Echinoside A from Pearsonothuria graeffei Exert the Cytotoxicity to MDA-MB-231 Cells via Mitochondrial Membrane and Modulation of PI3K/Akt/mTOR Pathway
2023-03-17LIHongyanCUIHuanhuanCONGPeixuXUJieXIEWancuiWANGYumingandXUEChanghu
LI Hongyan, CUI Huanhuan, CONG Peixu, XU Jie, XIE Wancui WANG Yuming,and XUE Changhu, *
Echinoside A fromExert the Cytotoxicity to MDA-MB-231 CellsMitochondrial Membrane and Modulation of PI3K/Akt/mTOR Pathway
LI Hongyan1),2), *, CUI Huanhuan2), CONG Peixu2), XU Jie2), XIE Wancui1), WANG Yuming2),and XUE Changhu2), *
1),,266042,2),,266003,
A kind of triterpene glycosides echinoside A (EA) was extracted from sea cucumber, and its yieldwas about 0.78%. The purity of EA was 99.0%, and its molecular weight was 1206 Da. EA was a linear tetrasaccharide attached to a pen- tacyclic triterpene aglycon. It inhibited the growth of MDA-MB-231 cells. The antitumor effect was related to elevate ROS level, decrease mitochondrial membrane potential, enhance caspase-3 expression, induce cells apoptosis and arrest cell cycle at G2/M phase. EA also dose-dependently suppressed the expressions of phophorylation proteins p-PI3K, p-Akt, and p-mTOR as analyzed by western blotting. These results suggested that EA caused MDA-MB-231 cells apoptosisintrinsic mitochondrial and PI3K/Akt/ mTOR pathway. EA can be a potential anti-breast cancer agent to enhance the clinical efficacy.
; echinoside A; cytotoxicity; PI3K/Akt/mTOR pathway
1 Introduction
Sea cucumber belongs to the invertebrate echinodermata and used as tonic food in China and other Asian countries. They contained various triterpene glycosides. So far, more than 700 kinds of triterpene glycoside have been isolated from sea cucumber (Bahrami and Franco, 2015). Triterpeneglycosides exhibited antitumor, antifungal, antiviral and he- molytic activities (Kim and Himaya, 2012; Park, 2014;Zhao., 2018). Among these activities, the antitumor activity was the most common biological property (Mon- dol., 2017). For example, eight triterpene diglycosides were isolated from sea cucumber, and five of them showed strong cytotoxicity on cancer cells HepG2 (hepatoma cancer), KB (epidermoid carcinoma),LNCaP (prostate cancer), MCF-7 (breast cancer), and SK- Mel2 (melanoma) (Cuong., 2017). Patagonicoside A fromand its desulfated derivative bothsuppressed the growth of Hep3B, MDA-MB231 and A549 cells (Careaga., 2009). Frondoside A from sea cucum-bercan inhibit the growth of breastcancer cells MDA-MB-231 with EC50of 2.5μmolL−1for 24h. Frondoside A increased the sub-G1 cell fraction through the activation of p53, caspases-9 and 3/7 cells death path- ways (Al Marzouqi., 2011). Frondoside A also induced human pancreatic cancer cells AsPC-1 apoptosisthe mi- tochondrial pathway and action of the caspase cascade (Li., 2008). The polar extract Frondonol-A5P from sea cucumbercaused G2/M phase cell cycle arrest and apoptosis in human pancreatic cancer cells S2013 and AsPC-1 (Roginsky., 2010). Cucumarioside A2-2 fromthe Far-Eastern sea cucumberhad cytostatic effect by blocking cell proliferation and DNA biosynthe- sis in S phase. It also induced tumor cells apoptosis in a caspase-dependent way (Menchinskaya., 2013). Fron-doside A and cucumarioside A2-2 both strongly induced leu- kemic cells apoptosis, and the apoptosis of cucumarioside A2-2 but not frondoside A was caspase-dependent (Jin.,2009). Stichoposide C frominduced human leukemia HL-60 and colorectal HL-60 cancer cells apop- tosis in a dose-dependent manner, and it also could activate Fas, caspase-8, caspase-3, cleavage of Bid, and mitochon- drial damage(Yun., 2012).
Sea cucumber triterpene glycosides have been reported to suppress the proliferation of various cancers (Aminin., 2015). Echinoside A (EA), as one of the most abun- dant saponins in sea cucumber, contains four monosaccha- rides with a sulfate group attached to C-4 of the first xylose residue (Kitagawa., 1980). EA exhibited various bio- logical activities, such as antitumor (Zhao., 2012), anti- obesity (Wang., 2014), stimulating hepatic fatty acid β-oxidation and suppressing fatty acid biosynthesis (Wang., 2014). Echinoside A inhibited the growth of prostate carcinomaand. Further mechanisms researchfound that EA induced cells apoptosis in a Top2-dependent manner (Li., 2010). The antitumor activity of echino- side A (EA) and ds-echinoside A (DSEA) fromwas compared. EA and DSEA exhibited marked anticancer activity in HepG2 cells by blocking cell-cycle progression and inducing apoptosis through mitochondrial pathway. DSEA-induced apoptosis was more potent than EA-induced apoptosis (Zhao., 2012). EA had a remarkable anti- tumor activity, but its detailed mechanisms still remain large- ly unclear.
