Development of high-strength magnesium alloys with excellent ignition-proof performance based on the oxidation and ignition mechanisms: A review
2023-03-24JingNiLiJinJianZengJingLiFulinWangFenghuaWangShuaiDongJieDong
Jing Ni,Li Jin,Jian Zeng,Jing Li,Fulin Wang,Fenghua Wang,Shuai Dong,Jie Dong
National Engineering Research Center of Light Alloy Net Forming and State Key Laboratory of Metal Matrix Composite,School of Materials Science and Engineering,Shanghai Jiao Tong University,Shanghai 200240,China
Abstract High reactivity and ease of ignition are the major obstacles for the application of Mg alloys in aerospace.Thus,the ignition mechanisms of Mg alloys should be investigated systematically,which can guide the ignition-proof alloy design.This article concludes the factors influencin the ignition resistance of Mg alloys from oxide fil and substrate microstructure,and also the mechanisms of alloying elements improving the ignition resistance.The low strength is another reason restricting the development of Mg alloys.Therefore,at the last section,Mg alloys with the combination of high strength and good ignition-proof performance are summarized,including Mg-Al-Ca based alloys,SEN (Mg-Al-Zn-Ca-Y) alloys as well as Mg-Y and Mg-Gd based alloys.Besides,the shortages and the future focus of theses alloys are also reviewed.The aim of this article is to promote the understanding of oxidation and ignition mechanisms of Mg alloys and to provide reference for the development of Mg alloys with high strength and excellent ignition-proof performance at the same time.
Keywords: High-strength and ignition-proof Mg alloys;High temperature oxidation;Oxide film Second phases;Alloying.
1.Introduction
Mg alloys have a lot of advantages,such as the low density,high specifi strength,high electromagnetic shielding performance and damping performance [1,2],which show wide application prospects in railway,car components,aerospace,medical instruments and 3C products [3–5].Moreover,due to the increasing awareness of environmental protection and emission reduction,Mg alloys,as the representative of the lightweight material,have attracted more and more attention recently [2,6].However,due to the low strength,application of Mg alloys is not as extensive as the Al alloys and steel[7–9].Besides,high surface reactivity of Mg leads to the ease of oxidation and ignition at high temperature.To avoid this problem,protective gasses are usually used in the melting,casting,and heat deformation processes of Mg alloys,which would cause increasing cost and environmental pollution [10,11].Meanwhile,flammabilit of Mg alloys would cause security problems,further raising the application threshold of Mg alloys [12].
A series of Mg alloys with excellent ignition resistance have been developed by alloying and optimizing processing technology [13].However,there is still no systematic understanding on the high temperature oxidation of Mg alloys in view of dynamic and thermodynamic aspects.On the other hand,there are limited researches contributing to the synergetic improvement of strength and ignition resistance and also few reviews concluding the Mg alloy systems with high strength and ignition resistance simultaneously.
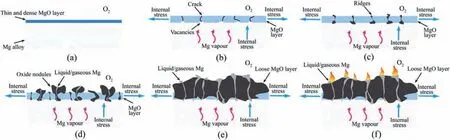
Fig.1.The diagram of the oxidation and ignition process of Mg alloys at the high temperature [16].
To promote the understanding of ignition-proof mechanisms and the development of the Mg alloys with a good combination of strength and ignition-proof performance,firstl,the high temperature oxidation and ignition process of Mg alloys are reviewed from dynamic and thermodynamic aspects to analyze the composition and structure of the oxide film And then shortages of the Pilling–Bedworth ratio (PBR)model which is commonly used to evaluate the protection of the oxide fil are concluded.Besides,the influenc of second phases on ignition resistance of Mg alloys is analyzed systematically.Finally,the mechanisms of alloying elements to improve ignition-proof performance and the Mg alloy systems with high strength and excellent ignition-proof performance are summarized.
2.Mechanisms underlying the oxidation and ignition process at the high temperature
Ignition of Mg alloys is closely related to the oxidation at the high temperature.Improving the oxidation resistance can decrease the evaporation rate of Mg alloys and improve the ignition resistance [14].The diagram of oxidation and ignition process is shown in Fig.1 and can be described as follows [11,15,16]: At the initial stage,thin and dense protective oxide fil is formed on the surface of Mg alloys.With the oxidation continuing,the oxide fil grows thicker and the internal stress of the oxide fil increases,promoting crack initiation.High vapor pressure of Mg alloys at the high temperature can also promote the crack formation.The cracks can act as the channels for the fast transportation of Mg and oxygen,which accelerates the oxidation rate.Besides,Mg can react with oxygen inside the cracks and then form the oxide ridges.Further growth of the ridges forms oxide nodules and the coalescence of them results in the porous oxide film Liquid Mg and its vapor can saturate in the oxide nodules and the porous oxide fil which increases the contact area with oxygen and results in the intensive oxidation process.A lot of heat can be generated during this stage.If the heat cannot be released sufficientl,local temperature will increase high enough,leading to the ignition of Mg alloys.
Based on the above description,it is seen that the ignition of Mg alloys is closely related to the properties of the oxide film As a result,to better understand the ignition-proof mechanisms,the oxide fil should be explored in much detail.
2.1.Oxide fil
The protection of the oxide fil depends on the composition and structure of the oxide film which are closely related to its formation process.In the following section,the oxidation process is analyzed firstl from the thermodynamics and dynamics aspects.Afterwards,the PBR model is also discussed comprehensively.
2.1.1.The composition and structure of oxide fil
2.1.1.1.Thermodynamics.The composition of oxide fil is influence by the change of Gibbs free energy (ΔG) during the chemical reaction [17]:
The calculation formula ofΔG is:
ΔGθrefers to the standard Gibbs free energy change;R is gas constant;T is oxidation temperature;αrepresents activity.
