Current therapeutic modalities and chemopreventive role of natural products in liver cancer: Progress and promise
2023-03-18AmitKumarSinghShivVardanSinghRameshKumarShashankKumarSabyasachiSenapatiAbhayPandey
Amit Kumar Singh,Shiv Vardan Singh,Ramesh Kumar,Shashank Kumar,Sabyasachi Senapati,Abhay K Pandey
Amit Kumar Singh,Department of Botany,Government Naveen Girls College,Balod (Hemchand Yadav University),Durg,Chattisgarh,India
Amit Kumar Singh,Shiv Vardan Singh,Ramesh Kumar,Abhay K Pandey,Department of Biochemistry,University of Allahabad,Prayagraj 211002,Uttar Pradesh,India
Ramesh Kumar,Shashank Kumar,Department of Biochemistry,School of Basic and Applied Sciences,Central University of Punjab,Bathinda 151401,Punjab,India
Sabyasachi Senapati,Department of Human Genetics and Molecular Medicine,Central University of Punjab,Bathinda 151401,Punjab,India
Abstract Liver cancer is a severe concern for public health officials since the clinical cases are increasing each year,with an estimated 5-year survival rate of 30%–35% after diagnosis.Hepatocellular carcinoma (HCC) constitutes a significant subtype of liver cancer (approximate75%) and is considered primary liver cancer.Treatment for liver cancer mainly depends on the stage of its progression,where surgery including,hepatectomy and liver transplantation,and ablation and radiotherapy are the prime choice.For advanced liver cancer,various drugs and immunotherapy are used as first-line treatment,whereas second-line treatment includes chemotherapeutic drugs from natural and synthetic origins.Sorafenib and lenvatinib are first-line therapies,while regorafenib and ramucirumab are secondline therapy.Various metabolic and signaling pathways such as Notch,JAK/STAT,Hippo,TGF-β,and Wnt have played a critical role during HCC progression.Dysbiosis has also been implicated in liver cancer.Drug-induced toxicity is a key obstacle in the treatment of liver cancer,necessitating the development of effective and safe medications,with natural compounds such as resveratrol,curcumin,diallyl sulfide,and others emerging as promising anticancer agents.This review highlights the current status of liver cancer research,signaling pathways,therapeutic targets,current treatment strategies and the chemopreventive role of various natural products in managing liver cancer.
Key Words: Liver cancer;Hepatocellular carcinoma;Signaling pathways;Therapeutic targets;Natural products;Chemopreventive
INTRODUCTION
The liver is the human body’s largest solid organ,and has a pivotal role in removing various blood toxins and maintaining bioenergetics and cellular metabolism[1,2].The liver is structured into four lobes that are made up of multiple lobules,each having a flowing duct toward the common hepatic duct,responsible for bile excretion[3].Changes in lifestyle patterns and excessive use of medicines,alcohol and intake of various unhygienic supplements,impose further stress and finally damage the liver[4,5].Excess alcohol and viral infections causing hepatitis are critical factors for liver cancer[6,7].Each year approximately 0.8 million new clinical cases of liver cancer are diagnosed.From this disease,approximately 830180 people died worldwide in 2020 alone,and this figure seems to be increasing daily,according to World Health Organization surveillance reports[8].Among various liver cancer types,hepatocellular carcinoma (HCC) is the most common type and accounts for approximately 85% of primary liver cancer cases and often occurs in people with chronic liver diseases.It is the most common and second leading cause of cancer-related deaths in Asian and sub-Saharan African countries.It is the sixth most common in western countries due to escalating hepatitis C burden along with nonalcoholic steatohepatitis and obesity[9,10].In patients with a preclinical history of chronic liver diseases and cirrhosis,the development of HCC is a complex process,including inflammatory damage leading to hepatocyte necrosis,regeneration and fibrotic deposition[11,12].
In recent years,multiple efforts have been made to manage HCC using various chemotherapeutic approaches,of which,targeted tyrosine kinase inhibitors,immunotherapy and anticancer combination therapies are the main ones[13].However,chemoresistance and initiation and progression of tumors mainly reprogram cellular metabolism,particularly during HCC development[14].These metabolic alterations are key factors promoting tumor growth,proliferation and requirements of cancer cells,such as increased energy production,macromolecular biosynthesis and maintenance of redox balance.The liver is the main site for contact with a variety of orally ingested therapeutic drugs,alcohol and other xenobiotics after intestinal absorption and this organ is susceptible to various chemicals[15,16].These chemicals cause serious complications such as acute and chronic hepatitis,granulomatous hepatitis,cholestasis with or without hepatitis,tumors and vascular disorders[17].
Among various factors responsible for HCC,viral hepatitis and resulting cirrhosis cover a significant proportion of clinical cases.Various viral infections cause upregulation of hexosamine and membrane lipid biosynthesis,by modulating glutamine-fructose-6-phosphate transaminase (GFAT)1 and choline kinase A expression[18,19].These findings have been further validated with some results where GFAT1 is upregulated in HCC patients and its overexpression enhances tumorigenic phenotypes,as observed duringin vitrostudies[20].Hepatitis B virus (HBV) also alters lipid metabolism,where viral proteins are known for inducing lipid accumulationviathe upregulation of sterol regulatory element-binding protein (SREBP)1,peroxisome proliferator-activated receptor (PPAR)γ,as well as lipogenic and adipogenic enzymes,which are also reported during HCC progression[21,22].HCC cells infected with hepatitis C virus (HCV) are also known to exhibit altered glycolysis and gluconeogenesis along with the activation of lipid-metabolism transcription factor PPARγ in human hepatocytes,similar to HCV infection[23].Some early findings of HCC include the CD36 gene role in free fatty acid uptake and its increased expression during chronic alcohol consumption,thus modulating lipid metabolism,upregulation of SREBP1c and PPARγ,and downregulation of sirtuin 1,collectively leading to impaired fatty acid oxidation[24,25].Nonalcoholic fatty liver disease also manifests alterations in mitochondrial and other metabolic pathways reminiscent of HCC metabolism.As mentioned earlier,modifications in the processes are primarily analogous to many contexts observed in HCC.However,there is still a need for a better understanding of various underlying mechanisms governing metabolic changes during HCC.
