Growth Performance of Complete Diallel Crosses Among New Varieties of Chinese Pearl Oyster Pinctada martensii
2023-03-17FANChaoZHANGXuekaiZHANGXingzhiYUEShaoboLIYangchunTANGLimingLIQiongzhenandWANGZhaoping
FAN Chao, ZHANG Xuekai, ZHANG Xingzhi, YUE Shaobo, LI Yangchun,TANG Liming, LI Qiongzhen, and WANG Zhaoping, *
Growth Performance of Complete Diallel Crosses Among New Varieties of Chinese Pearl Oyster
FAN Chao1), #, ZHANG Xuekai1), #, ZHANG Xingzhi2), YUE Shaobo1), LI Yangchun1),TANG Liming1), LI Qiongzhen2), and WANG Zhaoping1), *
1),,266003,2),,530021,
is an important shellfish for the production of sea pearls in China. To improve the growth performance of, we successfully established three complete diallel crosses using three new varieties of,which were namedHaiyou No. 1, Haixuan No. 1, and Nanke No. 1, to investigate the growth performance of each self-cross line and hybrid line in Bei- hai, Guangxi Zhuang Autonomous Region, China. Generally, high fertilization and hatching rates were observed in the experiment, sug- gesting that there was no sperm-egg recognition barrier among these threevarieties. The survival rate of the self-cross lines was relatively higher than that of hybrid lines, and ♀ ‘Haixuan No. 1’×♂ ‘Haiyou No. 1’ maintained a high survival rate during the grow-out stage. The shell length and width were affected by the genotypes as well as the interaction between the genotypes and environmental factors on day 400.The variances of the general combining abilities () and specific combining abilities () for shell length and width were the result of both additive genetic variance and nonadditive genetic variance.When Haixuan No. 1 was the female parent and Nanke No. 1 was the male parent, thefor shell length and width were both positive on day 400. The hete- rosis of Haixuan No. 1 and Nanke No. 1 in terms of shell length and width was positive.From the resulting combining ability and heterosis, ♀ ‘Haixuan No. 1’×♂ ‘Nanke No. 1’ was considered an ideal hatchery method to improve the growth performance of. Our results therefore demonstrated that crossbreeding among these three new varieties can further improve the growth per- formance of.
; crossbreeding; growth performance; combining ability; heterosis
1 Introduction
Producing a hybrid animal that has a better performancethan each of its parents due to heterosis is an effective way to improve production in aquaculture (Newkirk, 1980). In recent years, increasingly successful hybridizations between different populations have been achieved in bivalve breed- ing, such as Manila clam(Huo., 2015), eastern oyster(Rawsonand Feindel, 2012), and Pacific oyster(Yin and Hedgecock, 2019).
Family selection is applied to improve and select traits on the basis of family and hybrids, under conditions in which the environmental conditions are as consistent as possible, and is the essence of the selection of genotypes (Zhang,2016). Studies have shown that family selection can im-prove the traits of shellfish. Family selection in Pacific oys- ters has nearly doubled the yield over that of wild stocks (Langdon., 2003). Zhang (2016) found that the hy- brids of zebra clam ♀ × orange strain ♂ ofshowed heterosis in hatching, growth, metamorpho- sis, and survival. Zhang. (2017) established recipro- cal hybrid families of different geographical populations ofand found that reciprocal crosses had a higher survival rate than the pure stock crosses. Wang. (2010) found that seven growth-related traits had lar- ger additive genetic variances in the offspring offamilies. Therefore, the use of the hybrid fami- lies may be beneficial to improve the production and achi- eve the effect of trait improvement.
