恶性肿瘤相关肝脓肿患者的临床特征分析
2023-04-29张谷芬姚娜毕铭辕张野康文连建奇王临旭汪春付
张谷芬 姚娜 毕铭辕 张野 康文 连建奇 王临旭 汪春付

摘要:目的 對恶性肿瘤相关肝脓肿的临床特点进行分析和总结,早期判断病情进展,及时有效治疗。方法 回顾性分析2005年3月—2018年7月空军军医大学第二附属医院收治的371例肝脓肿患者的临床资料。其中34例恶性肿瘤相关肝脓肿患者作为肿瘤组,按照约1∶2比例、时间匹配的原则,随机选择非恶性肿瘤相关肝脓肿患者(n=70)作为非肿瘤组,将两组的临床特点进行比较。正态分布的计量资料两组间比较采用成组t检验;非正态分布的计量资料两组间比较采用Mann-Whitney U检验;计数资料两组间比较采用χ2检验或Fisher检验。结果 肿瘤组中肝胆系统肿瘤22例(64.7%),胃肠道肿瘤7例(20.6%),非消化道肿瘤5例(14.7%)。肿瘤组合并腹部手术史及肝硬化比例(44.1%、26.5%)高于较非肿瘤组(7.1%、7.1%)(χ2值分别为20.142、7.338,P值均<0.05);入院急性生理与慢性健康评分>16分患者比例高于非肿瘤组(44.1% vs 15.7%, χ2=9.846,P=0.002)。肿瘤组白蛋白低于非肿瘤组[(27.2±5.2) g/L vs (30.8±2.6) g/L, t=-3.131,P=0.002],而总胆红素显著高于非肿瘤组[54(13~313) μmol/L vs 33(7~96) μmol/L, U=1 816.0,P<0.001]。肿瘤组以大肠埃希菌为主(23.5%),非肿瘤组以肺炎克雷伯菌为主(27.1%),前者两种以上细菌感染更为多见(11.8% vs 2.8%)。影像学提示肿瘤组多发脓肿更为多见(47.1% vs 24.3%,χ2=5.479,P=0.019)。与非肿瘤组相比,肿瘤组平均住院天数长(U=1 728.5,P<0.001)、治疗失败率高(P=0.005)。结论 恶性肿瘤相关肝脓肿多合并肝胆肿瘤,致病菌以大肠埃希菌为主,多部位脓肿较常见,预后较差。临床应选择合适抗生素,联合穿刺引流,针对高危人群,必要时可降低手术干预门槛,降低病死率。
关键词:肝脓肿; 肿瘤; 预后
基金项目:陕西省重点研发计划(2022SF-186)
Clinical features of patients with malignant tumor-related pyogenic liver abscess
ZHANG Gufen, YAO Na, BI Mingyuan, ZHANG Ye, KANG Wen, LIAN Jianqi, WANG Linxu, WANG Chunfu. (Department of Infectious Diseases, The Second Affiliated of Air Force Medical University, Xian 710038, China)
Corresponding author:
WANG Chunfu, wcf402@163.com (ORCID:0000-0003-0879-3933)
Abstract:
Objective To investigate the clinical features of malignant tumor-related pyogenic liver abscess (PLA), and to provide a basis for early judgment of disease progression and timely and effective treatment. Methods A retrospective analysis was performed for the clinical data of 371 patients with PLA who were admitted to the Second Affiliated of Air Force Medical University, from March 2005 to July 2018, among whom 34 patients with malignant tumor-related PLA were enrolled as tumor group, and after matching for time and at a ratio of 1∶2, 70 patients without malignant tumor-related PLA were enrolled as non-tumor group. Clinical features were compared between the two groups. The group t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test or the Fishers exact test was used for comparison of categorical data between groups. Results In the tumor group, there were 22 patients with hepatobiliary tumor (64.7%), 7 patients with gastrointestinal tumor (20.6%), and 5 patients with non-gastrointestinal tumor (14.7%). Compared with the non-tumor group, the tumor group had a significantly higher proportion of patients with a history of abdominal surgery (44.1% vs 7.1%, χ2=20.142, P<0.05), liver cirrhosis (26.5% vs 7.1%, χ2=7.338, P<0.05), or an Acute Physiology and Chronic Health Evaluation Ⅱ score of >16 (44.1% vs 15.7%, χ2=9.846, P=0.002). Compared with the non-tumor group in terms of laboratory examination, the tumor group had a significantly lower level of albumin [(27.2±5.2) g/L vs (30.8±2.6) g/L, t=-3.131, P=0.002] and a significantly higher level of total