Surgical resection is the primary choice for treating HCC,where recurrence and metastasis mostly occur,thus limiting proper treatment for HCC.Due to the minimal number of drugs available for the treatment of HCC,chemotherapy has remained insufficient for successful management of HCC[26,27].Although the first-line and second-line therapies can increase life span for several months,these have serious side effects and resistance problems[28].Since natural products are promising and cost-effective against various illnesses,it seems reasonable to focus on HCC management using natural products where anticancer drugs are limited[2,4,29].This review focuses on HCC and its associated pathways,descriptive illustration of various natural products,along with their anticancer properties.This review provides information to investigate further regarding liver cancer,signaling pathways,therapeutic targets,current treatment strategies and the chemopreventive role of various natural products.
LIVER CANCER
Molecular signaling pathways associated with hepatic cancer
The liver is highly exposed to foreign materials,and their continuous processing is required for the body’s normal functioning.Alcohol consumption imposes stress on hepatic cells.This condition worsens when combined with a genetic defect in hepatic cells.These factors,either alone or in combination,alter the molecular signaling events responsible for controlled cellular proliferation and differentiation,ultimately leading to hepatic cancer[23].Targeting these signaling pathways by therapeutic molecules is an important strategy.Inhibition of hepatic cancer-associated signaling pathways ameliorates cancer hallmarks such as increased cellular proliferation,reduced apoptosis,migration,and angiogenesis[24,25].This section discusses recent advances in molecular signaling pathways associated with the different stages (initiation and development) of liver cancer and their therapeutic target potential.Critical signaling pathways related to HCC include transforming growth factor (TGF)-β,Wnt/B-catenin,Hedgehog,Notch,epidermal growth factor (EGF),hepatocyte growth factor (HGF),vascular endothelial growth factor (VEGF),Janus kinase (JAK)/STAT3,and Hippo signaling pathways[30].
The human liver possesses regeneration potential,and highly controlled molecular mechanisms regulate its repair and regeneration.The Notch signaling pathway is involved in the repair and regeneration of the liver,but its malfunction (loss or gain of function) is associated with hepatic diseases,including cancer[31].Notch1 upregulation has been found in most hepatic cancer patients.Molecular profiling studies have revealed Notch target genes such asHes1andHey1in hepatic cancer patients with increased cellular proliferation,reduced apoptosis,increased metastasis and angiogenesis in hepatic cancer cells[32,33].Notch signaling crosstalk with other molecular pathways (such as hypoxia signaling) is associated with hepatic cancer[34].Cytokine signaling pathways such as JAK/STAT (Janus kinase/signal transducer and activator of transcription) have been involved in viral escape in virusinduced HCC[35].Viral invasion and liver injury stimulate hepatocytes and Kupffer cells to secrete sonic hedgehog (SHH) ligands.The ligand triggers Smoothened (Smo) receptor by interacting with the Patched protein,which initiates the Hippo signaling pathway in hepatic cancer cells.The activation of the Hippo signaling pathway results in increased transcription of effector genes (cyclin D,c-Myc,MMP,and CD133,etc.),affecting cell proliferation,invasion,and stemness properties of hepatic cancer cells[36-38].
The TGF-β signaling pathway promotes epithelial to mesenchymal transition,angiogenesis,macrophage maturation,cancer stem cell population,and cellular proliferation in HCC.Crosstalk of TGF-β with other pathways (EGF,Wnt,SHH,etc.) is associated with liver cancer[39,40].Increased Wnt ligand expression and/or mutations in the molecular components of the Wnt signaling pathway results in hyperactivation of the pathway in hepatic cancer cells.The binding of Wnt ligand to its receptor,followed by production of free β-catenin and its translocation to the nucleus,activates transcription of target genes (CD44,EpCAM,cyclin D1,c-Myc,etc.)[41].Transcription of target genes ultimately increases cellular proliferation,stemness,angiogenesis and migration potential in hepatic cancer cells.Wnt signaling response to a hypoxic condition in the tumor microenvironment increases stemness potential in hepatic tumor cells.Like other solid tumors,liver cancer cells secrete various growth factors such as platelet-derived growth factor (PDGF),fibroblast growth factor (FGF),HGF,and VEGF.These factors in turn induce angiogenesis to ensure the appropriate supply of nutrients and oxygen.Liver cancer is the result of chronic liver cirrhosis,which ultimately takes the shape of advanced HCC.Available clinical data show that protein mutation increases as the disease progresses from the initiation stage to highly advanced cancer[42].Mutation inTERTgene (catalytic subunit of telomerase reverse transcriptase) is associated with increased cellular proliferation in liver cancer cells.Clinical data revealed that TERT promoter mutation increased up to 10 times in HCC cells compared with low-grade dysplastic nodules[43].It indicates that mutation plays an important role in the initiation and progression of the pathological stage of HCC.Besides,other mutations are only involved at the later stage of the disease progression and produce more genetic diversified subtypes[44].
Recent development in therapeutic targets in liver cancer
Sorafenib is a first-line chemotherapeutic agent approved for advanced HCC.It is a multikinase inhibitor targeting Raf,EGFR,VEGF receptor (VEGFR),PDGF receptor (PDGFR),FMS-like tyrosine kinase-3 (FLT3) and c-kit[45,46].Clinical studies have revealed that sorafenib inhibits hepatic tumor growth and angiogenesis in advanced stage,but its prolonged exposure induces resistance[47-50].Recently it has been reported that the second-line drugs such as lenvatinib,regorafenib and ipilimumab have a better therapeutic outcome,and increase overall disease-free survival in liver cancer patients[51].Increased tumor growth and distance metastasis in sorafenib resistance patients and lower overall survival rates in sorafenib-treated liver cancer patients necessitate exploring new and potential therapeutic targets in liver cancer.Exploration of newer therapeutic agents and combinatorial drug regimens may also be explored to target the disease and increase the therapeutic outcome in patients.The current treatment strategy for liver cancer (first- and second-line therapies) is discussed in more detail in the subsequent section of this review.