XbOacan be formed prior to MgO ifΔG is smaller than 0.If XbOais more protective than MgO,adding X would be beneficia to the ignition resistance.ΔG<0 is realized when element content exceeds the equilibrium concentration at a certain temperature [18].We can useΔG to design ignitionproof Mg alloys preliminarily.Inoue et al.[19] calculated theΔGθfor 2b/aX+O2→2/aXbOaof different elements at varying temperature and classifie them into less-reactiveelement and reactive-element groups,as shown in Fig.2.Thus,ΔGθfor reaction (1) of reactive elements is smaller than 0 within this temperature range and as a result,XbOais easy to be formed.The ignition temperature of Mg99X1alloys is shown in Fig.3.It indicates that Mg alloys containing reactive element tends to show higher ignition temperature than pure Mg,while Mg alloys containing less reactive element shows similar ignition temperature to pure Mg.Based on the calculating result,we can select the alloying elements to improve ignition-proof performance.
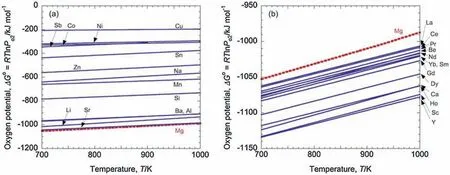
Fig.2.(a)ΔGθ of Li,Na,Al,Si,Mn,Co,Ni,Cu,Zn,Sr,Sn,Sb,and Ba at different temperature.(b)ΔGθ of Be,Ca,Sc,Y,La,Ce,Pr,Nd,Sm,Gd,Dy,Ho,and Yb at different temperature [19].

Fig.3.Ignition temperatures of Mg99X1 alloys [19].
It should be noted that Mg alloys can also form protective fil even thoughΔG is larger than 0.On one hand,after the prior oxidation of Mg,the concentration of X increases with the consumption of Mg.X may segregate [20] at the surface and when the concentration of X achieves the equilibrium concentration,XbOacan be formed [21,22].On the other hand,although XbOacan’t be formed,element X may segregate at the grain boundaries of the oxide and the cracks of MgO,which blocks the channels for the fast diffusion of Mg and oxygen,suppressing oxidation of Mg alloys [21,23].
Even though the calculation ofΔG is an important method to analyze the oxide fil composition,it is pointed out that there is no strong connection betweenΔG and the ignition point[14].Therefore,usingΔG can make a preliminary analysis on the oxide fil composition,but the fina composition and structure need the consideration of dynamic and other factors.
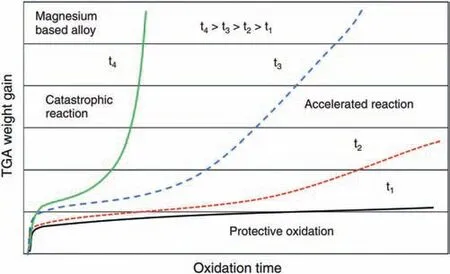
Fig.4.Oxidation dynamics under different temperature of Mg alloys [11].
2.1.1.2.Dynamics.Oxidation dynamics is another important factor to understand the oxidation mechanisms and the oxide fil structure.Oxidation dynamics and its mechanisms depend on the oxidation temperature and time [11,24].There is a critical temperature for Mg alloys [25].When the temperature is lower than the critical temperature,oxidation rate is slow and growth of oxide fil follows the parabolic law.However,when the temperature is higher than the critical value,oxidation process is divided into two stages,i.e.,the initial incubation period following the parabolic law and the accelerating oxidation stage following the linear law [26].Fig.4 shows the oxidation kinetics at different temperature.It is found that with increasing temperature,the accelerating oxidation stage appears and the incubation period becomes shorter [11].The duration of incubation period is an important factor to evaluate the oxidation resistance of Mg alloys.To be specific extending the incubation (parabolic oxidation)stage can improve the oxidation and ignition resistance [16].
Transition from the parabolic oxidation to the linear oxidation is caused by the change of oxide fil morphology [11].Oxide fil is dense and protective in the parabolic oxidation process,while in the linear oxidation stage,voids and cracks are formed in the oxide film which makes the fil lose protection [16,27].Czerwinski et al.[11,27] pointed out that the formation of non-protective oxide fil at the linear oxidation stage is attributed to the generation and coalescence of the oxide nodules,whose strcucture is illustrated in Fig.5.
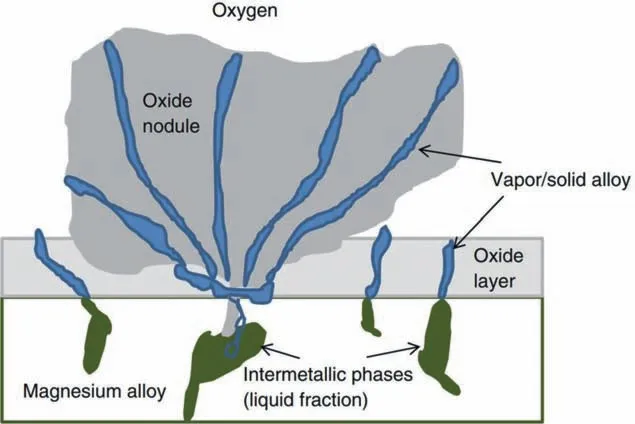
Fig.5.The structure of the oxide nodule [11].