bilirubin [54(13~313) μmol/L vs 33(7~96) μmol/L, U=1 816.0, P<0.001]. Escherichia coli was the main pathogen in the tumor group (23.5%), while Klebsiella pneumonia was the main pathogen in the non-tumor group (23.5%), and compared with the non-tumor group, the tumor group had a significantly higher proportion of patients infected with more than two types of bacteria (11.8% vs 2.8%). Radiological examination showed that the tumor group had a significantly higher proportion of patients with multiple abscesses than the non-tumor group (47.1% vs 24.3%, χ2=5.479, P=0.019). Compared with the non-tumor group, the tumor group had a significantly longer mean length of hospital stay (U=1 728.5, P<0.001) and a significantly higher treatment failure rate (P=0.005). Conclusion Patients with malignant tumor-related PLA often have hepatobiliary tumor, with Escherichia coli as the main pathogen. Abscesses at multiple sites are common, and patients tend to have a poor prognosis. Appropriate antibiotics combined with percutaneous drainage should be used in clinical practice, and for the high-risk population, the threshold for surgical intervention can be lowered to reduce mortality.
Key words:
Liver Abscess; Neoplasms; Prognosis
Research funding:
Shaanxi Provincial Key Research and Development Program(2022SF-186)
细菌性肝脓肿(pyogenic liver abscess,PLA)作为肝脏最常见的感染性病变,随着诊疗技术的进步,诊断率和治愈率都在不断上升,其病死率已降至10%以下[1]。PLA的病因也随着社会环境而变化,除糖尿病和胆道疾病等常见合并疾病外,肿瘤也成为PLA常见的共存疾病。多项研究[2-4]提示恶性肿瘤相关性肝脓肿发病率为5%~25%,恶性肿瘤是PLA患者死亡的独立危险因素。据报道[3],伴有恶性肿瘤的PLA患者病死率是无癌患者的2倍。我国对恶性肿瘤相关肝脓肿报道较少,尚需进一步总结临床经验以期早期诊断和优化治疗。回顾性分析恶性肿瘤相关肝脓肿患者的临床资料,并对其临床特点进行了归纳和总结,现报道如下。
1 资料与方法
1.1 研究对象 选取2005年3月—2018年7月本院收治的371例PLA患者,其中34例恶性肿瘤相关肝脓肿患者作为肿瘤组,按照约1∶2比例、时间匹配原则,简单随机法选择非恶性肿瘤相关肝脓肿患者70例作为非肿瘤组。因出现发热、乏力、腹痛等经CT和B超等影像学检查或/和经脓肿穿刺诊断,并排除阿米巴或结核性肝脓肿。
1.2 治疗方法 所有患者均采用抗感染治疗。肿瘤组2例单用抗感染治疗,4例联合手术治疗,28例联合B超或CT引导下脓肿穿刺引流。非肿瘤组9例单用抗感染治疗,61例联合B超或CT引导下脓肿穿刺引流。肿瘤组初始治疗方案多为含β-内酰胺酶抑制剂抗生素(19/34,55.8%),9例调整抗感染方案,其中5例更换为碳青霉烯类药物,2例对碳青霉烯类耐药,更换为替加环素,2例加用万古霉素。非肿瘤组初始治疗方案多选用三代头孢联合奥硝唑(30/70,42.8%),6例调整抗感染方案为含β-内酰胺酶抑制剂抗生素。
1.3 统计学方法 应用SPSS 22.0统计软件进行数据分析。符合正态分布的计量资料以x±s表示,两组间比较采用成组t检验;不符合正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验或Fisher检验。P<0.05为差异有统计学意义。
2 结果
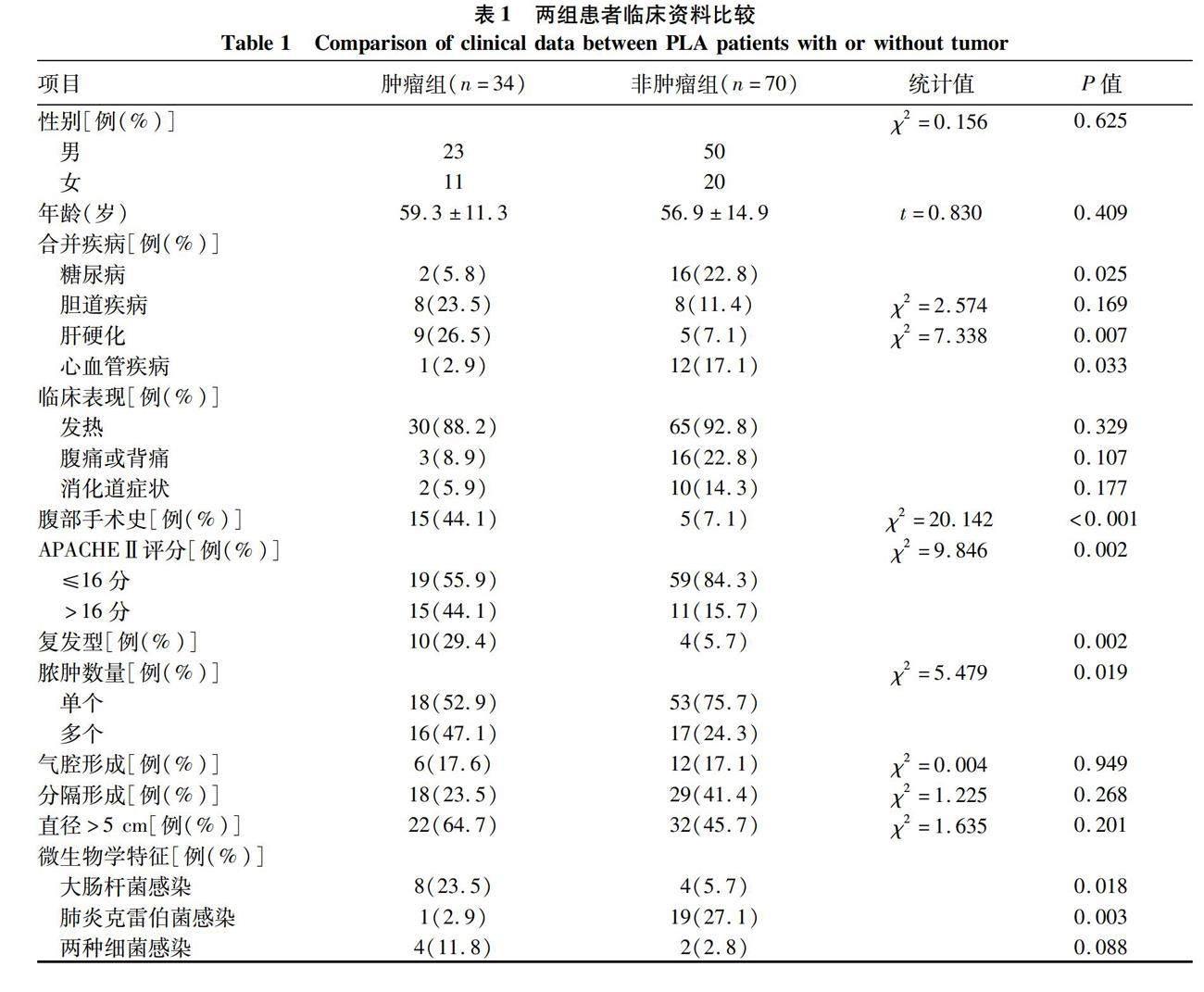
2.1 一般资料 34例肿瘤组患者中,肝胆系统肿瘤22例(64.7%),包括肝癌17例,胆囊癌5例,胆管癌1例;胃肠道肿瘤7例(20.6%),包括胃癌4例,结肠癌2例,胰腺癌1例;非消化道肿瘤5例(14.7%),包括淋巴瘤2例,宫颈癌1例,肺癌1例,纤维母细胞瘤1例和颌下腺肿瘤1例,肝脓肿均不早于肿瘤诊断。
在合并疾病当中,肿瘤组合并糖尿病、心血管疾病较非肿瘤组比例低,而合并肝硬化较非肿瘤组高(P值均<0.05)。肿瘤组入院急性生理与慢性健康评分(APACHEⅡ评分)>16分比例更高,同时既往有腹部手术史患者较非肿瘤组高(P值均<0.05)。两组患者的症状均以发热最为常见,部分患者出现腹痛或胸痛或消化道症状,整体典型症状比例不高。肿瘤组出现复发二次住院的比例为29.4%,较非肿瘤组(5.7%)高(P=0.002)。两组患者均以单发脓肿多见,但肿瘤组更易出现多发脓肿(χ2=5.479,P=0.019)(表1)。
2.2 两组病原学特征和药敏情况比较 34例恶性肿瘤相关肝脓肿患者血液/脓液培养阳性14例(41.2%),其中大肠埃希菌8例,链球菌属2例,葡萄球菌属2例,肠球菌属2例,肺炎克雷伯菌1例,其他肠杆菌属3例,合并2种以上病原菌4例。70例非肿瘤组中培养阳性29例(41.4%),其中肺炎克雷伯菌19例,大肠埃希菌4例,链球菌属4例,肠球菌属4例,其他肠杆菌1例,葡萄球菌属1例,霉菌1例,合并2种以上病原菌2例(表1)。20株肺炎克雷伯菌中含产超广谱β-内酰胺酶阳性肠杆菌2株(10%),12株大肠埃希菌中含产超广谱-内酰胺酶阳性肠杆菌3株(25%),含产碳青霉烯酶的肠杆菌2株(16.7%)。肺炎克雷伯菌菌株对碳青霉烯类药物敏感,对除氨苄青霉素以外的大多数抗菌药物耐药率低。大肠埃希菌对除氨苄青霉素外的大多数抗菌药物的耐药率高于肺炎克雷伯菌。