Luoet al[52] identified emerging targets in liver cancer by utilizing comprehensive and integrated multiomics analysis.The study identified potential signaling pathways (Tp53/RB1,Wnt/β-catenin,PI3/Akt/mTOR,JAK/STAT,MAPK and TGF-β) and molecular events (telomere maintenance,cellular differentiation,chromatin remodeling and oxidative stress) in liver cancer.Mutation-mediated protein activation (CCND1,CTNNB1,TERT,PIK3CA,KRAS,KEAP1,NFE2L2,JAK3,FGF4,FGF19,and FGF3)and inactivation (TP53,Rb1,CDKN24,CHN2B,ATM,AXIN1,APC,ZNRF3,HNF1A,APOB,ALB,ARID1A/B,ARID2,SMARC2,BAP1,BRD7,KMT2C,PTEN,TSC1,TSC2,RPS6KA3,and ACVR2A) are associated with the pathophysiology of liver cancer and have emerged as therapeutic targets for hepatic cancer[52].β2-spectrin (SPTBN1),a cytoskeleton protein is essential for the development of various organs,including the liver.It performs both structural (establishment and maintenance of cellular structure) and functional (apoptosis,cell adhesion,and cell cycle regulation) role[53].Recently it has been reported that SPTBN1 induces lipogenesis-mediated liver cancer in high-fat diet fed experimental mice.The study proposed SPTBN1 as a potential therapeutic target for liver cancer[54].Craiget al[55]studied the expression profile of cancer testis antigens (CTA) proteins in HCC.CTA was overexpressed in HCC patients and associated with poor overall survival and prognosis.Further experimental evidence of the study showed that melanoma-associated antigens family A (MAGE-A),a member of the CTA family,is responsible for increasing cellular proliferation,and decreased apoptosis and aggressiveness in HCC experimental models.The study revealed that MAGEA3 is involved in the developing hepatic carcinoma and could serve as a potential novel target for the disease[55].Glypican (GPC)-3,a heparin sulfate proteoglycan,was significantly overexpressed in >80% of HCC patients and was positively associated with poor diagnosis in the patients[56,57].Clinical studies showed that targeting GPC-3 by developed antibodies significantly increased disease progression-free survival in patients with overexpressed GPC-3 in comparison with patients with low GPC-3 levels.Combination of chemotherapy and the immunotoxin (antibody + exotoxin) mediated GPC-3 targeting showed better therapeutic outcomes in liver cancer patients[58-60].These facts indicate the therapeutic potential of GPC-3 proteins in liver cancer.Interaction between HGF and its receptor c-Met is important in liver regeneration.Overexpression and/or mutation in c-kit have been positively associated with liver cancer[61].Direct or indirect (viadifferent signaling pathways) interaction among HGF and c-kit increases the cellular growth,angiogenesis and metastasis in liver cancer cells[62].Preclinical and clinical studies reported that interrupting the association between HGF and c-kit resulted in a potential therapeutic response in liver cancer[63-65].Thus HGF and/or c-kit are potential therapeutic targets in liver cancer.Various studies showed that cancer cells rewire their metabolic pathways to fulfil their increased need for nutritional requirement.Liver cancer cells also reprogram their lipid metabolic pathway to combat their increased nutritional requirements,which ultimately help in cellular proliferation,growth and survival.Preclinical studies have shown that biosynthesis of lipids and desaturation process play an important role in liver cancer initiation,progression and survival.Popeet al[66]beautifully reviewed aberrant biochemical/molecular players of lipid metabolism as potential therapeutic targets in liver cancer[66,67].Overexpression of lipid metabolism enzymes such as fatty acid synthetase,ATP citrate lyase,stearoyl-CoA desaturase (SCD)-1,and acetyl CoA carboxylase have been associated with various cancers including liver cancer.Targeting these enzymes with small molecules showed a potential tumor-suppressive nature in experimental models of liver cancer.There is a need to study some enzyme inhibitors in the clinical trial,such as SCD-1 inhibitors[68-70].
miRNAs are short-length noncoding RNAs involved in regulating gene expression and thus controlling the normal physiology and disease pathophysiology by normal and abrupt expression,respectively[71].Modulating miRNAs by therapeutic molecules,and/or using their respective inhibitors or mimics is an important strategy to target cancer at the gene level[72].The study showed that aberrant expression of miRNAs (miR34,miR36,miR21,miR203,miR17,miR83,miR93,miR221,etc.)in liver cancer cells is associated with the increased cellular proliferation,metastasis,angiogenesis,drug resistance,cell survival and reduced apoptosis[72].A miRNA-based mouse model of HCC has been developed to study inflammation,tumor initiation,metabolic alteration,and hepatocyte differentiation[73].The therapeutic potential of miRNAs in liver cancer has been shown by utilizing the miRNA inhibition/replacement approach.One studyidentified miR-550a,miR-574,miR-424,let-7i,miR-549,miR-518 and miR-512 as being significantly associated with overall survival,using bioinformatics tools that indicated their therapeutic potential.The study proposed that these miRNAs should be studied in detail for their therapeutic potential in liver cancer experimental models[74].Daiet al[75] compared the publically available liver cancer miRNA expression data with the human HCC (hepatitis B positive and negative) data (generated by the study group).The study identified miR-0308-3p as a novel miRNA associated with HBV-positive HCC.miRNA suppresses liver cancer cell proliferation and arrests cells in the G1/S phase by targetingCDK6andcyclin1genes[75].These results show that the miR-0308-3p is a novel therapeutic target in liver cancer.Shaoet al[76]developed personalized miRNA cocktail therapy by combining nanotechnology and gene therapy to treat liver cancer.The research group encapsulated mimics (of miR-199a/b-3p) and inhibitor (of miR-10b) into a polymer-based nanoplatform (PCACP).Thein vitroandin vivoexperiments showed the better anticancer potential of the PCACP/miR-cocktail system in comparison with mimic or inhibitor treatment alone in liver cancer experimental models[76].This study showed a novel potential strategy to treat liver cancer by combining nanotechnology and gene therapy.Wanget al[77] studied the relation between LINC01018 (a long noncoding RNA),miR-182-5p and FOXO1 protein in HCC.There was poor expression of the long noncoding RNA and FOXO1,and higher expression of miR-182-5p in the HCC patient samples.Forced expression of LINC01018 inin vitroandin vivoexperimental models showed decreased cellular proliferation and induced apoptosis with increased miR-182-5p levels.The study showed liver cancer therapeutic potential of LINC01018 by miR-182-5p sponge-mediated downregulation of FOXO1 expression[77].