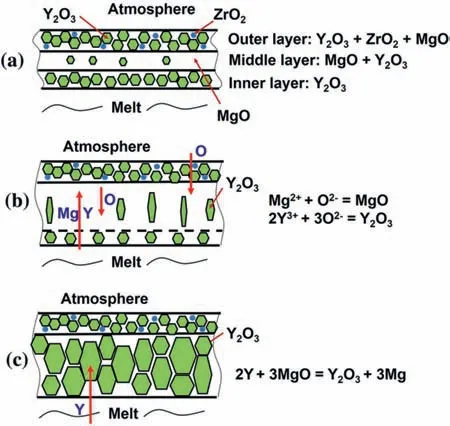
Fig.6.The structure transformation of the oxide scale on WE43 controlled by solid diffusion [33].
Oxidation mechanisms of different stages are also different.At the initial parabolic stage,the growth of the oxide fil is controlled by the solid diffusion [27,28],while the growth of the oxide fil at the linear stage is mainly controlled by the Mg evaporation [11].There is competitive oxidation of alloying elements in the initial oxide fil [29,30].Different diffusion rate of metal cations and oxygen ions causes different oxidation order and position of elements,which affects the fina composition and structure of the oxide fil[28,31,32].For example,WE43 alloys would form oxide fil with three layers initially at 1000 K as shown in Fig.6a.Because Y is difficul to diffuse in Y2O3and the diffusion of O in MgO is slower than Mg and Y,the oxidation process is expected to be the reaction of outward Mg and inward O at outer/middle layer interface,promoting the growth of middle MgO,as shown in Fig.6b.The consumption of Mg leads to the enrichment of Y and then promotes the occurrence of reaction 2Y3++3MgO=Y2O3+3Mg2+,resulting in the inner layer with more Y2O3as shown in Fig.6c [33].

Fig.7.The model of diffusion-controlled oxidation of Mg alloys [34].
However,the outward diffusion of cations leads to the inward diffusion of cation vacancies as seen in Fig.7,which forms voids at the metal/fil interface and causes the local stress concentration,resulting in cracks [27,30,34].Meanwhile,as oxidation reaction proceeds,oxide fil becomes thicker and internal stress of the oxide increases,which can also promote the formation of cracks.Besides,it is reported that with the thickening of the oxide film MgO transforms from a monocrystalline layer to a polycrystalline layer,which may activate the grain boundary diffusion and then increase the oxidation rate [25].Namely,when oxide fil thickness reaches a critical value,cracks are formed and the accelerating oxidation occurs.
Linear oxidation is closely related to oxide nodules and the formation of oxide nodules is controlled by Mg vapor [27].Subsequently,Mg vapor plays an important role at this stage.During linear oxidation process,porous and loose oxide fil can no longer suppress the evaporation of Mg and protect the substrate,which results in the intense oxidation and the following ignition.
High temperature oxidation and ignition of Mg alloys is a complex process,which is related to the oxide fil closely.Thermodynamic and dynamic factors are of vital importance to high temperature oxidation process.Nevertheless,there is few definit thermodynamic and dynamic parameters about the high temperature oxidation and ignition of Mg alloys.
2.1.2.Properties of oxide fil
The composition and structure of oxide fil have a great influenc on the fil properties and finall affect the oxidation and ignition resistance.Continuous and dense oxide fil can act as the barrier for Mg and oxygen,preventing further oxidation of the substrate.While porous and loose oxide fil provides fast transportation channels for Mg and oxygen and can’t protect the substrate.PBR [35] is a commonly used model to evaluate the protection of the oxide fil and it is define as:
Voxis the volume of metal oxides.VMis the volume of metal consumed to form metal oxides.MoxandMMare the molecular weight of metal oxides and metals,respectively.nis the metal atom numbers in one molecular of metal oxide;ρMandρoxare the density of metals and metal oxides.
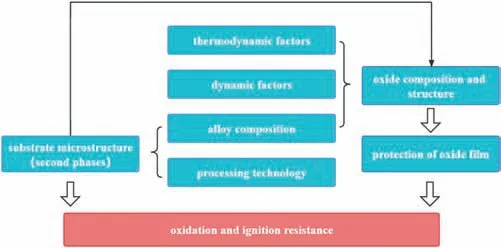
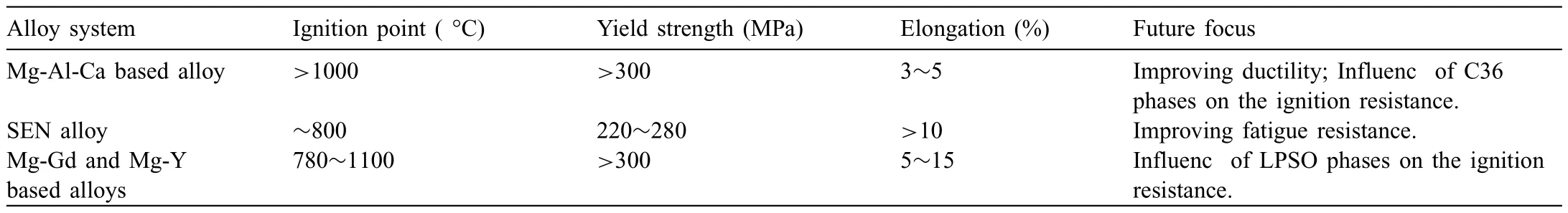
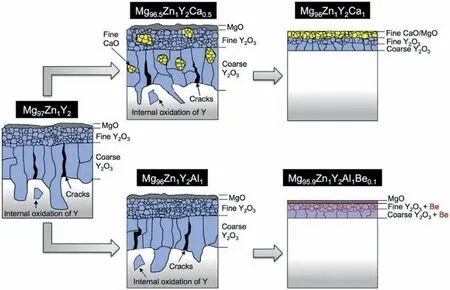
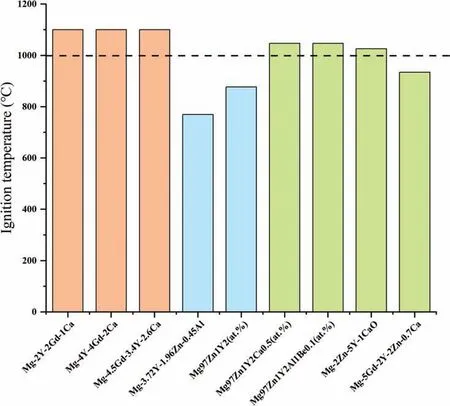
When PBR is lower than 1,the oxide fil is porous and loose and can’t protect the substrate;When PBR is larger than 1 and lower than 2,oxide fil is continuous and dense and can protect the substrate effectively;When PBR is larger than 2,the internal stress of the fil is too large,which causes the formation of cracks and the oxide fil also can’t provide protection.The PBR of MgO is 0.81 [36],so the oxidation resistance of pure Mg is poor.Adding Y,Gd,Nd,Be and other elements [37–39] would form oxide with 1 The PBR model is most commonly used to analyze the ignition resistance of Mg alloys.However,the PBR model also has several disadvantages,mainly including: (1) PBR values of XaObon Mg alloys are calculated based on their pure metal X rather than the Mg alloys.For example,PBR of CaO is 0.64,but adding Ca to Mg alloys can form dense fil and improve ignition-proof property.The reason is that the substrate is Mg alloys rather than pure Ca.By changing the substrate,Inoue et al.[40] calculated the PBR of CaO/Mg-10Al-5Ca and the result is 1.17,which shows that the addition of Ca indeed can improve the ignition resistance of Mg alloys.Although it has been realized that the substrate composition would influenc the PBR value,the PBR of XaOb/X is still directly used to evaluate the ignition proof performance,which is mainly due to the lack of PBR values of different substrates. (2) PBR is not accurate and perfect enough,especially there is a composite oxide fil formed.When the oxide fil is multilayer,the adhesion between different layers should be taken into consideration.For example,Inoue [40] used RAV(the anionic volume ratio) to evaluate the continuity between different layers of Mg-10Al-5Ca.RAVof CaO/Mg-10Al-5Ca and CaO/MgO are larger than 1 and smaller than 2,showing the oxide fil is compressive and continuous,which explained the high ignition temperature