatol, 2021, 26(11): 1293-1297. DOI: 10.3969/j.issn.1008-1704.2021.11.027.
賈锶琦, 窦晓光. 法尼醇X受体及其激动剂与肝脏疾病[J]. 肝脏, 2021, 26(11): 1293-1297. DOI: 10.3969/j.issn.1008-1704.2021.11.027.
[16]ISAACS-TEN A, ECHEANDIA M, MORENO-GONZALEZ M, et al. Intestinal microbiome-macrophage crosstalk contributes to cholestatic liver disease by promoting intestinal permeability[J]. Hepatology, 2020, 72(6): 2090-2108. DOI: 10.1002/hep.31228.
[17]HAO H, CAO L, JIANG C, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis[J]. Cell Metab, 2017, 25(4): 856-867. e5. DOI: 10.1016/j.cmet.2017.03.007.
[18]HUANG F, ZHENG X, MA X, et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism[J]. Nat Commun, 2019, 10(1): 4971. DOI: 10.1038/s41467-019-12896-x.
[19]WAHLSTRM A, KOVATCHEVA-DATCHARY P, STHLMAN M, et al. Crosstalk between bile acids and gut microbiota and its impact on farnesoid X receptor signalling[J]. Dig Dis, 2017, 35(3): 246-250. DOI: 10.1159/000450982.
[20]LI SL. Effect of FXR on LPS-induced macrophage inflammatory response and intestinal barrier injury in mice[D]. Chinese Peoples Liberation Army (PLA) Medical School, 2019.
李淑玲. FXR对LPS诱导的巨噬细胞炎症反应及小鼠肠道屏障损伤的作用研究[D]. 中国人民解放军医学院, 2019.
[21]VERBEKE L, FARRE R, VERBINNEN B, et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats[J]. Am J Pathol, 2015, 185(2): 409-419. DOI: 10.1016/j.ajpath.2014.10.009.
[22]ZHOU JL, WANG ZX, ZHOU SM, et al. Composition and functional change of intestinal microbiota in infantile cholestasis[J]. J Clin Hepatol, 2021, 37(1): 126-130. DOI: 10.3969/j.issn.1001-5256.2021.01.025.
周建利, 王朝霞, 周少明, 等. 婴儿胆汁淤积的肠道菌群组成及功能变化[J]. 临床肝胆病杂志, 2021, 37(1): 126-130. DOI: 10.3969/j.issn.1001-5256.2021.01.025.
[23]PAN F, ZHANG L, LI M, et al. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice[J]. Microbiome, 2018, 6(1): 54. DOI: 10.1186/s40168-018-0440-5.
[24]WANG H, HE S, XIN J, et al. Psychoactive effects of lactobacillus johnsonii against restraint stress-induced memory dysfunction in mice through modulating intestinal inflammation and permeability-a study based on the gut-brain axis hypothesis[J]. Front Pharmacol, 2021, 12: 662148. DOI: 10.3389/fphar.2021.662148.
[25]ZHANG H, LIU M, LIU X, et al. Bifidobacterium animalis ssp. lactis 420 mitigates autoimmune hepatitis through regulating intestinal barrier and liver immune cells[J]. Front Immunol, 2020, 11: 569104. DOI: 10.3389/fimmu.2020.569104.
[26]MANGIN I, DOSSOU-YOVO F, LVQUE C, et al. Oral administration of viable Bifidobacterium pseudolongum strain Patronus modified colonic microbiota and increased mucus layer thickness in rat[J]. FEMS Microbiol Ecol, 2018, 94(11): fiy177. DOI: 10.1093/femsec/fiy177.
[27]SEREGIN SS, GOLOVCHENKO N, SCHAF B, et al. NLRP6 protects Il10-/- mice from colitis by limiting colonization of akkermansia muciniphila[J]. Cell Rep, 2017, 19(4): 733-745. DOI: 10.1016/j.celrep.2017.03.080.
收稿日期:
2022-08-04;錄用日期:2022-10-25
本文编辑:林姣