Current treatment strategies for liver cancer
The dysregulated cell cycle,apoptosis,and many other key signaling pathways are linked to HCC pathogenesis.Chemotherapeutic approaches similar to different types of cancer are also reported with a limited number of drugs for the cure of HCC and various side effects.Sorafenib,an oral multitargeted tyrosine kinase inhibitor has been used as first-line treatment for advanced HCC,showing increased survival of approximately 12 mo compared with controls[78].Various antiangiogenic agents such as bevacizumab (human monoclonal antibody directed against VEGF) and erlotinib (EGF receptor tyrosine kinase inhibitor) have also been studied and shown effective results in early studies[79].Until 2016,sorafenib was the only FDA-approved first-line treatment for HCC,whereas lenvatinib has also been identified and is in use for advanced HCC[80].Sorafenib acts as an inhibitor of intracellular tyrosine and serine/threonine protein kinases such as VEGF,VEGFR,PDGFR,c-Raf and b-Raf MAP kinases,which in turn induces autophagy.Due to drug resistance and side effects such as liver fibrosis,clinical usage of sorafenib is limited[63].Long-term exposure to sorafenib also induces cancer cells with less E-cadherin content making them more invasive.Some second-line treatments are also available for HCC,including regorafenib,ramucirumab and cabozantinib,which are rarely used and are less efficient[28,51,79].It is reported that chemotherapeutic drugs used for HCC treatments are limited in number and seem to be less effective,considering their efficacy,bioavailability and side effects.Considering the side effects of ongoing therapies,scientific pieces of evidence are also suggestive for the use of natural products for the management of HCC,since they can inhibit viral infection,inflammation,oxidative stress,metabolic disorders,angiogenesis and metastatic activity,which are known as prime contributors in HCC[2,80,81].Hence,there is strong demand for searching novel plant-based drugs for managing HCC with fewer side effects and less chemotoxicity.Therefore,several drugs are used to treat HCC to target the inhibition of some of these processes (Figure 1).The current therapeutic interventions for patients with HCC are divided into first- and second-line therapies.The pharmacological features of these drugs are discussed in the following section of this review.
First line therapies
Sorafenib: Sorafenib (BAY 43-9006,Nexavar) is the first-ever systemic drug as well as a standard therapeutic agent approved by the US FDA for treatment of liver cancer patients who cannot undergo surgical resection or liver transplantation[79].Sorafenib was the only first-line treatment in the last 10 years until the FDA approved lenvatinib as a frontline therapy in 2018.It is a tyrosine kinase inhibitor that targets VEGFR1 and VEGFR2 and PDGFR-β.It activates AMP-activated protein kinase (AMPK)that can block the formation of tumor blood vessels and inhibit proliferation of liver cancer cells[82].For individuals with HCC,sorafenib has a clear advantage in terms of survival.Sorafenib improved overall survival considerably compared with placebo in two phase III clinical randomized controlled trials (10.7 movs7.9 mo and 6.5 movs4.2 mo).However,the side effects associated with these clinical trials were diarrhea,tiredness,and hand–foot skin response[80,82].
Several factors hinder more people from obtaining benefits after sorafenib treatment.Because of the genetic variability of HCC and other factors,around 40% of people with HCC can benefit from sorafenib.Sorafenib was more beneficial for some patient categories in several trials.The two clinical studies mentioned above featured only a small number of patients;all of whom had good liver function.These individuals were termed Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol(SHARP)-eligible patients,and only SHARP-eligible individuals benefited from sorafenib treatment[79,82].Furthermore,the effectiveness of sorafenib is greater in HCV-infected individuals than in others who have always been resistant to sorafenib.Primary resistance is another term for the unclear mechanism of this phenomenon[82,83].However,some research has uncovered probable explanations.Gene polymorphism may be a crucial factor influencing sorafenib function.Polymorphisms in the ATP binding cassette (ABC) subfamily B member 1 (ABCB1),ATP binding cassette subfamily G member 2(ABCG2),solute carrier family 15 member 2 (SLC15A2) and endothelial nitric oxide synthase (eNOS)have been linked to the action of sorafenib[83].This was confirmed by Silvia and co-workers who reported that β-caryophyllene oxide inhibits ABC proteins and causes HCC cells to become chemosensitized to sorafenib[84].
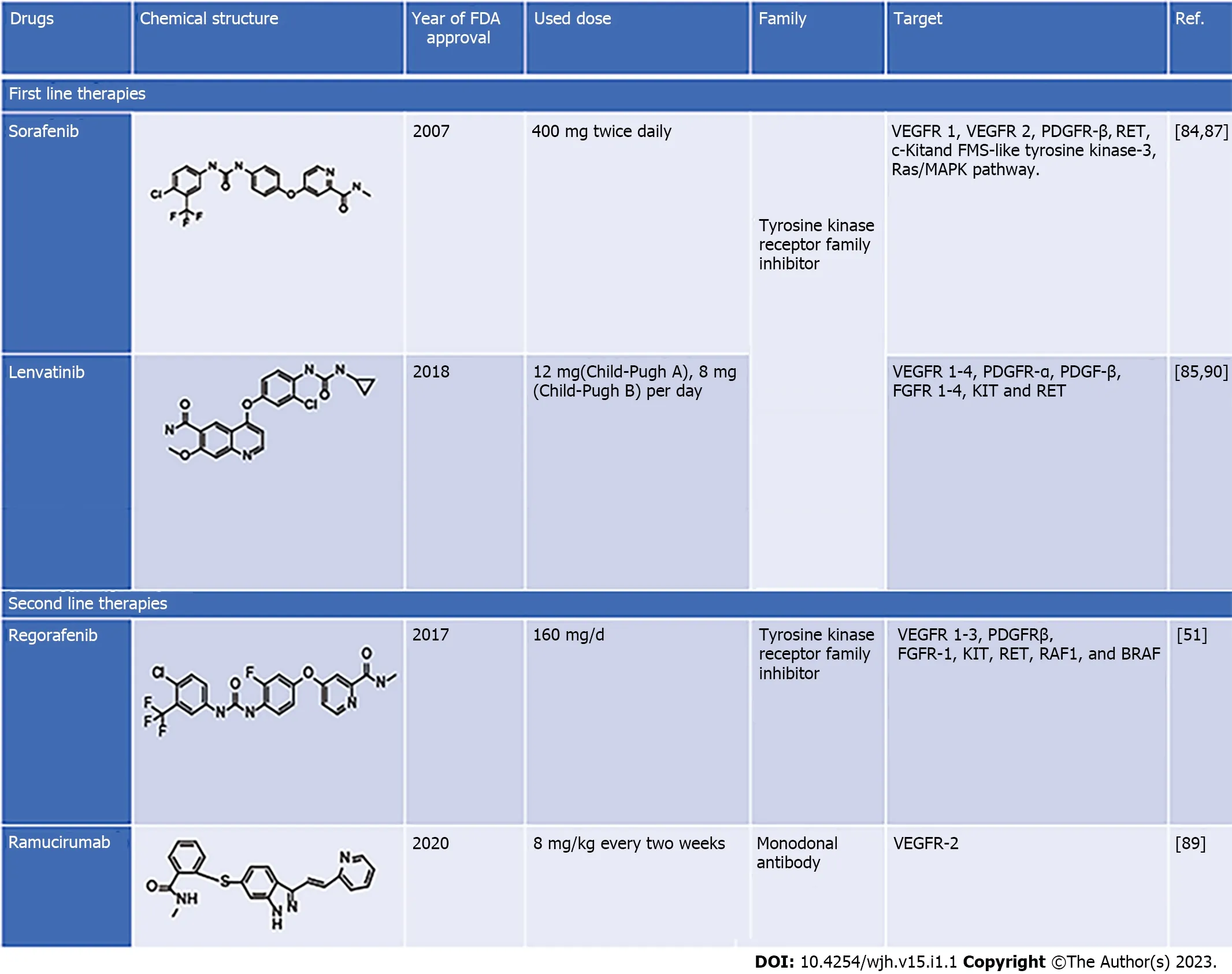
Figure 1 First- and second-line therapies and their targets.