2.3 实验室检查指标 与非肿瘤组相比,肿瘤组白蛋白明显降低,总胆红素明显升高(P值均<0.05)(表2)。2.4 两组并发症发生率比较 PLA常见的并发症为肺部感染、胸腔积液、败血症、腹膜炎以及其他部位合并脓肿。肿瘤组更易合并其他部位的脓肿,与非肿瘤组相比差异有统计学意义(P=0.005)(表3)。
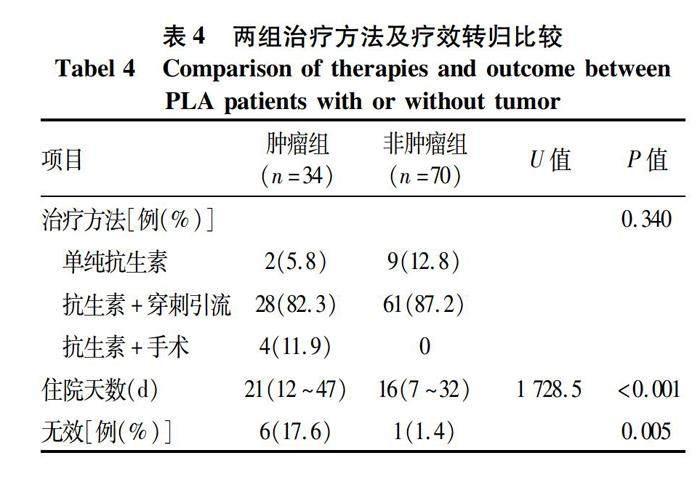
2.5 两组患者治疗及预后比较 两组患者均主要采用抗感染联合B超或CT引导下脓肿穿刺引流,治疗方法比较差异无统计学意义(P=0.340)。肿瘤组住院期间治疗无效3例,死亡2例,自动出院1例,治疗总体无效率高达17.6%,平均住院天数明显长于非肿瘤组(U=1 728.5,P<0.001),且治疗效果较非肿瘤组差(P=0.005)(表4)。此外,非肿瘤组初始治疗方案多选用三代头孢联合奥硝唑(30/70,42.8%);肿瘤组初始治疗方案多为含β-内酰胺酶抑制剂抗生素(19/34,55.8%),且出现更换首次使用抗生素的概率较非肿瘤组高(14.7% vs 8.6%,P=0.030)。
3 讨论
恶性肿瘤相关性肝脓肿是除糖尿病[5]、胆源性肝脓肿外常见的肝脓肿类型。国内外个案报道多见于肝癌介入治疗后肝脓肿的形成[6-9],包括经股動脉化疗栓塞,热消融(射频消融、微波消融)等。随着医疗技术的进步,恶性肿瘤患者生存时间延长,肿瘤相关PLA发病率较前上升,但预后普遍较差。多篇文献[4,10-12]报道,恶性肿瘤是PLA死亡的危险因素,对于合并恶性肿瘤的化脓性肝脓肿患者而言,早期诊断及合理治疗是临床的关键。
本研究中恶性肿瘤相关肝脓肿的发病率为9.2%,这与Li等[13]报道的2005—2018年总患病率9.99%基本一致。恶性PLA可分为3个子类别:原发性肝肿瘤的继发性感染、继发性转移性肝病的感染,以及自发性坏死。本研究中肿瘤来源以原发性肝癌、胆囊癌或胆管癌为主,其次为胃肠道肿瘤[14],此外还有淋巴瘤、宫颈癌等。原发性肝癌介入治疗后出现肝脓肿比例较高,这与报道一致,考虑介入治疗后出现自发性坏死继发细菌感染,也可能引起胆道阻塞,导致胆管炎和PLA的发生。有报道[15-16]表明,PLA可能是原发性肝癌的最初表现,临床上需及时有效判断是否有肝脓肿或肝转移,以免误诊。肿瘤组合并肝硬化和腹部手术史多见,考虑肝硬化是肝癌发生的高危因素,因此所占比例较高。既往也有研究[17]报道肝脓肿合并肝硬化比例高达62.5%。肝胆系统的手术可能会干扰肝脏的血液供应,导致缺血性坏死。此外,手术并发症胆道狭窄等临床操作可能会导致易感组织的继发感染。
肝脓肿临床表现的不典型可能与发病年龄较大,而老年人痛阈较高,或糖尿病周围神经病变有关,为临床诊断增加了难度[18]。实验室检查方面,肿瘤相关PLA更易出现白蛋白的降低和总胆红素的升高,考虑与肿瘤疾病的消耗性营养不良有关,同时肝脓肿也可导致机体消耗增加,有文献[19]提出营养水平降低是PLA预后不良的独立危险因素。而肝胆系肿瘤易出现恶性梗阻性黄疸,增加了继发逆行感染导致菌血症的可能性,因此高胆红素血症也被报道是肝脓肿预后的影响因素[4]。