PI3K/Akt/mTOR signaling pathway can regulate the in- tracellular functions of cell proliferation, growth, and sur- vival. Dysregulation of PI3K/Akt/mTOR signaling path- way was detected in breast cancers (Du Rusquec., 2020). As reported by the literature, this pathway was ac- tivated in triple-negative breast cancer (TNBC) (Pascual and Turner, 2019). In this text, the cytotoxicity effect of EAon MDA-MB-231 cells was investigated via detecting ROSlevel, cell apoptosis, cell cycle, mitochondrial membrane potential, caspase-3 expression, and the PI3K/Akt/mTOR signaling pathway.
2 Materials and Methods
2.1 Materials and Reagents
Sea cucumber (was acquired in aquatic mar- ket of Qingdao, Shangdong Province, China. MCF-7, MDA- MB-231 and BT474 breast cancer cells were purchased fromthe American Type Culture Collection. Phospho-Akt, phos- pho-PI3K, phospho-mTOR, anti-β-actin, Goat anti-mouse IgG, and Goat anti-rabbit IgG antibodies were purchased from CST (Beverly, MA). All reagents such as methanol, ethanol, NH4HCO3were all chemical grade without further purified.
2.2 Preparation and Structure Identification of Echinoside A
Echinoside A (EA) was extracted fromaccord- ing to the previous studies(Kitagawa., 1980; Dong., 2008). In brief, sea cucumber body wall was ground- ed into powders and extracted with 60% ethanol at room temperature for three times. The extracts were concentrated and isolated on an HP-20 resin column by eluting with dif-ferent concentrations of ethanol. The fraction that eluted with 80% ethanol was collected, and concentrated to ac-quire the triterpene glycosides. The sea cucumber saponins were purified on silica gel column and reversed silica gel column in sequence to acquire the fraction EA. The purity of EA was analyzed by HPLC. A ZORBAX Eclipse XDB- C18 column (4.6mm×150mm, 5μm) was used and detect- ed at 205nm. The mobile phase was acetonitrile and 0.1% NH4HCO3with a flow rate of 1.0mLmin−1. EA’s mole-cular weight and structure was identified with ESI/MS andNMR spectroscopy (Kitagawa., 1980; Dong., 2008).
2.3 CCK-8 Assay
The cytotoxicity of EA on breast cancer cells was car- ried out with CCK-8 cell proliferation and cytotoxicity as- say kit. Briefly, MCF-7, MDA-MB-231, and BT474 cells (2×104cells per well) were planted into 96-well plates andincubated overnight. EA with different concentrations (0.5, 1.0, 2.0, 4.0, and 8.0μgmL−1) was added and incubated cells for 24h and 48h. Then 10μL CCK-8 was added, and the plate was incubated further for 4h at 37℃. The absor- bance was measured at 450nm with a microplate reader (BioRad, USA).
2.4 Apoptosis Assay
The apoptotic ratio of EA on MDA-MB-231 cells was measured with an Annexin V-FITC Apoptosis Detection Kit (Invitrogen, USA). Cells at a concentration of 1×105cells per well were added into 6-well plates for 12h. Then 2.0, 4.0, and 8.0μgmL−1of EA was added respectivelyand incubated cells for 48h. Then cells were digested with try-psin, and stained with the apoptosis detection kit. The fluo- rescence intensity of cells was detected by a flow cytome- ter (ACEA, Agilent, USA).
2.5 Cell Cycle Assay
Whether EA would influence the cell cycle of MDA- MB-231 cells was detected by the fluorescent dye Propi- dium Iodide (PI). Briefly, MDA-MB-231 cells at a densityof 1×105cells per well were added into 6-well plates over- night. EA at the concentrations of 2.0, 4.0, and 8.0μgmL−1treated cells for 24h, then cells were harvested and fixed over-night. After cells were washed with PBS, they were dyed with PI for flow cytometry detection.
2.6 Reactive Oxygen Species Assay
Reactive oxygen species (ROS) level in EA treated cellswas detected by flow cytometry. 1×105MDA-MB-231 cells per well were added into 6-well plates to produce adherent cells, and EA at a concentration of 2.0, 4.0, and 8.0μgmL−1was used to treat cells for 48h. Then cells were collected with trypsin, and stained with the fluorescent dye DCFH- DA according to the kit’s instructions. Changes of cells’ fluorescent intensity were measured with flow cytometry.