of Mg-10Al-5Ca well.Besides,Villegas-Armenta proposed EPBR (Effective Pilling–Bedworth ratio) [41] to evaluate protection of oxide fil formed on the Mg-2.5Sr-1Ca ternary alloy and the calculated result is also consistent with the excellent oxidation resistance.However,all these models need further development and complement. (3) Using PBR alone is not enough to understand the ignition-proof mechanisms.For example,adding Al to Mg can form Al2O3with PBR>1,but the ignition-proof performance of Mg-Al alloys is poor.The reason is that adding Al to Mg forms large amounts of low-melting phases,promoting the evaporation of Mg,the formation of cracks,and then resulting in the poor ignition-proof performance [42].Therefore,we should take multiple factors into consideration to evaluate the oxidation and ignition resistance of Mg alloys. (4) It is obvious that the oxidation resistance of Mg alloys is closely related to the crack resistance of the oxide film However,the mechanical properties,rather than the PBR,has a more direct relationship with the crack resistance of the oxide fil [43].Higher strength and toughness can withstand higher internal stress and inhibit the formation of cracks,which can extend the protective oxidation period and improve the ignition-proof performance [44].Tan et al.[45] found that adding Be can improve the strength and crack resistance of the oxide fil by the solution strengthening and fin grain strengthening,and as a result,oxidation resistance is improved.This oxide reinforcement model has already been proven to be valid in several Mg alloys [43]. Except for the oxide film the substrate microstructure,especially the second phases,also plays an important role in the ignition resistance of Mg alloys.The melting point,size and volume fraction of the second phases have a great influenc on the oxidation rate and ignition resistance [46]. Fig.8.The EDS results of (a) area of map scanning(b) Mg (c) Al (d) O for AZ91D after oxidation [42]. The low-melting phases could melt before the Mg matrix and then form the liquid areas,which promotes the evaporation of Mg and the generation of cracks in the oxide film finall deteriorating the ignition-proof performance of Mg alloys [17,47].Besides,low-melting phases can cause selective oxidation and form serrated interface,increasing the contact area between oxygen and Mg.Li et al.[42] further found that the prior melting of the low-melting phases which distribute along grain boundaries can form cracks in the Mg substrate,promoting the oxidation of the substrate.As illustrated in Fig.8,the EDS results show that there is oxygen in the cracks,indicating that these cracks act as the diffusion path for oxygen and promote the internal oxidation. In a word,low-melting phases play a negative role in the ignition resistance of Mg alloys.This is the reason why Al and Zn are harmful to the ignition resistance of Mg alloys[35,38,48–50].Adding Ca or rare earth elements in moderate amount can suppress the formation of low-melting phases and form thermally stable phases to improve the ignition resistance relatively[51–53].But large amounts of high-melting phases could cause the depletion of solute atoms around the second phases and the inhomogeneity of the microstructure,resulting in porous oxide areas around the second phases and non-uniform oxide film Besides,forming high-melting phases consumes so many Ca or rare earth elements that the solute atoms are not adequate to form protective fil [54,55].Rapid solidificatio is effective to suppress the formation of high-melting phases,extend solubility and form uniform microstructure to improve the oxidation and ignition resistance[56–58].The ignition temperature of AM50-Y alloys prepared by furnace cooling and rapid cooling is shown in Fig.9 [57].It can be seen that the alloy treated by rapid solidificatio shows better ignition resistance.This is because the cooling rate of rapid solidificatio is so high that there is no time to form high-melting phases,which improves the solubility of Y in the Mg matrix and leads to the enough Y atoms to form protective film Fig.9.The ignition point of AM50-Y alloys treated by furnace and rapid cooling [57]. Fig.10.Factors influenin the oxidation and ignition resistance of Mg alloys. Fig.11.The mechanism of reative element effect in Mg alloys [11]. Moreover,it is found that the different diffusion and oxidation behaviors of the second phases and Mg matrix could cause different composition,structure and properties of the oxide film Aydin et al.[59] found that the initial formation of Nd2O3is favored on the second phases and the formation of MgO is favored on theα-Mg phase.Hence,the oxide fil formed on the Mg-6Nd alloy consists of MgO-Nd2O3composite scale and Nd2O3-rich subscale.While there is no Nd2O3-rich subscale formed on the Mg-0.5Nd alloys without the second precipitations.They believed that the formation of Nd2O3-rich subscale is related to the initial formation of Nd2O3on the second phases.Besides,they found that the oxide on the second phases is thicker than that on theα-Mg.Ming et al.