Chok等[20]研究表明肝细胞癌伴化脓性肝脓肿患者的住院病死率高达40.9%。肿瘤相关PLA一般合并消耗性营养不良,基线生理评分低,且恶性肿瘤组多以肝胆肿瘤为主,而胆源性肝脓肿的主要病原菌仍多见大肠埃希菌[21]。有研究[22]表明大肠埃希菌肝脓肿预后较差,病死率较高,复发率也较高。Chen等[23]研究了關于预后的关键因素,包括高龄、感染性休克、生理状态较差(如低蛋白血症,急性肾衰竭和高胆红素血症),APACHEⅡ评分≥16分等。 本研究中恶性肿瘤PLA患者的入院APACHEⅡ评分更高,考虑该组患者具有较高的死亡风险。肿瘤组病原学培养合并大肠埃希菌和链球菌多见,这与非肿瘤组多合并肺炎克雷伯菌不同,合并2种以上细菌感染较非肿瘤组多见。考虑混合感染的协同效应可能导致组织损伤的进展,降低宿主本身的防御功能,抑制吞噬杀伤,诱发脓肿并增强了混合感染菌的毒力。肿瘤组中大肠埃希菌为主要致病菌,且含2株碳青霉烯类耐药株。近年来全球耐碳青霉烯革兰阴性菌流行情况不容乐观,由于感染耐碳青霉烯类肠杆菌的病死率高,且具有潜在广泛传播的能力,因此对于肿瘤相关PLA患者,经验性选择抗菌药物时可首选含酶抑制剂类药物如哌拉西林他唑巴坦,多重耐药肠杆菌感染首选碳青霉烯类以期早期控制病情,降低病死率[24]。
影像学在肝脓肿的诊断和治疗中发挥着越来越重要的作用。本研究中,肿瘤组更常见多发肝脓肿,与既往研究[8]一致。本研究超声引导下穿刺肝脓肿并引流,未出现并发症,证实超声引导下肝脓肿穿刺治疗的有效性及安全性[25]。肿瘤组有4例患者进行了肝切除术,因样本量较少,与非肿瘤组的差异尚待大样本研究证实。由于脓肿的分隔较多加大了引流的难度,因此对于肿瘤合并多腔脓肿的患者,早期手术可能被认为是必要合理的有效治疗方式[1],但是能否有效降低患者病死率,需要更多的前瞻性研究。
肿瘤组治疗失败率高于非肿瘤组,差异有统计学意义。既往研究[23,26]表明,男性、恶性肿瘤、早期急性呼吸窘迫综合征、多器官衰竭是影响PLA预后的高危因素。本研究中肿瘤相关PLA治疗方面更换首次抗生素频率高,住院时间长,治疗无效率高,出现复发概率高,均提示恶性肿瘤PLA预后较差,治疗难度大。有文献[13]报道,肝胆介入治疗、乙型肝炎、多发脓肿、门静脉栓塞和胆管扩张是肿瘤相关PLA预后的独立影响因素。本研究由于样本量较少,多因素分析数据结果可信度不高,因此未行预后影响因素分析。有关研究仍需大样本前瞻性的临床分析。
综上所述,恶性肿瘤相关肝脓肿以肝胆系统肿瘤为主,多合并胆源性感染,大肠埃希菌为主,复发率及治疗失败率较高,易出现多发脓肿及总胆红素的升高,临床上出现此类高危人群,应及时调整抗生素,联合穿刺引流,必要时可降低手术干预的门槛,早期控制病情,降低病死率。
伦理学声明:本研究方案经由空军军医大学第二附属医院伦理委员会审批,批号为TDLL-第202210-01号。
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:张谷芬负责撰写论文;王临旭、毕铭辕、康文参与收集数据;张野、姚娜修改论文;汪春付、连建奇负责课题设计,资料分析,指导撰写文章并最后定稿。
参考文献:
[1]
RAHIMIAN J, WILSON T, ORAM V, et al. Pyogenic liver abscess: recent trends in etiology and mortality[J]. Clin Infect Dis, 2004, 39(11): 1654-1659. DOI: 10.1086/425616.