2.7 Mitochondrial Membrane Potential Assay
The effect of EA on cells’s mitochondrial membrane po- tential (MMP) was detected with a flow cytometor after staining with the JC-1 fluorescent dye. 1×105MDA-MB- 231 cells per well were added into 6-well plates. Then cellswere treated with 2.0, 4.0, or8.0μgmL−1EA for 48h. Cells were collected and stained with JC-1 dye following the kit’s instructions. Changes of fluorescent intensity were mea- sured by flow cytometry.
2.8 Caspase-3 Assay
The expression level of caspase-3 in EA treated MDA- MB-231 cells were measured by flow cytometry. In brief, after the cells were treated with 2.0, 4.0, and 8.0μgmL−1EA for 48h, they were collected and stained with PE activecaspase-3 apoptosis kit. The fluorescent intensity was ana- lyzed by flow cytometry.
2.9 Western Blot Assay
Dysregulation of PI3K/Akt/mTOR pathway is one of the prominent features in breast cancer (Donnella., 2018).As previous reported, this pathway is related to breast can- cer cells’ apoptosis (Miricescu., 2021). Changes of the main proteins’ content in this pathway of MDA-MB-231 cells were measured by western blotting. After cells were treated with EA for 48h, the proteins in cells were collect- ed with RIPA buffer (Solarbio, China). The protease inhi- bitor and phosphatase inhibitor were added in RIPA buffer. The quantity of proteins in samples was analyzed with BCAprotein assay kit (Solarbio, China). 20μg of protein sam- ples were isolated on 10% polyacrylamide gels, and then shifted onto a polyvinylidene fluoride membrane. After seal-ed in blocking solution, the PVDF membrane was incubatedwith p-PI3K, p-Akt, p-mTOR, and β-actin antibody (1:1000) (CST, USA). After the PVDF membrane was rinsed with TBST, it was labeled with the HRP-conjugated secondary antibodies. Finally, the proteins were visualized by an en-
hanced chemiluminescense kit from Beyotime Biotechno- logy (Nanjing, China), and the proteins expression level was quantified with Image J software.
2.10 Data Analysis
Data were repeated three times and expressed as mean±SD (standard deviation). GraphPad Prism 5.0 software was used to analyze the data.<0.05 was considered sta- tistically significant.
3 Results and Discussion
3.1 Structural Characterization of EA
The triterpene glycosides EA was isolated from, and the yield was about 0.78% relative to the dry weight of sea cucumber. As detected by HPLC (Fig.1a), the purity of EA was about 99.0%, and it met the requirements for further experiment. The molecular weight of EA was 1206Da analyzed by MS, and its structural characteriza- tion was identified with1H and13C NMR spectra (Figs.1a, b). By comparing with the results reported by Dong. (2008), the probable structure of EA was listed in Fig.1b. EA contained a linear tetrasaccharide attached to a penta- cyclic triterpene aglycon.
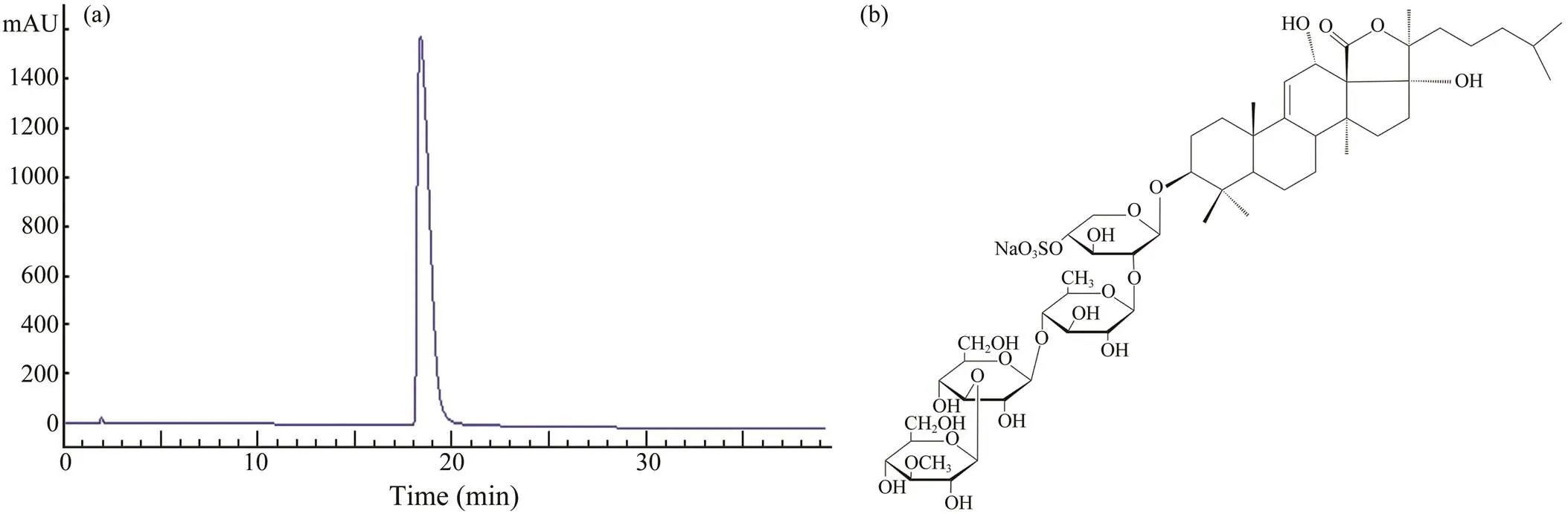
Fig.1 Purity of echinoside A analyzed by HPLC (a), and the probable chemical structure of echinoside A (b).