Lenvatinib:Lenvatinib (E7080,Lenvima) is an antitumor drug that belongs to the quinoline carboxiamides.The IUPAC name of lenvatinib is 4-[3-chloro-4-(cyclopropylcarbamoylamino) phenoxy]-7-methoxyquinoline-6-carboxamide.Lenvatinib acts as multikinase inhibitorviatargeting VEGFR 1-4,PDGFR-α,PDGFR-β,FGFR 1-4,tyrosine kinase receptor (KIT) and rearranged during transfection receptor (RET) that leads to angiogenesis inhibition,and reduced vascular permeability of the tumor microenvironment[28].Lenvatinib is an effective drug that increases overall survival in patients with advance HCC and whose tumor cannot be removed by surgery.In a phase I clinical trial,lenvatinib (12 and 8 mg) was effective in patients with advanced HCC and Child–Pugh class A or B.The adverse effects observed during 12 mg daily lenvatinib oral treatment were hypertension,decreased body weight,loss of appetite fatigue,and diarrhea[28,70].A Phase II clinical trial was conducted to evaluate the effectiveness of lenvatinib in advanced unresectable HCC.The trial was conducted on 46 patients who received 12 mg lenvatinib orally once daily for 28 d,and lenvatinib demonstrated high efficacy with a good toxicity profile.However,the efficacy of lenvatinib was influenced by body weight[85,86].
Second-line therapies
Regorafenib:Regorafenib (BAY-73-4506) with the brand name Stivagra is an oral multikinase receptor antagonist developed by Bayer and approved by the US FDA in June 2017 to treat unresectable advanced liver cancers.Despite its structural similarity with sorafenib,regorafenib showed more effectiveness in inhibiting the activities of various protein kinases associated with neovascularization (VEGFR 1–3 and tyrosine kinase with immunoglobulin-like loops and epidermal growth factor homology domain-2 (TIE2),oncogenesis (KIT,RET,Raf1 and BRAF) and tumor microenvironment (PDGFR-β,PDGFR-α and FGFR) with better drug tolerance profile[87,88].HCC patients treated with regorafenib(160 mg/d for 28 d) showed better overall survival,i.e.,10.6 mo compared with 7.8 mo in the placebo group in a randomized,double-blind,placebo-controlled phase III trial.However,the main side effect was hypertension,unlike body weight loss,hepatorenal dysfunction,and fatigue in sorafenib-treated individuals[87,88].
Ramucirumab:Ramucirumab,sold under brand name Cyramza and others,is a recombinant monoclonal antibody (IgG) that targets VEGF2 and blocks its binding to VEGFR ligands.The anticancer activity of ramucirumab as second-line therapy was evaluated in Phase II clinical trials in advanced HCC patients with a high level of α-fetoprotein.These trials found that individuals who received ramucirumab had a better overall survival rate than those who received placebo;the drug was well tolerated and had an acceptable toxicity profile[89].
Future promising therapeutic drugs
Pirfenidone: Pirifenidone (Esbriet®) is an orally administered antifibrotic,antioxidant,and anti-inflammatory drug that has been studied in clinical and preclinical trials to treat hepatic and idiopathic pulmonary fibrosis[86].Pirifenidone was effective in causing cell cycle arrest at G0/G1,eventually inhibiting cell proliferation in anin vitromodel.Similarly,it induces apoptosis in HepG2 cellsviaWnt/β-catenin signaling pathway.Pirifenidone has also been demonstrated to be a potent antifibrotic agent at a dose of 300 mg/kg in a carbon tetrachloride-induced HCC mouse model.However,the cellular mechanisms behind the responses elicited by pirifenidone remain unknown[70,86].Figure 1 summarizes the pharmacological properties of drugs used in liver cancer[86].
GUT MICROBIOTA AND LIVER CANCER
Multiple lines of scientific evidence have suggested the significant contribution of gut microbes to critical aspects of human health.Even though the gut microbiota offers substantial benefits to the host,particularly in terms of immunity and metabolic activities,there is still growing evidence of the role of gut microbes in several pathological conditions.They promote disease progression not just locally,as in chronic inflammatory bowel syndrome,but also in other parts of the body,such as liver,brain and heart[91].Similarly,there is mounting evidence that the gut microbiota plays a significant role in carcinogenesisviaits local and long-distance effects.The liver is intimately connected to the gut through the portal vein.The liver is directly exposed to microbial metabolites and microbe-associated molecular patterns (MAMPs) that can induce inflammatory reactions through pattern-recognition receptors,and receive nutrient-rich blood from the gut.The multilayer epithelial barrier is responsible for minimal hepatic exposure to MAMPs.Although,as in chronic liver diseases,altered gut barrier and microbiota composition increases the incidence of inflammation and progression of liver disorder and thus raises the risk of HCC[92].