[2]SEETO RK, ROCKEY DC. Pyogenic liver abscess. Changes in etiology, management, and outcome[J]. Medicine (Baltimore), 1996, 75(2): 99-113. DOI: 10.1097/00005792-199603000-00006.
[3]YEH TS, JAN YY, JENG LB, et al. Pyogenic liver abscesses in patients with malignant disease: a report of 52 cases treated at a single institution[J]. Arch Surg, 1998, 133(3): 242-245. DOI: 10.1001/archsurg.133.3.242.
[4]MAVILIA MG, MOLINA M, WU GY. The evolving nature of hepatic abscess: A review[J]. J Clin Transl Hepatol, 2016, 4(2): 158-168. DOI: 10.14218/JCTH.2016.00004.
[5]XIAO J, XIN XJ. Analysis of clinical characteristics of pyogenic liver abscess patients with diabetes mellitus[J]. China Med Herald, 2021, 18(14): 128-131.
肖娟, 辛小娟. 细菌性肝脓肿合并糖尿病患者的临床特征分析[J]. 中国医药导报, 2021, 18(14): 128-131.
[6]
LV WF, LU D, HE YS, et al. Liver abscess formation following transarterial chemoembolization: Clinical features, risk factors, bacteria spectrum, and percutaneous catheter drainage[J]. Medicine (Baltimore), 2016, 95(17): e3503. DOI: 10.1097/MD.0000000000003503.
[7]de BARE T, ROCHE A, AMENABAR JM, et al. Liver abscess formation after local treatment of liver tumors[J]. Hepatology, 1996, 23(6): 1436-1440. DOI: 10.1002/hep.510230620.
[8]FACCIORUSSO A, DI MASO M, MUSCATIELLO N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis[J]. Dig Liver Dis, 2016, 48(6): 571-577. DOI: 10.1016/j.dld.2016.02.005.
[9]LARDIRE-DEGUELTE S, RAGOT E, AMROUN K, et al. Hepatic abscess: Diagnosis and management[J]. J Visc Surg, 2015, 152(4): 231-243. DOI: 10.1016/j.jviscsurg.2015.01.013.
[10]MUKTHINUTHALAPATI V, ATTAR BM, PARRA-RODRIGUEZ L, et al. Risk factors, management, and outcomes of pyogenic liver abscess in a us safety net hospital[J]. Dig Dis Sci, 2020, 65(5): 1529-1538. DOI: 10.1007/s10620-019-05851-9.
[11]YU SL, WENG XH. Antimicrobial therapy in adult patients with bacterial liver abscess[J]. J Prac Hepatol, 2015, 18(4): 337-339. DOI: 10.3969/j.issn.1672-5069.2015.04.001.
虞勝镭, 翁心华. 成人细菌性肝脓肿的抗感染治疗要点与进展[J]. 实用肝脏病杂志, 2015, 18(4): 337-339. DOI: 10.3969/j.issn.1672-5069.2015.04.001.
[12]ROSSI G, NGUYEN Y, LAFONT E, et al. Large retrospective study analysing predictive factors of primary treatment failure, recurrence and death in pyogenic liver abscesses[J]. Infection, 2022, 50(5): 1205-1215. DOI: 10.1007/s15010-022-01793-z.
[13]LI W, WU C, QIN M, et al. The aura of malignant tumor: Clinical analysis of malignant tumor-related pyogenic liver abscess[J]. Medicine (Baltimore), 2020, 99(9): e19282. DOI: 10.1097/MD.0000000000019282.
[14]WANG Y, WANG Y, LIU K, et al. Pyogenic liver abscess as initial presentation of colon cancer: a case report[J]. Gastroenterol Nurs, 2020, 43(6): 470-473. DOI: 10.1097/SGA.0000000000000558.