Generally, the dry weight of one sea cucumber was about 10g, and the yield of EA inwas about 0.78%, so the content of EA in one sea cucumber was about 78mg. For an adult woman of 60kg, her body water was 50% of her weight (Mattoo., 2020). After eating one sea cucumber, the concentration of EA in her body was 2.6μgmL−1. In this text, the concentration of EA was set as 0.5, 1.0, 2.0, 4.0, and 8.0μgmL−1for its cytotoxic activity to breast cancer cells.
3.2 CCK-8 Analysis
Theefficacy and cytotoxicity of EA against breastcancer cells was measured by CCK-8 assay. After cells were treated with 8.0μgmL−1EA for 48h, the inhibition rates on MCF-7, BT474, and MDA-MB-231 cells was about65.0%, 68.3%, and 64.6%, respectively (Figs.2a, b, c). EA suppressed MCF-7, BT474, and MDA-MB-231 cells’ growth time- and dose-dependently. When 0.5, 1.0, 2.0, 4.0, 8.0μgmL−1of EA and 5.0μgmL−1Dox incubated MDA-MB- 231 cells for 48h, the changes of cells’ morphology were captured with phase contrast microscope. As shown in Fig. 2d, cells were decreased in number, and tended to become rounded, individualized, and condensed with the increase of EA’s concentrations. This suggested that the treatments of EA led to the repression of MDA-MB-231 cells.
At the concentrations from 0.5μgmL−1to 8.0μgmL−1, EA exhibited anti-proliferative effect to breast cancer cells. So one sea cucumber (2.6μgmL−1EA for an adult woman) can effectively prevent the occurrence and progress of breastcancer, and sea cucumber was an efficient dietary supple- ment in protecting breast cancer patients.
3.3 ROS Analysis
The homeostasis of intracellular ROS level is vital for cells’ survival and growth. After MDA-MB-231 cells were incubated with different concentrations of EA, the fluore- scent dye DCFH-DA was used to detect ROS level. In Figs. 3a and 3d, it showed that EA significantly elevated ROS levels in MDA-MB-231 cells by comparing with the con- trol group. This demonstrated that EA induced MDA-MB-231 cells apoptosis possiblyproducing excess intracel- lular ROS. Elevation of intracellular ROS in cancer cells could lead to the damage of cells, and this phenomenon wasobserved in clinic (Redza-Dutordoir and Averill-Bates, 2016). The damage to cancer cells via increasing ROS level could enhance the chemotherapy effect (Galadari., 2017).

Fig.2 Cytotoxic effect of EA on breast cancer cells MCF-7 (a), BT474 (b), and MDA-MB-231 (c) for 24h and 48h. Mor- phology of MDA-MB-231 cells after incubated with different concentrations of EA (0, 0.5, 1.0, 2.0, 4.0, and 8.0μgmL−1) and Dox (5.0μgmL−1) for 48h (d).
3.4 Mitochondrial Membrane Potential (MMP) Analysis
Mitochondrial dysfunction has been shown to participate in the induction of apoptosis, and the elevation of ROS level causes the decrease of MMP. Changes of MMP in cells were usually detected by the florescent dye JC-1 and quantified by flow cytometry (Figs.3b, e). By comparing with the control group, the fluorescence of Green/Red ra- tio in EA group was noticeably increased dose-dependent- ly. This indicated that EA leaded to severe mitochondrial dysfunction in MDA-MB-231 cells.
3.5 Caspase-3 Analysis
Caspase-3 is considered as a primary executor of apop- tosis. The expression level of caspase-3 in MDA-MB-231 cells was measured with a quantitative flow cytometric ana- lysis. By contrast with the control group, EA at the concen- trations of 2.0, 4.0, 8.0μgmL−1could enhance caspase-3’s expression, and this effect remarkably increased dose-de- pendently (Figs.3c, f). These results demonstrated that EA could induce caspase-3-dependent apoptosis.