[60] found that the oxide scale formed onα-Mg is protective,while the oxide formed on Mg2Ca is not stable and easy to peel off,resulting in poor protection. It should be noted that although second phases could influenc the oxidation and ignition resistance,oxide fil is the decisive factor.The above section reviews how the factors affect the oxidation and ignition resistance of Mg alloys.The composition and structure of the oxide film which is influence by the thermodynamic factors,dynamic factors and alloy composition,is decisive to the protection properties of the oxide film and finall affect the oxidation and the ignition resistance of Mg alloys.On the other hand,the substrate microstructure which is influence by the alloy composition and the processing technology also influence the oxidation and ignition resistance directly.Besides,the substrate microstructure can also influenc the oxide composition and structure and thus exert an indirect effect on the oxidation and ignition resistance.They are concluded in Fig.10. Not only the low ignition resistance,the low strength also limits the wide application of Mg alloys.The common processing method to fabricate Mg alloys with high strength and high ignition resistance simultaneously is as follows: alloying Mg firstl to improve the ignition-proof performance and strength,then through the thermal processing to further optimize the mechanical properties.The influenc of alloying and thermal processing on the mechanical properties of Mg alloys has been studied and concluded in many reviews.Thus,this article mainly concentrates on the influenc of alloying on the ignition resistance of Mg alloys.Afterwards,alloy systems with high strength and excellent ignition resistance that have been developed are also concluded in this section. Kim et al.[36] proposed the selection rules of alloying elements to improve the ignition resistance.The alloying elements used to improve the ignition resistance are expected to be with a high solubility in Mg matrix,a PBR>1 and a negativeΔG for the reaction aMgO+bX →XbOa+aMg. According to the effect of alloying elements,we classify the common alloying elements into three categories: (1) Elements deteriorating the ignition resistance,such as Al,Zn,Fe and Ni,which would form low-melting phases or impurities [12,25].(2) Elements without impact on the ignition resistance like Zr [61].(3) Elements improving the ignition resistance,such as Ca,Be,Sr and rare elements including Y,Gd,Nd,Ce,Er etc.[25,36].Moderate addition of these elements can improve the ignition-proof performance,mainly due to the formation of protective oxide film And there are three special influenc mechanisms of these elements:reactive element effect (REE),surface active element effect (SAEE)and third element effect (TEE). REE: reactive elements have high affinit to oxygen and thus they are easy to react with oxygen and then form the reactive-element oxide.Common reactive elements include Be,Ca and rare earth elements.The mechanism of REE refers to that the reactive elements could diffuse to the boundaries of MgO and exist in the form of cations or oxide,which could inhibit the outward diffusion of Mg2+effectively.As a result,the inward diffusion of O2−becomes the main process of the oxidation.Since the inward diffusion rate is very low,the oxidation rate is reduced effectively [11,19,62].The mechanisms of REE in Mg is illustrated in Fig.11 [11]. Fig.12.The mechanism of Sr surface activity in Mg-6Sr [63]. SAEE: surface-active elements tend to segregate at the alloy surface,decreasing the surface energy.Aydin et al.[63,64]have illustrated the mechanisms of surface-active elements systematically.Surface-active elements diffuse to the alloy surface to lower the surface tension,which can decrease the activity and the vapor pressure of Mg and thus contribute to the improvement of the ignition resistance.On the other hand,as the cracks form in the oxide film surface-active elements with high affinit to oxygen could react with oxygen rapidly to repair the oxide film Sr is a common surface-active element in Mg.As illustrated in Fig.12a,due to the surface segregation of Sr,inner SrO rich oxide can be formed.Fig.12b further shows that when cracks are generated,Sr atoms react with oxygen and form SrO instantly to prevent the oxidation of Mg substrate.Based on the previous investigations,alloying elements with larger Wigner–Seitz radius (nm) are more possible to exhibit the surface-active effect.Wigner-Seitz radius of some elements is shown in Table 1,it is seen that Sr,Ca,Ce,Nd,Sn,Se,and Te may exert surface-active effect in Mg.Note that the surface-active element effect is negligible in solid metal because the diffusion rate is very low. Table 1 Wigner–Seitz radius of some elements [63]. Table 2 Properties and future focus of the three alloy systems. TEE: the third element effect refers to the addition of element C in the A-B binary alloy (A is Mg and B is the most reactive element).C has an oxygen affinit intermediate between A and B and the addition of element C can reduce the critical content of B to form the protective oxide film The specifi mechanism is that element C can act as “oxygen getter” to lower the vapor pressure of oxygen and slow down the inward diffusion of oxygen,which thus reduces the internal oxidation content of B and turns the internal oxidation of B into the external oxidation.Consequently,the critical content of B to form protective fil is decreased [65–67].Fan et al.[68,69] found that Ca could perform TEE in Mg-Y alloys,decreasing the critical addition content of Y to form Y2O3.Besides,Fan et al.[69] figure out that the third elements not only act as “oxygen getter”,but also can increase the activity of B.At present,there are few researches focusing on the TEE in Mg alloys.The mechanisms of the third elements in Mg alloys need further experimental verification Besides,the simultaneous addition of two or more elements in the Mg matrix can exert synergetic effect and thus obtain a better ignition-proof performance than only adding single element [21].For example,AZ91 added 20 ppm (wt.)Be and 0.5 wt.