is a warm water shellfish that is mainly dis-tributed in Guangdong Province, Guangxi Zhuang Autono-mous Region, and Hainan Province, China, and along the southern coast of Japan. The ‘southern pearl’ produced byhas long enjoyed a good reputation worldwide. It has a high value in the cultured sea pearl production in- dustry in China and Japan (Qiu., 2013). In 1965, Chi- na successfully developed artificial breeding technology for, and then established large-scale artificial breed- ing of the species, which greatly improved the survival rateof the seedings. However, due to a lack of in-depth research on genetic resource management measures and supportingtechnologies, a series of problems have arisen in thebreeding industry with the continuous expansion of the breeding scale. For example, the long-term use of a small number of parents for breeding and the disorderly introduc- tion of hybridization has resulted in serious genetic charac- ter differentiation, seedling degeneration, small individual size, slow growth, reduced stress resistance, and secretion activity in the breeding population, and has finally result- ed in the reduced growth performance of(Gu., 2009). Although the diversity of coastal habitats ishigh, they are vulnerable to human activities, such as over- fishing. Wild pearl oysters are therefore in serious decline. The decline is so serious that it is impossible to isolate ge- netically improved farmed stock from natural populations. This has resulted in the stagnation of the sea pearl indus- try, and the industry has even begun to regress in China. Therefore, it is of great significance to develop genetic im-provements in, and cultivate new varieties with fast growth and strong resistance to develop pearl oyster production and revitalize the industry in China. Chinese re- searchers have carried out a series of breeding programs, including population selection (Deng and Du, 2009; Wang., 2011), cross breeding (Gu., 2011), and molecu-lar marker assisted breeding (Shi., 2009). Through thesestudies, new varieties ofwith genetic advantages, such as Haixuan No. 1 (Du, 2015), Haiyou No. 1 (Wang., 2006), and Nanke No. 1 (He., 2016), have been cultivated. These new varieties have the characteristics of fast growth, large shell width, and a strong secreting ability.
There have been some studies on the hybridization ofdifferent populations of(Wang., 2010; Peng., 2015; Fan., 2016; Yang., 2018). To our knowledge, however, no studies on the establishment of families among the new varieties ofhavebeen reported.In this study, complete diallel cross lines wereestablished usingHaiyou No. 1, Haixuan No. 1,and Nanke No. 1.Hatching and growth were measured,and heterosis and combining ability were evaluated. The different lines were compared to select the lines with the fastest growth rate, largest shell width, and greatest suit-ability for pearl nucleus implantation. The outcomes of the study can provide guidance for improving the growth per- formance of.
2 Materials and Methods
2.1 Broodstocks
We transferred two-year-oldHaiyou No. 1 (Y), Haixuan No. 1 (X), and Nanke No. 1 (K) with well- developed gonads from Sanya in Hainan Province and Zhan- jiang in Guangdong Province to the Guangxi Comprehen- sive Test Station Hatchery of the National Shellfish Indus- try Technology System at Guangxi Academy of Fisheries Sciences in Beihai in Guangxi Zhuang Autonomous Region in February 2019. There were 500 individuals of each va- riety. The attachments on the surface of the shell were re- moved with an oyster knife. The oysters were then placed into scallop cages and reared in filtered seawater for seven days. The pearl oysters were fed on sufficientevery 6h. The feeding amount was 100000 to 200000 cellsmL−1d−1. All water was exchanged once a day and the water temperature was maintained at 22–24℃and salinity was kept at 30.
2.2 Establishment of Families
Before family establishment, the shell widths of pearl oysters were measured with an electronic vernier caliper (accuracy 0.01mm). Individuals in the top 10% of shell widths in each population were selected to identify gender and maturity. Three females and three males from each po-pulation were selected as parents. Gametes from mature broodstocks were obtained by dissection. The egg suspen- sion was passed through an 80μm nylon screen to removetissue debris. For each stock, egg suspensions from three females were divided equally into nine 5L buckets, with each female supplying three buckets. Then a few drops of ammonia (2–3mmolL−1) were dropped into the egg sus- pensions, and the oocytes maturity was observed during the process of oocyte soaking(Ohta., 2007). Semen was extracted from the gonad of each male with a pipette, and then divided equally into three 1L beakers. After it was ob- served that all oocytes were mature, a few drops of ammo- nia were added to the seminal fluid, and the sperm activitywas observed immediately. Eggs were examined to rule out the occurrence of uncontrolled fertilization before formal fertilization. When it was observed that most of the sperm was activated, an appropriate amount of seminal fluid was poured into the oocyte fluid for fertilization to produce 3×3 complete diallel cross families. The method of the es- tablishment of complete diallel cross families is shown in Table 1 and each family was conducted in triplicate. The fertilized eggs were pooled and placed into a 100L bucket for hatching at a density of 10–15 eggsmL−1. The tempe- rature of the hatching water was maintained at 23–26℃and the salinity was kept at 30.