[15]CHONG VH, LIM KS. Pyogenic liver abscess as the first manifestation of hepatobiliary malignancy[J]. Hepatobiliary Pancreat Dis Int, 2009, 8(5): 547-550.
[16]DESALEGN H, TESFAYE A, SHUME P. Pyogenic liver abscess presenting as an initial manifestation of underlying hepatocellular cancer: A case report in ethiopia[J]. Ethiop J Health Sci, 2022, 32(3): 665-668. DOI: 10.4314/ejhs.v32i3.24.
[17]ZHANG CL, GUO JJ, JIA TY, et al. Clinical and pathogenic characteristics in 75 patients with pyogenic liver abscess[J]. Infect Dis Info, 2014, 27(3): 157-159. DOI: 1007-8134(2014)03-0157-04.
[18]WANG Y, LI WK, SU JY, et al. Clinical characteristics of bacterial liver abscess and its risk factors in ICU[J]. J Clin Exp Med, 2022, 21(20): 2233-2238.
王蕓, 李文坤, 苏珈仪, 等. 细菌性肝脓肿临床特征及其入住重症监护室危险因素分析[J]. 临床和实验医学杂志, 2022, 21(20): 2233-2238.
[19]XU J, ZHOU X, ZHENG C. The geriatric nutritional risk index independently predicts adverse outcomes in patients with pyogenic liver abscess[J]. BMC Geriatr, 2019, 19(1): 14. DOI: 10.1186/s12877-019-1030-5.
[20]CHOK KS, CHEUNG TT, CHAN AC, et al. Liver resection for de novo hepatocellular carcinoma complicated by pyogenic liver abscess: A clinical challenge[J]. World J Surg, 2016, 40(2): 412-418. DOI: 10.1007/s00268-015-3239-6.
[21]ZHUANG HX, HUANG WP. Analysis of pyogenic liver abscesses of biliary and cryptogenic origin[J]. Mod Med J China, 2017, 19(9): 23-26. DOI: 10.3969/j.issn.1672-9463.2017.09.007.
庄涵虚, 黄伟平. 胆源性肝脓肿和隐源性肝脓肿临床特征分析[J]. 中国现代医药杂志, 2017, 19(9): 23-26. DOI: 10.3969/j.issn.1672-9463.2017.09.007.
[22]RUIZ-HERNNDEZ JJ, CONDE-MARTEL A, SERRANO-FUENTES M, et al. Pyogenic liver abscesses due to Escherichia coli are still related to worse outcomes[J]. Ir J Med Sci, 2020, 189(1): 155-161. DOI: 10.1007/s11845-019-02041-4.
[23]CHEN SC, TSAI SJ, CHEN CH, et al. Predictors of mortality in patients with pyogenic liver abscess[J]. Neth J Med, 2008, 66(5): 196-203.
[24]YAO N, KANG W, LIAN JQ, et al. Clinical features of liver abscess versus[J]. J Clin Hepatol, 2020, 36(9): 2010-2014. DOI: 10.3969/j.issn.1001-5256.2020.09.020.
姚娜, 康文, 连建奇, 等. 肺炎克雷伯菌肝脓肿与大肠埃希菌肝脓肿临床特点对比分析[J].临床肝胆病杂志, 2020, 36(9): 2010-2014. DOI: 10.3969/j.issn.1001-5256.2020.09.020.
[25]TAN YM, CHUNG AY, CHOW PK, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm[J]. Ann Surg, 2005, 241(3): 485-490. DOI: 10.1097/01.sla.0000154265.14006.47.
[26]CHEN CH, WU SS, CHANG HC, et al. Initial presentations and final outcomes of primary pyogenic liver abscess: a cross-sectional study[J]. BMC Gastroenterol, 2014, 14: 133. DOI: 10.1186/1471-230X-14-133.
收稿日期:
2022-09-08;录用日期:2022-10-17
本文编辑:林姣