% Ca shows higher ignition point than that of AZ91 with the addition of 60 ppm (wt.) Be [21]. Except for the element type,the content of alloying element is also important.Generally,there is an optimal alloying content at which Mg alloy shows the best ignition resistance.When the concentration of the element exceeds this critical value,the ignition resistance is no longer improved apparently and even undermined with further addition [55,57].Besides,excessive addition of elements could lead to the increasing cost. To promote the application of Mg alloys in aerospace and other fields it is necessary to synergistically improve the strength and ignition resistance.Tekumalla and Gupta[70] concluded the strength and ignition point of common Mg alloys,as shown in Fig.13.It can be seen that the modifie commercial alloys,C36 type alloys and LPSO alloys tend to have both high strength and high ignition point.Based on previous researches,this article reviews Mg alloy systems with both high strength and good ignition-proof performance,aiming to provide reference for the development of new Mg alloys with great comprehensive performance. 3.2.1.Mg-Al-Ca based alloys The yield strength of Mg-Al-Ca based alloys can reach 300 MPa,and in some cases even higher than 400 MPa [71–73].High strength is attributed to the Mg2Ca,Al2Ca and other thermally stable phases,which could pin grain boundaries and suppress the growth of grains.After thermal processing,the strength of alloys would be further improved due to precipitation strengthening,fin grain strengthening,solid solution strengthening and some other strengthening mechanisms[72,74,75].Besides,the ignition point of this alloy system is higher than 1000 °C in general.Reasons for the high ignition point are as follows: Adding Ca to Mg-Al alloys could suppress the formation of low-melting phases,β-Mg17Al12or improve their thermal stability[21].Besides,adding Ca would form the protective oxide fil containing CaO [16]. Fig.13.The ignition point and yield strength of common Mg alloys [70]. Kawamura [76] developed C36-Mg-Al-Ca alloys with high strength and ignition point,called as “KUMADAI nonflammabl Mg alloys”.The yield strength of them is as high as 400 MPa and the ignition point is higher than 1090 °C.They recently reported a series of new Mg-Al-Ca alloys with high strength and high ignition point,including Mg-4.5Al-2.5Ca alloy,Mg-5Al-3Ca alloy,Mg-6Al-4Ca alloy and Mg-4Al-2Ca-0.03Be alloy,whose yield strength reaches 318 ∼363 MPa and ignition point is 1343∼1408 K [77].Inoue et al.[40]investigated the ignition-proof performance of C36-Mg-Al-Ca alloys systematically and found that the high ignition resistance of Mg-10Al-5Ca is attributed to the formation of protective oxide film The protective fil is composed of fin CaO outermost layer,fin MgO intermediate layer,and coarse MgO innermost layer.Adding other elements into the Mg-Al-Ca alloy systems is reported as another way to further improve the strength and ignition-proof performance recently.Han et al.[78] developed a non-flammabl high strength Mg-4.5Al-4.5Ca-Mn alloy,whose ignition point is as high as 1040 °C.Besides,this alloy cannot be ignited during flammabilit test because Ca reacts with oxygen rapidly to repair the fil once cracks are formed in the oxide film The yield strength and ultimate tensile strength of this extruded alloy is 310 MPa and 330 MPa,respectively.The high strength attributes to grain refinemen strengthening and dispersion strengthening.Chu et al.[79] reported a series of Mg-Al-Ca-Mn alloys with Ca/Al ≥1,the yield strength,ultimate tensile strength and ignition point of which are 287 ∼318 MPa,324 ∼350 MPa and 1292∼1344 K,respectively.Grain refinement dispersion strengthening and solid solution strengthening improve the strength synergistically.The oxide fil consisting of Al2Ca,MnAl2O4and CaO results in the high ignition point. Mg-Al-Ca alloys are promising to be applied widely due to their high strength and excellent ignition resistance,but there are some questions needed to be solved.The microstructure of these Mg-Al-Ca alloys in casting state generally consists ofα-Mg and C36-(Mg,Al)2Ca and thus their high ignition point may be associated with C36 phases.Therefore,it’s necessary to study the relationship between the ignition resistance and C36 phases deeply.Moreover,Mg-Al-Ca alloys with high strength and high ignition point usually contain a large amount of Ca,resulting in poor ductility (3%∼5%).Consequently,to apply these Mg-Al-Ca alloys,the ductility of this alloy system needs to be improved. 3.2.2.Mg-Al-Zn-Ca-Y alloys AZ series alloys are extensively used in the modern industry,but their ignition point is very low due to the existence of Al and Zn.Simultaneous addition of a small amount of Ca and Y can eliminate the low-melting phases and form protective oxide film contributing to the improvement of the oxidation and ignition resistance.Besides,a small amount of Ca and Y can inhibit the formation of brittle phases containing Ca [80–83].Mg-Al-Zn-Ca-Y alloys with excellent performance are called as SEN alloys.The “S”,“E” and “N” represent stiffness,environmentally friendly and non-flammable respectively [84].Thermal processing could further improve the mechanical properties of SEN alloys due to the grain refinement precipitation strengthening and other strengthening mechanisms.At present,the ignition point of SEN alloys can be developed as high as 800 °C,which is higher than that of AZ series alloys,AM series alloys and WE43.The yield strength is ranged from 220 MPa to 270 MPa in general,and their ductility is also relatively high,which is usually higher than 10%. Kim et al.