3.6 Cell Cycle Analysis
In order to identify the underlying mechanisms of EA on the inhibition of MDA-MB-231 cells, the cell cycle was detected by flow cytometry. After cells were treated with EA for 48h, the fluorescence PI dye was used to stain cells. As presented in Figs.4a, c, the population of cells in G2/M phase significantly elevated, while that in G0/G1 phase sig- nificantly reduced. This indicated that EA could inhibit the growth of MDA-MB-231 cells via arresting the cell cycle in G2/M phase.
3.7 Apoptosis Analysis
In order to explore whether EA suppressed the prolifera- tion of MDA-MB-231 cells through inducing apoptosis, af- ter cells were incubated with EA for 48h, cells were col- lected and stained with the apoptosis detection kit. The flowcytometry quantitative analysis indicated EA can lead to MDA-MB-231 cells apoptosis dose-dependently (Figs.4b, d). This effect was identical to the up-regulation of ROS and caspase-3 in MDA-MB-231 cells.
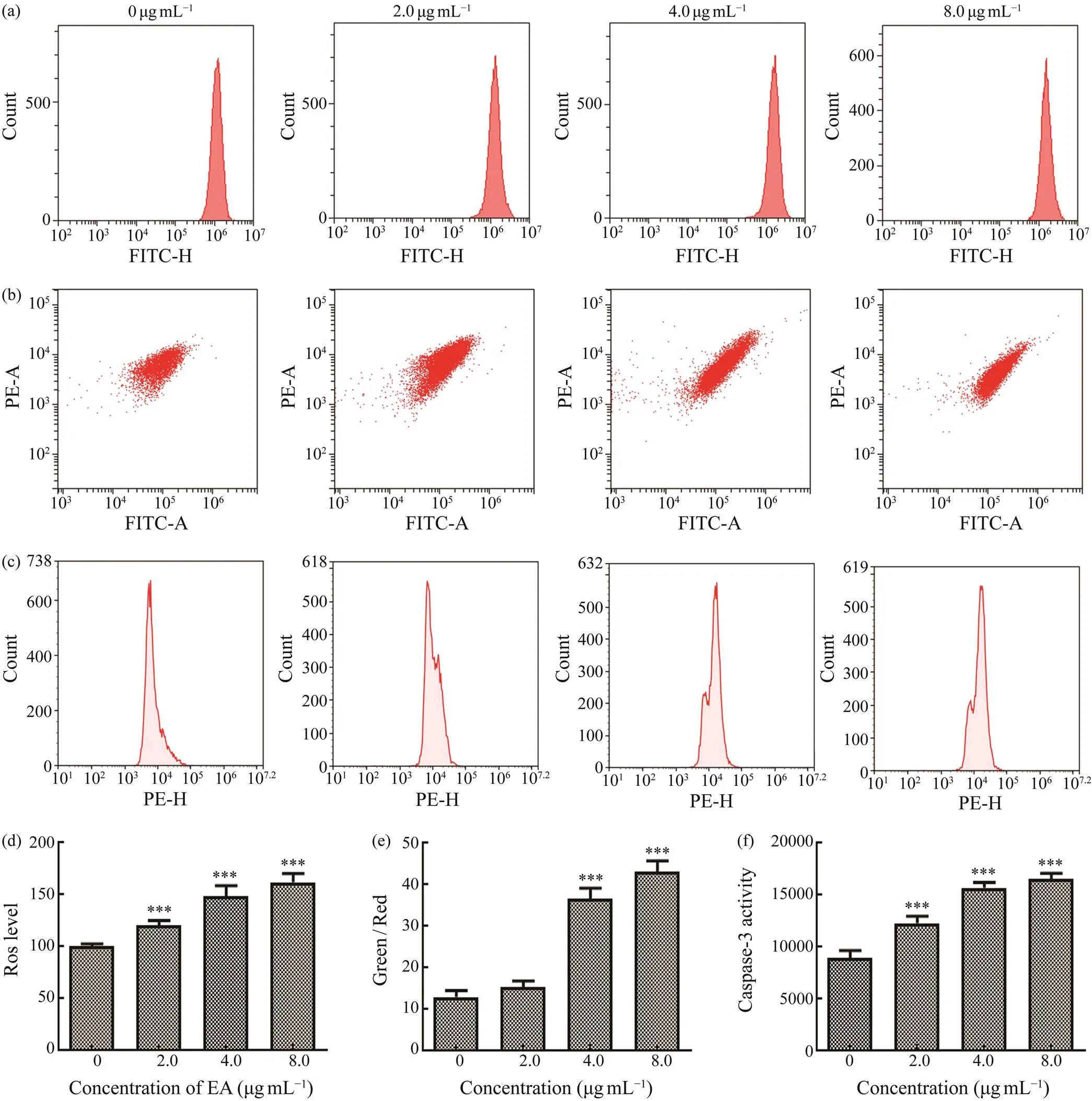
Fig.3 Effect of EA with different concentrations (2.0, 4.0, and 8.0μgmL−1) on ROS level (a, d), MMP (b, e), and caspase- 3 expression (c, f) in breast cancer cells MDA-MB-231. Significant differences compared with control group are indicated by ***P<0.001.