According to accumulating scientific evidence,intestinal dysbiosis appears to have a significant role in developing chronic liver disease and HCC.Metagenomic studies have demonstrated significant changes in the gut microbiota composition in a variety of chronic liver diseases as well as in people with cirrhosis[93].Patients with advanced liver disease and cirrhosis have an increase in potentially harmful bacteria and a decrease in microorganisms with beneficial qualities in their gut microbiomes[94,95].
Toll-like receptor (TLR)4 is found in various liver resident cells such as Kupffer cells,hepatic stellate cells (HSCs),endothelial cells,and hepatocytes.A study conducted by Dapito and colleagues in bone marrow chimeric mice concluded that the presence of TLR4 on these liver-resident cells promotes fibrogenesis and hepatocarcinogenesis[96].Lipopolysaccharide (LPS),a Gram-negative bacterial cell wall component,is produced through the leaky gut and mainly targets Kupffer cells and HSCs,which appears to increase the incidence of hepatocarcinogenesis.Activation of TLR4 in HSCs causes nuclear factor (NF)-κB-mediated increased expression of epiregulin,a hepatic mitogen belonging to the EGF family,and reported to have strong mitogenic potential in hepatic cells[96,97].The finding was confirmed when hepatocarcinogenesis decreased in epiregulin-deficient rats treated withN-nitrosodiethylamine (DEN)-CCl4.Another important method through which the LPS–TLR4 axis promotes HCC development is through prevention of NF-κB-mediated hepatocyte apoptosis[96,97].
BIOACTIVE NATURAL PRODUCTS AGAINST LIVER CANCER AND MOLECULAR MECHANISMS INVOLVED
For centuries,bioactive natural products from plants have been extensively used to treat many human diseases.Recent molecular evidence explains their modes of action,metabolic regulation,and identification of their biological targets.This evidence adds value to their potential use in the chemoprevention of HCC.The promising candidate bioactive natural products are discussed in this section,where their possible role in liver cancer therapy has been reported.
In vitro studies
In the last two decades,growing evidence has suggested an affirmative role of resveratrol (polyphenolic natural product) in the chemoprevention of liver cancer.Its application is limited due to its poor bioavailability.Previously,resveratrol was shown to negatively regulate the cellular proliferation of rat hepatoma and human hepatoblastoma cell line HepG2 at 1–150 μmol/L concentration[98].Decreased proliferation and invasion of HepG2 cells and AH109A rat ascites hepatoma cells were also reported.In subsequent studies,resveratrol induced apoptosis inin vitrostudies using HepG2and H4IIE rat hepatoma cells[98].Notaset al[99] showed that even 2 h treatment with resveratrol (10-6–1 μmol/L) interfered with DNA replication and caused cell cycle arrest.Roncoroniet al[100] using SK-ChA-1 human cholangiocarcinoma cells in a multicellular tumor spheroid model showed arrest of cell cycle at G1/S phase,at a concentration up to 64 μmol/L resveratrol.Resveratrol limited cellular proliferation and mobility by activating autophagy through p53 and inhibiting phosphoinositide 3-kinase/Akt in MHCC-97H cells.Autophagy thus explained the increased chemopreventive property of resveratrol.A study on HepG2 and Hep3B cells identified that resveratrol regulates the PTEN/Akt signaling pathway through downregulation of membrane-associated RING-CH (MARCH1),which ultimately aggravates apoptosis and inhibits cellular growth[101].
A curcumin analog,CUR3d,inhibited the proliferation of liver cancer cells at 100 μmol/L,which was due to downregulation of PI3K/Akt and inhibition of the NF-κB pathway,which are responsible for cancer cell growth[102].In another study,supplementation of curcumin (1 g/kg) significantly inhibited the growth and liver metastasis of colorectal cancer cells[103].Microemulsion formulation improve 1225 times the water solubility of myricetin and enhanced its antiproliferative activity against human liver cancer cells (HepG2)[104].
Extract of immature plum induced extrinsic apoptosis in HepG2 cells as demonstrated by caspase-1,-3 and -8 activation as well as DNA fragmentation[105].Two natural polyphenolic compounds (epicatechin and gallocatechin gallate) were quantified in the extract and might be responsible for the anticancer potential[106].The garlic extracts consist of multiple organosulfur components and flavanols that obstruct different stages of the carcinogenic process.Diallyl sulfide is one of the important component of garlic extract and has inhibited diethylnitrosamine (DEN) induced HCC.Another constituent of Allium extracts,S-allyl cysteine,has established antiproliferative and metastatic activity in the management of HCC[107].6-Shogaol and 6-gingerol are the most common active constituents in ginger that display anticancer activity against hepatoma cell lines by triggering reactive oxygen species(ROS)-mediated apoptosis and controlling expressionof matrix metalloproteinases (MMP)-9 and tissue inhibitor of metalloproteinase-1[108].In vitroandin vivoactivities of many natural products are depicted in Table 1.
In vivo studies
Intraperitoneal resveratrol administration (1 mg/kg) for 7 d in male Wistar rats implanted with AH-130 hepatoma cells arrested tumor growth.Liuet al[109] showed the immunomodulatory role of resveratrol(500,1000,1500 mg/kg for 10 d) in BALB/c mice implanted with H22 hepatoma cells.Rajasekaranet al[110] studied the chemopreventive role of resveratrol in a model of DEN-induced HCC in male Wistar rats.It induced apoptosis by PARP cleavage,caspase-3 activation,p53 upregulation and cytochrome c release when given early at a dose of 200 mg/kg.Gaoet al[111] tested the chemopreventive property of resveratrol in MHCC97-H-inoculated athymic nude mice.The study identified its antitumor activity by downregulating the HGF/c-Met signaling pathway.Resveratrol-gold nanoparticles have shown improved anticancer effects compared with resveratrol alone in HEPG2 cells and xenografted BALC/c nude mice[112].