[83] improved the ignition point of AZ31 and AZ61 to 1041 K and 1047 K,respectively,by adding 1 wt.%Ca and 0.6 wt.% Y.Their mechanical properties are improved apparently after rolling.The yield strength and ultimate tensile strength of the rolled AZ61-1Ca-0.6Y is as high as 225.7 MPa and 323.4 MPa,respectively.Moreover,its elongation is closely to 20% because the amount of brittle and coarse Ca-containing phases is limited.Go et al.[82] found that the chip ignition temperature of AZ80 alloy is increased by 200 °C to 820 °C after adding 0.3 wt.% Ca and 0.2 wt.% Y.After low-speed indirect extrusion,AZ80-0.3Ca-0.2Y shows high yield strength(379.3 MPa)and ultimate tensile strength (421.7 MPa) as well as appropriate elongation(11.3%).The satisfying mechanical properties are the result of grain boundary strengthening by fin DRXed grains,precipitation hardening by nanosized Mg17Al12precipitates,and strain hardening by non-DRXed grains with a strong basal texture.Gneiger et al.[81] studied the flammabilit of AZ31 alloy and AZ31-0.5Ca-0.2Y.They found that AZ31 alloy can be ignited and burned while AZ31-0.5Ca-0.2Y cannot be ignited.The flammabilit test results are displayed in Fig.14.This study shows that synergetic addition of Ca and Y can not only improve the ignition point,but also benefi to the fla resistance. Fig.14.The flammabilit test results of (a) AZ31 and (b) AZ31-0.5Ca-0.2Y[81]. Due to the high mechanical properties and ignition resistance,the thermal processed SEN alloys are promising to apply to aerospace.Besides,because SEN alloys contain small amounts of rare earth elements,their application can also cut the cost.However,it is found that although adding small amounts of Ca and Y to AZ series alloys improves the strength and ductility,the fatigue resistance is decreased due to Ca and Y can form undissolved particles which cause stress concentration.Fatigue cracks can nucleate around these particles,resulting in the poor fatigue resistance [84].Therefore,future development of SEN alloys should focus on minimizing the negative effects of undissolved phases to improve the fatigue resistance. 3.2.3.Mg-Gd and Mg-Y based alloys Gd and Y have high solubility in Mg matrix at high temperature and the solubility decreases with the decreasing temperature,showing prominent precipitation strengthening effect[85–87].Besides,Gd and Y show high affinit to oxygen and are easy to form protective oxide film Therefore,Gd and Y meet the three conditions in the last section concluded by Kim and thus can effectively improve the oxidation and ignition resistance [36,50,54,88,89]. Mg-Gd and Mg-Y based alloys have become a research focus in recent years due to their excellent performance,especially Mg-Gd-Y based alloys.Dvorsky et al.[90] designed Mg-2Y-2Gd-1Ca alloy and Mg-4Y-4Gd-2Ca alloy,whose ignition temperature is higher than 1100 °C.Kubasek et al.[91] also developed Mg-4.5Gd-3.4Y-2.6Ca alloy with high ignition point (1100 °C) and good mechanical properties,i.e.,yield strength of 229 MPa,ultimate tensile strength of 302 MPa and elongation of 10%.The high ignition point is attributed to the formation of stable (Gd,Y)2O3oxide fil and the outstanding mechanical properties benefi from the combined effect of fin DRXed grains and weak texture. Adding other elements to Mg-Gd or Mg-Y alloys can also obtain excellent performance.Wu et al.[92] found that Mg-15.3Gd-1.8Ag-0.2Zr (GQ152K) alloy shows high yield strength and ultimate tensile strength,i.e.,309 MPa and 421 MPa,respectively,after solution and aging treatment.Oxide scale of this alloy consists of Gd2O3and Ag2O,which is protective,thus,the alloy shows great ignition resistance with the ignition point of 1208 K.In Mg-Y alloys,adding Ca could play the third element effect and then improve the ignition-proof property [61,93].However,the strength of Mg-Y alloys with the addition of Ca needs further improvement. Adding Zn to Mg-Gd or Mg-Y alloys would form long period stacking ordering(LPSO)phases.The Mg alloys containing LPSO phases usually show high strength [86,94–97].For example,by using rapid solidificatio powder metallurgy (RS P/M),Kawamura et al.[98] prepared Mg97Zn1Y2(at.%) alloy with high yield strength of 600 MPa.The ultrahigh strength is thought to be related to the fin grains and homogeneous dispersion of the nanocrystalline particles.Yamasaki et al.[99] also obtained high strength Mg97Zn1Y2(at.%) by extrusion,whose yield strength is 350 MPa.Meanwhile,Mg alloys containing LPSO phases also exhibit good ignition resistance.Kawamura [76] developed “KUMADAI heat-resistant Mg alloys”based on LPSO alloys,whose ignition point,yield strength and elongation are 780∼940 °C,359∼520 MPa and 5∼15%,respectively.MgY3.72Zn1.96Al0.45(wt.%) designed by Tan et al.[100]has better ignition-proof performance with the ignition point of 770°C,which is higher than that of the common commercial Mg alloys.Besides,this Mg alloy also has high yield strength of 360 MPa at the same time.The moderate ignition-proof performance is attributed to the protective oxide fil formed by Al and Y.The perfect mechanical properties originate from the LPSO phases,fin DRXed grains and the solid solution strengthening of Al and Y. Adding other elements to the Mg alloys containing LPSO phases could further improve the mechanical properties and the ignition resistance.Inoue et al.