3.8 Western Blotting Analysis
PI3K/Akt/mTOR pathway plays an important role in the progression, development, and maintenance of cancer (Al-zahrani, 2019). Previous literatures have demonstrated that overexpression of phosphorylated PI3K, Akt, and mTOR has been identified in breast cancer cells (Donnella., 2018; Du Rusquec., 2020; Nunnery and Mayer, 2020).For exploring the influence of EA on the expressions of phosphorylated proteins PI3K, Akt, and mTOR in MDA- MB-231 cells, the proteins’ contents were analyzed by wes- tern blotting. In Figs.5a and 5b, it showed that the treat- ment of EA decreased the expressions of p-PI3K, p-Akt, and p-mTOR dose-dependently. The results emphasized the role of PI3K/Akt/mTOR pathway in leading to the anti- breast cancer effect of EA. The antitumor effect of EA wassimilar to that of sea cucumber saponin Frondoside A. Frondoside A inhibited the colony formation, migration, and invasion of MDA-MB-231 cells, and PI3K/Akt signals wasalso one of the antitumor mechanisms that involved in this effect (Park., 2012).
Triterpene glycosides from sea cucumbers exerted their antitumor activitydifferent pathways. For example, Fron- doside A and Cucumarioside A2-2 caused leukemic cells apoptosis caspase-dependently (Jin., 2009). Cucuma- rioside A2–2 fromcould block the cell cycle of Ehrlich carcinoma cells in S phase, and induce cellsapoptosis caspase- and p53-dependently (Menchinskaya., 2013). Philinopside A fromremarkably suppressed HMECs cells’ growth, migration, and tube formation dose-dependently (Tong., 2005). Philinopside E (PE) fromexerted the anti-angiogenic activitysuppressing VEGFR2 signaling (Tian., 2005) and KDR phosphorylation in HMECs (Tian., 2007). Holothutin A1 and 24-Dehydeiechinoside A fromexerted significant anti-metastatic activi- ty by inhibiting the expressions of MMP-9 and VEGF in HepG2 cells (Zhao., 2010). In recent years, the anti- tumor activity of triterpene glycosides from sea cucumber has attracted great attention, and their antitumor molecu- lar mechanisms should be investigated further in detail.

Fig.4 Effect of EA with different concentrations (2.0, 4.0, and 8.0μgmL−1) on cell cycle (a, c) and apoptosis (b, d) of breast cancer cells MDA-MB-231. Significant differences compared with control group are indicated by * P<0.05; *** P<0.001.
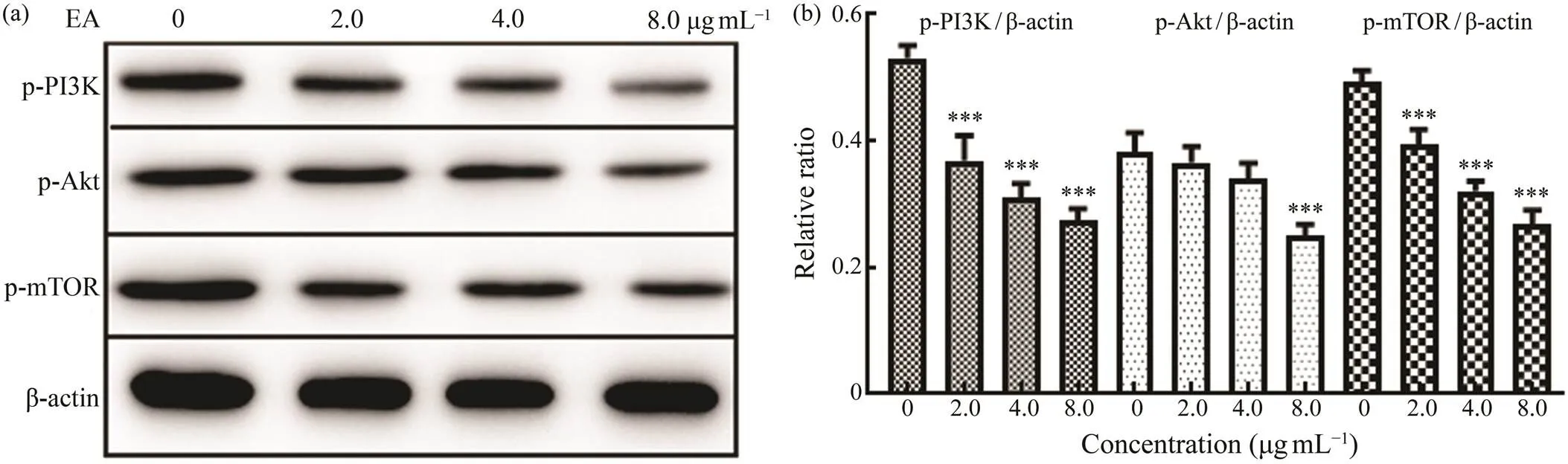
Fig.5 Impact of EA at the concentrations of 2.0, 4.0, and 8.0μgmL−1 on the protein expression in PI3K/Akt/mTOR sig- naling pathway as detected by western blotting. (a), the expression levels of proteins p-PI3K, p-Akt, p-mTOR, and β-actin in MDA-MB-231 cells analyzed with western blotting; (b), the expression level of proteins relative to β-actin in western blot- ting. Significant differences compared with control group are indicated by *** P<0.001.