Lycium polysaccharide portion (LPP) is the most crucial part ofLycium barbarumthat has abundant biological activities such as antioxidant,neuroprotective,immunoprotective,antitumor,and glucose metabolism regulatory activities.LPP inhibited the propagation of hepatocytes and led to apoptosis of liver hepatocytes,thus indicating its anticancer role.A clinical trial showed that consumption of LPP juice leads to elevation in interleukin (IL-2),IgG,serum antioxidants levels,lymphocyte count and reduced levels of lipid peroxides[113].Berberine mediates anticancer activity by inhibiting antiapoptotic protein Bcl-2,and activating the caspase cascade and proapoptotic pathway of Egr1-NAG-1(nonsteroidal anti-inflammatory drug-activated gene).Berberine facilitates phosphorylation of AMPK,thus increasing the concentration of p-AMPK/total AMPK.The AMPK-mediated mitochondrial/caspase pathway by raising the Bax/Bcl-2 ratio may be responsible for the anticancer activity of berberine.Long-lasting polyethylene glycol-based liposomal berberine displayedin vivoandin vitroanti-HCC activity[114].Paclitaxel-loaded nanoparticles,followed by galactosamine conjugation on the formed nanoparticles,were effective in reducing the tumor size through apoptosis activation and cell cycle arrest[115].The efficacy of many natural products against liver cancer is shown in Table 1.
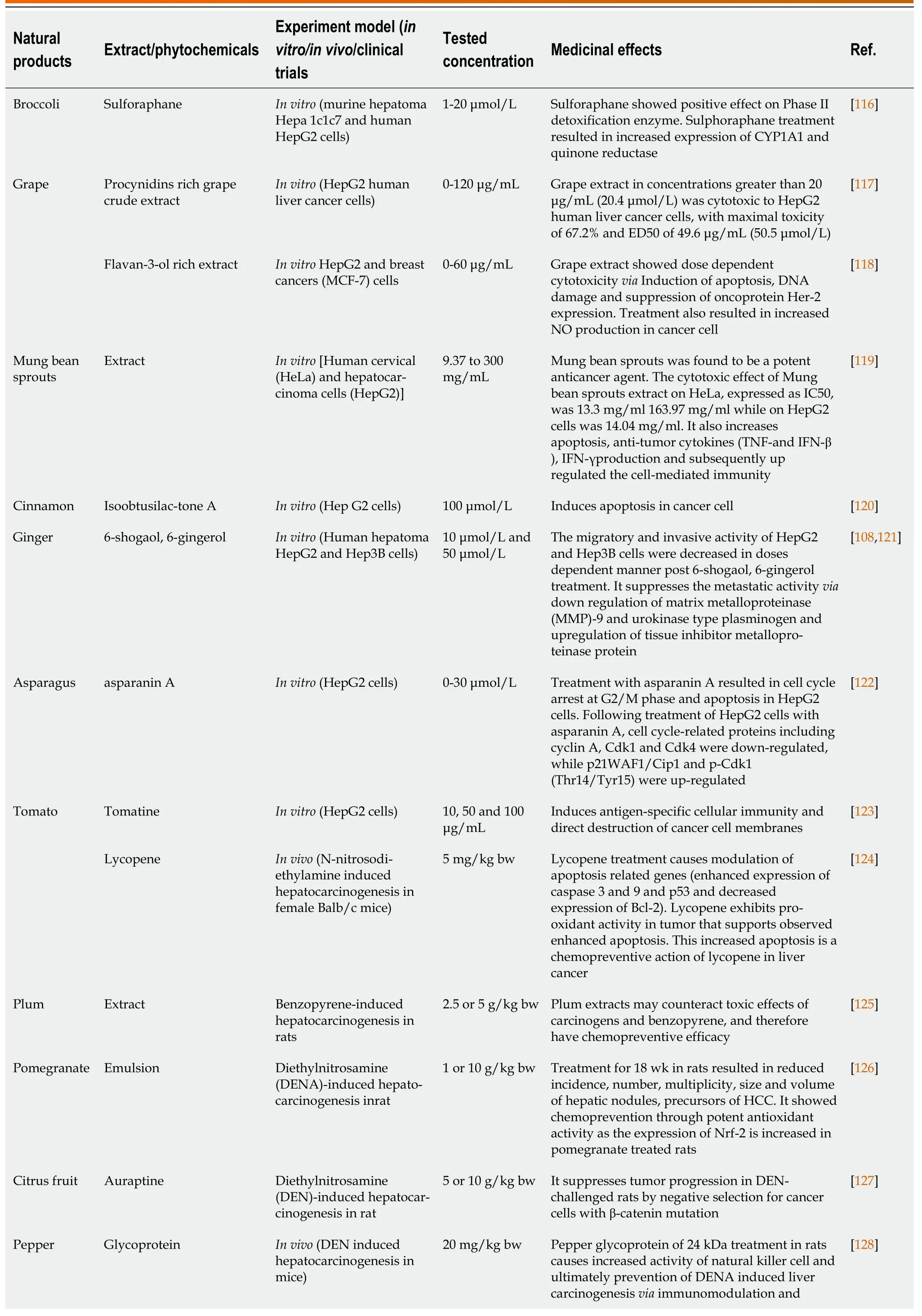
Table 1 Effect of natural products on liver cancer

promotion of apoptosis
CHALLENGES AND WAY FORWARD IN NATURAL-PRODUCT-BASED ANTI-LIVER CANCER THERAPEUTICS
Natural products have become a focus of attention in anticancer drug discovery due to unsolved problems related to current chemotherapy,such as drug resistance and toxicity.It should be noted that from 1940 to 2014,approximately 50% of the small molecules approved for cancer treatment were either natural compounds or their derivatives[129,130].Natural products for anticancer therapy have had some therapeutic limitations,which affect therapeutic outcome,lower bioavailability,and selected and targeted delivery.This section highlights these issues,recent advances in the field,and future potential.Advancements in computational biology/pharmacology/chemistry and high-throughputin vitroscreening of natural anticancer drugs have highly accelerated the drug discovery process,resulting in a lead molecule.Most of the time,it is frustrating to obtain unsatisfactory activity of the lead natural molecule inin vivoexperiments and/or clinical studies,which results in lesser activity and nonselectivity for a given therapeutic target.It has been proposed that delivering natural products to a targeted site using an appropriate delivery system may improve the efficacy by increasing their bioavailability.The process may also decrease the off-target effects and toxicity related issues in a given therapy[131].Different means of drug delivery or appropriate vehicles have been discussed elsewhere[132].The use of these tools/vehicles is dependent on their biocompatibility,degradability and functional limitations.However,the concept is promising but has its limitations (rapid elimination from the body,toxicity and inflammation),which still need to be addressed[132,133].