[101] measured the ignition point of Mg97Zn1Y2(at.%) and the result was 1150 K,which was increased to 1320 K after adding Ca or simultaneously adding Al and Be.The reasons are as follows:Adding Ca or Be could reduce the thickness of the oxide fil and the residual stress in the film contributing to the formation of continuous and uniform oxide film Besides,adding Ca or Be could inhibit the inner oxidation of Y effectively.These effects are illustrated in Fig.15.Lim et al.[102] designed Mg92Zn2Y5-1CaO (WZO5210) (wt.%) alloy with a good combination of high ignition resistance and high strength,i.e.,ignition point of 1025.6 °C,yield strength of 310 MPa and ultimate tensile strength of 350 MPa.The high strength and high ignition point are attributed to the fin microstructure and the protective oxide fil respectively.Later,the authors developed Mg-5Gd-2Y-2Zn-0.7Ca alloy,which exhibits excellent comprehensive properties with ignition point of 934 °C,yield strength of 407 MPa,ultimate tensile strength of 424 MPa and elongation of 7% [103].This alloy shows quite high strength,whose yield strength is higher than 400 MPa.However,the mechanisms need further investigation. Fig.15.The acting mechanism of Ca and Be in the oxidation process of Mg97Zn1Y2 [101]. Fig.16.The ignition points of Mg-Gd and Mg-Y alloys mentioned above. The LPSO alloys reviewed above show moderate ignition point.The main reason is that rare elements (Y and Gd) can form protective oxide film On the other hand,it is reported that the LPSO phases may play a role in the improvement of ignition resistance [70].Fig.16 concludes the ignition points of Mg-Gd and Mg-Y based alloys mentioned above,which shows that the ignition points of the Mg-LPSO alloys containing Zn are relatively low and are hard to reach 1000 °C unless Ca element or other elements are added.Thus,it is proposed that the influenc of LPSO phases on ignition resistance is negligible while the oxide fil which is controlled by the alloy composition is decisive to the ignition resistance.But the actual effect of LPSO phases on ignition resistance needs further experimental verification Table 2 concludes the properties and the future focus of the above three high strength and ignition-proof alloy systems.It should be noted that the measurement method of ignition point and the variation of size and shape of the sample in different studies have a significan effect on the results. This article systematically reviews the high temperature oxidation and ignition mechanisms.Besides,the development of Mg alloys with high strength and excellent ignition-proof performance are also concluded in detail.We draw a series of conclusions as follows: (1) The composition and structure of the oxide fil is influence by the thermodynamic and dynamic factors.Protection of oxide fil depends on the oxide fil structure.The PBR model is most commonly used to evaluate the protection properties of the oxide film However,there are some deficiencie in the PBR model,which are mainly reflecte in the lack of PBR values of different substrate alloys and ignoring the effect of continuity between different oxide layers,second precipitations in the matrix,mechanical properties of the oxide film etc. (2) Second phases are always emphasized when it comes to the influenc of the substrate microstructure on the oxidation and ignition resistance.Large amount of second phases is detrimental to the oxidation and ignition resistance.However,the Mg alloys with LPSO phases or C36 phases show good ignition-proof performance,but whether the LPSO and C36 phases can improve the ignition-proof performance have not been verified (3) Mg alloy systems with both high strength and excellent ignition-proof performance are fabricated by alloying and subsequent thermal processing generally.Three high-strength and ignition-proof Mg alloy systems are summarized in this article,including Mg-Al-Ca based alloys,Mg-Al-Zn-Ca-Y alloys as well as Mg-Y and Mg-Gd based alloys.Their high strength attributes to the combined effects of fin grain strengthening,precipitation strengthening and solid solution strengthening,etc.The excellent ignition-proof performance is due to the dense and protective oxide film However,almost all studies have ignored the effect of the thermal processing on the ignition-proof performance,and the reasons are still unclear. Declaration of Competing InterestNone. Acknowledgments The authors gratefully acknowledge the financia supports from the National Key Research and Development Plan (Grant No.2021YFB3701100),and the National Natural Science Foundation of China (Grant No.U2241231,No.52071206).2.2.Substrate microstructure



3.Development of Mg alloys with high strength and excellent ignition-proof performance
3.1.The influenc of alloying on the ignition resistance


3.2.High-strength and ignition-proof Mg alloy systems


4.Conclusions
杂志排行
Journal of Magnesium and Alloys的其它文章
- Development and application of magnesium alloy parts for automotive OEMs: A review
- Recent advances in surface endothelialization of the magnesium alloy stent materials
- Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications
- Exploring the contribution of oxygen reduction reaction to Mg corrosion by modeling assisted local analysis
- Microstructures,mechanical properties,corrosion,and biocompatibility of extruded Mg-Zr-Sr-Ho alloys for biodegradable implant applications
- Fabrication of a model specimen for understanding micro-galvanic corrosion at the boundary of α-Mg and β-Mg17Al12