4 Conclusions
Sea cucumber saponin EA fromexhibited an excellent antitumor effect on MDA-MB-231 cells. The cytotoxicity of EA to MDA-MB-231 cells was involv- ed in elevating intracellular ROS levels, arrest of cell cy- cle, down-regulation of MMP, up-regulation of caspase-3, and decreasing the expression of p-PI3K, p-Akt, and p-mTOR. The antitumor mechanisms of EA still require to be further explored. This study demonstrated that EA was a promising antitumor agent for the treatment of breast can- cer.
Acknowledgements
This work was supported by the National Key R&D Pro-gram of China (No. 2018YFC0311206),the China Post-doctoral Science Foundation (No. 2018M642706), and the Postdoctoral Innovation Program of Shandong Province.
Al Marzouqi, N., Iratni, R., Nemmar, A., Arafat, K., Ahmed Al Sultan, M., Yasin, J.,., 2011. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growthof breast tumor xenografts.,668:25-34.
Alzahrani, A. S., 2019. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside.,59: 125-132.
Aminin, D. L., Menchinskaya, E. S., Pisliagin, E. A., Silchenko, A. S., Avilov, S. A., and Kalinin, V. I., 2015. Anticancer acti- vity of sea cucumber triterpene glycosides.,13: 1202-1223.
Bahrami, Y., and Franco, C. M., 2015. Structure elucidation of new acetylated saponins, Lessoniosides A, B, C, D, and E, and non-acetylated saponins, Lessoniosides F and G, from the vis- cera of the sea cucumber.,13: 597-617.
Careaga, V. P., Bueno, C., Muniain, C., Alche, L., and Maier, M. S., 2009. Antiproliferative, cytotoxic and hemolytic activities of a triterpene glycoside fromand its de- sulfated analog.,55: 60-68.
Cuong, N. X., Vien, L. T., Hoang, L., Hanh, T. T. H., Thao, D. T., Thanh, N. V.,., 2017. Cytotoxic triterpene diglycosides from the sea cucumber.&,27: 2939-2942.
Dong, P., Xue, C.H., Sheng, W.J., Xu, J., and Li, Z.J., 2008. Study on the determination of total triterpene glycosides in sea cucumbers.,27: 28-32.
Donnella, H. J., Webber, J. T., Levin, R. S., Camarda, R., Mom- cilovic, O., Bayani, N.,., 2018. Kinome rewiring reveals AURKA limits PI3K-pathway inhibitor efficacy in breast can- cer.,14: 768-777.
Du Rusquec, P., Blonz, C., Frenel, J. S., and Campone, M., 2020. Targeting the PI3K/Akt/mTOR pathway in estrogen-receptor positive HER2 negative advanced breast cancer.,12: 1-12.
Galadari, S., Rahman, A., Pallichankandy, S., and Thayyullathil, F., 2017. Reactive oxygen species and cancer paradox: To pro- mote or to suppress?,104: 144-164.
Jin, J. O., Shastina, V. V., Shin, S. W., Xu, Q., Park, J. I., Rasska- zov, V. A.,., 2009. Differential effects of triterpene gly- cosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells.,583: 697-702.
Kim, S. K., and Himaya, S. W. A., 2012. Triterpene glycosides from sea cucumbers and their biological activities., 65: 297-319.
Kitagawa, I., Inamoto, T., Fuchida, M., Okada, S., Kobayashi, M.,Nishino, T.,., 1980. Structures of echinoside A and B, two antifungal oligoglycosides from the sea cucumber(Jaeger).,28: 1651-1653.
Li, M., Miao, Z. H., Chen, Z., Chen, Q., Gui, M., Lin, L. P.,.,2010. Echinoside A., a new marine-derived anticancer saponin, targets topoisomerase2α by unique interference with its DNA binding and catalytic cyels.,21:597-607.
Li, X., Roginsky, A. B., Ding, X. Z., Woodward, C., Collin, P., Newman, R. A.,., 2008. Review of the apoptosis pathwaysin pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, Frondoside A.,1138: 181-198.
Mattoo, T.K., Lu, H., Ayers, E., and Thomas, R., 2020. Total body water by BIA in children and young adults with normal and excessive weight., 15:e0239212.