To consider the efficacy of natural products in living systems,it is essential to understand their pharmacokinetics.Absorption,distribution,metabolism and excretion may primarily affect the therapeutic outcome of the natural products.Absorption of a particular drug is influenced by the mode of administration,i.e.,whether it is oral,intravenous or inhalation.In each case,the drug shows different kinetic behavior in relation to its therapeutic outcome.Factors such as permeability of barriers,pH of cellular/body compartments,binding affinity with the off-targets and their fat solubility affect the distribution of the natural products in the body.Drug metabolism in the liver or gut introduces alterations in the structure of natural products,as well as irreversible secretion of the drugs through the hepatobiliary system or kidneys,which affects the plasma level of the drug and its efficacy.Few reports are available on the pharmacokinetics of natural products (such as glycyrrhetinic acid,curcumin,ethiodized oil) in liver cancer experimental models or patients[103,104,134].Most of the lead anti-liver cancer natural products have not yet been studied for the above pharmacokinetic parameters in experimental models.Information on the pharmacokinetic parameters of the particular natural products may shed light on the efforts that should be taken to improve their therapeutic efficacy inin vivoexperimental models and liver cancer patients.
New approaches have been introduced to improve the natural product delivery and specifically target liver cancer cells.Previously it has been reported that tissue-targeted drug delivery significantly enhances the therapeutic efficacy of anti-liver cancer drugs,confining their bioavailability within the tumor.The concept of tissue targeted drug delivery also minimizes the side effects such as toxicity by reducing systemic bioavailability to other organs of the body.Anti-liver cancer drugs combined with a delivery system providing galactose residues have been utilized to target liver cells (with asialoglycoprotein receptors) specifically[135,136].Liposomes have been used as carriers for anticancer drugs due to various advantages such as improved drug stability in the body without altering the structural integrity of the drug[136,137].Liet al[138] studied the effect of natural product encapsulated galactosylated liposomes (NPEGLs) to assess their anticancer activity and liver cancer cell selectivity.The study found that anti-liver cancer activity of the NPEGLs was significantly increased compared with normal natural product–liposomes and free natural product treatment in liver cancer cells.Enrichment of NPEGLs with galactosylated stearate significantly increased the uptake of the delivery system by the liver cancer cells compared with gastric and non-small cell lung cancer cells[139].
Toxicity due to the off-target effect of the anticancer therapeutic drug is also an important problem in managing liver cancer at the clinical level.It is challenging to increase bioavailability and decrease offtarget effects of anti-liver cancer natural products without compromising therapeutic efficacy.This situation is more difficult when increased effectiveness of the product is required.Nanotechnologybased approaches are promising to provide the solution to this problem.It is possible to deliver the natural products using nanotechnology-based strategies,which not only increase the product’s biological activity but also enhance its bioavailability.Targeted delivery using these strategies also lowers toxicity by reducing the systemic circulation of the product.Geraet al[140] synthesized a phytocomposite nanoparticle and studied its anticancer efficacy in liver cancer cells.The naturalcompound-based nanoparticles produced significantly higher antiproliferative activity in liver cancer cells in comparison with free natural product (non-nanoparticle form).The study suggested that the increased activity of the nano-formulation of the natural product in comparison with its non-nano form might be attributed to its well dispersed,small-sized particles,and thereby increased cellular uptake.The study also suggested that the attraction of the formulation towards the acidic environment of liver cancer cells enhances the output of targeted therapy with less or no effect on normal cells.Thus,this type of strategy,in combination with other approaches (such as receptor targeting),could be utilized to selectively target liver cancer cells to avoid the off-target effects and increase the drug’s bioavailability[141,142].
CONCLUSION
In recent years,liver cancer has emerged as a significant public health concern worldwide.Various factors such as viral infection,alcohol abuse,drug-induced liver injury,or a high fat diet are the leading causes of mortality due to liver diseases.Different signaling pathways,including TGF-β,Wnt/B-catenin,Hedgehog,Notch,EGF,VEGF,JAK and Hippo,are responsible for the progression of liver cancer.Firstand second-line treatments produced better therapeutic outcomes than chemotherapy and increased overall disease-free survival in liver cancer patients.Intestinal dysbiosis appears to have a significant role in developing chronic liver diseases.The available modes of treatment include numerous side effects that could be minimized with the use of natural products such as resveratrol,curcumin,diallyl sulfide and many more.However,natural-product-based anticancer therapy also has some limitations,mainly concerning the therapeutic outcome,lower bioavailability,and newer targeted delivery approaches.Targeted drug delivery using NPEGLs and nano-formulations increased the biological activity and bioavailability of the drugs.
ACKNOWLEDGMENTS
Shashank Kumar and Sabyasachi Senapati acknowledge DST-FIST facilities of the Department of Biochemistry and Department of Human Genetics and Molecular Medicine,Central University of Punjab,Bathinda,Punjab.All the authors also acknowledge DST-FIST and UGC-SAP facilities of the Department of Biochemistry,University of Allahabad,Prayagraj,India.
FOOTNOTES
Author contributions: Pandey AK conceptualized the idea;Singh AK,Singh SV,Kumar R,Kumar S and Senapati S performed the literature search and wrote the first draft of the manuscript;Kumar R and Singh SV validated the references;Kumar S and Pandey AK critically reviewed and revised the manuscript;Pandey AK supervised the project;all authors have read and approved the final version of the manuscript.
Conflict-of-interest statement: All authors report no relevant conflict of interest for this article.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin: India
ORCID number: Amit Kumar Singh 0000-0002-4438-4427;Shiv Vardan Singh 0000-0002-6720-5719;Ramesh Kumar 0000-0002-8236-5016;Shashank Kumar 0000-0002-9622-0512;Sabyasachi Senapati 0000-0002-9448-7432;Abhay K Pandey 0000-0002-4774-3085.
S-Editor: Wu YXJ
L-Editor: Kerr C
P-Editor: Wu YXJ
杂志排行
World Journal of Hepatology的其它文章
- Therapeutic interventions of acute and chronic liver disorders: A comprehensive review
- Acute-on-chronic liver failure in patients with severe acute respiratory syndrome coronavirus 2 infection
- Detection of colorectal adenomas using artificial intelligence models in patients with chronic hepatitis C
- Prognostic role of ring finger and WD repeat domain 3 and immune cell infiltration in hepatocellular carcinoma
- Rising incidence,progression and changing patterns of liver disease in Wales 1999-2019
- Influence of non-alcoholic fatty liver disease on non-variceal upper gastrointestinal bleeding: A nationwide analysis