Menchinskaya, E. S., Pislyagin, E. A., Kovalchyk, S. N., Davy- dova, V. N., Silchenko, A. S., Avilov, S. A.,., 2013. Antitu-mor activity of cucumarioside A2-2.,59: 181-191.
Miricescu, D., Totan, A., Stanescu-Spinu, I. I., and Badoiu, S. C., 2021. PI3K/AKT/mTOR signalling pathway in breast cancer: From molecular landscape to clinical aspects.,22: 173-196.
Mondol, M. A. M., Shin, H. J., Rahman, M. A., and Islam, M. T., 2017. Sea cucumber glycosides: Chemical structures, produc- ing species and important biological properties.,15:317-351.
Nunnery, S. E., and Mayer, I. A., 2020. Targeting the PI3K/AKT/mTOR pathway in hormone-positive breast cancer.,80: 1685-1697.
Park, J. I., Bae, H. R., Kim, C. G., Stonik, V. A., and Kwak, J. Y., 2014. Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers.,2: 1-14.
Park, S. Y., Kim, Y. H., Kim, Y., and Lee, S. J., 2012. FrondosideA has an anti-invasive effect by inhibiting TPA-induced MMP- 9 activation via NF-κB and AP-1 signaling in human breast cancer cells.,41: 933-940.
Pascual, J., and Turner, N. C., 2019. Targeting the PI3-kinase path- way in triple-negative breast cancer.,30 (7): 1051-1060.
Redza-Dutordoir, M., and Averill-Bates, D. A., 2016. Activation of apoptosis signalling pathways by reactive oxygen species., 1863: 2977-2992.
Roginsky, A. B., Ding, X. Z., Woodward, C., Ujiki, M. B., Singh, B., Bell, R.H.,., 2010. Anti-pancreatic cancer effects of a polar extract from the edible sea cucumber, 39: 646-652.
Tian, F., Zhang, X., Tong, Y., Yi, Y., Zhang, S., Li, L.,., 2005. PE, a new sulfated saponin from sea cucumber, exhibits anti-angiogenic and anti-tumor activitiesand.,48: 874-882.
Tian, F., Zhu, C.H., Zhang, X.W., Xie, X., Xin, X.L., Yi, Y.H.,., 2007. Philinopside E, a new sulfated saponin from sea cucumber, blocks the interaction between kinase insert domain-containing receptor (KDR) and alphavbeta3 integrin via bind- ing to the extracellular domain of KDR.,72: 545-552.
Tong, Y., Zhang, X., Tian, F., Yi, Y., Xu, Q., Li, L.,., 2005. Philinopside A, a novel marine-derived compound possesing dual anti-angiogenetic and anti-tumor effects. International Jour-nal of Cancer,114: 843-853.
Wang, Y., Wang, J., Yanagita, R. C., Liu, C., Hu, X., Dong, P.,., 2014. Effects of two sulfated triterpene saponins echinoside A and holothurin A on the inhibition of dietary fat absorption and obesity reduction.,78: 139-146.
Yun, S. H., Park, E. S., Shin, S. W., Na, Y. W., Han, J. Y., Jeong, J. S.,., 2012. Stichoposide C induces apoptosis through thegeneration of ceramide in leukemia and colorectal cancer cells and showsantitumor activity.,18: 5934-5948.
Zhao, Q., Xue, Y., Liu, Z. D., Li, H., Wang, J. F., Li, Z. J.,., 2010. Differential effects of sulfated triterpene glycosides, Ho- lothurin A1, and 24-Dehydroechinoside A, on antimetastasic activity via regulation of the MMP-9 signal pathway.,75: 280-288.
Zhao, Q., Xue, Y., Wang, J. F., Li, H., Long, T. T., Li, Z.,., 2012.andanti-tumour activities of echinoside A and ds-echinoside A from.&,92: 965-974.
Zhao, Y. C., Xue, C. H., Zhang, T. T., and Wang, Y. M., 2018. Sa- ponins from sea cucumber and their biological activities.,66: 7222-7237.
(July 9, 2021; revised November 8, 2021; accepted March 22, 2022)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
Corresponding authors. E-mail: lihongyan1447@163.com
E-mail: xuech@ouc.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Quality Changes and Safety Evaluation of Ready-to-Eat Roasted Antarctic Krill (Euphausia superba) During Storage at Room Temperature (25℃)
- The Influence of Sea Sprays on Drag Coefficient at High Wind Speed
- Highly Efficient Heavy-Metal-Ion Removal from Shellfish Processing Liquid with Low Protein and Polysaccharide Loss by Hybrid Mesoporous Silica Diol-APDC-SBA15
- Ship Weather Routing Based on Hybrid Genetic Algorithm Under Complicated Sea Conditions
- L-Band Analysis of the Effects of Oil Slicks on Sea Wave Characteristics
- A Method for Reducing Ocean Wave-Induced Magnetic Noises in Shallow-Water MT Data Using a Complex Adaptive Filter