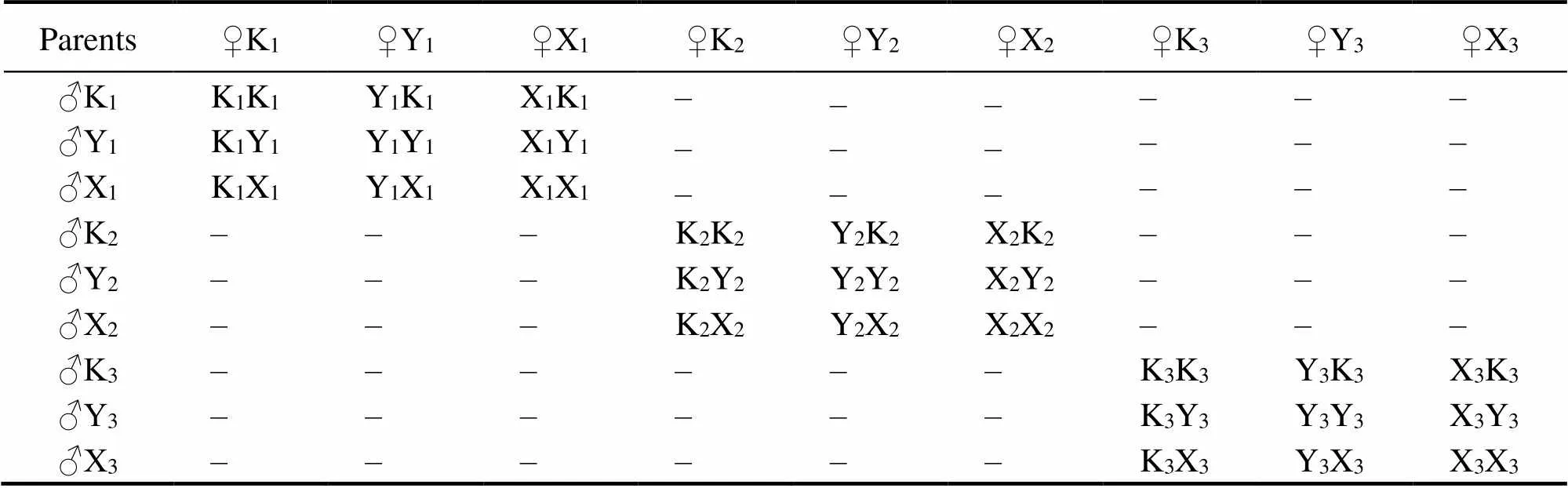
Table 1 Mating strategy of families of P. martensii
Notes: The dominant characters of ‘Y’ are fast growth and increase of superior pearl percentage of one-year-old pearl oyster. The dominant characters of ‘X’ are fast growth rate, large shell width coefficient, and a strong secreting ability. The dominant characters of ‘K’ are large shell height and width, increase of superior pearl percentage.
2.3 Larval Rearing, Spat Nursery, and Grow-out
The fertilized eggs developed into D-veliger larvae inabout 20h. Larvae were collected after being passed througha 40μm nylon screen and the density of each family was adjusted to 5–8 individualsmL−1. The density of each fa- mily was adjusted regularly during the cultivation to elimi- nate the effect of density. The larvae were fed onbefore they reached a size of 110–120μm.With the growth of the spat,andwere added to the diet. Feeding was gradually in- creased from 3000 to 50000 cellsmL−1d−1. The proportion of the three phytoplankton was 1:1:1. The water was com- pletely exchanged with filtered seawater once a day.
The spat from each replication were gathered into poly- ethylene mesh bags with a 2mm aperture at a density of 120–150 individuals/bag when the shell length of juveniles was about 2–3mm. Then, they were respectively transfer- red to three culture sites in Beihai in Guangxi Zhuang Au- tonomous Region. The three culture sites were named Zhu- lin (Z), Huolu (H), and Qingshantou (Q). The locations andcoordinates are shown in Figs.1a and 1b. The mesh bagsat Zhulin were hung on a floating raft in a large pond (Fig.1c).The mesh bags at Huolu and Qingshantou were suspend- ed on off-bottom piles, which were located in the shoal off- shore (Fig.1d). The biofouling on the mesh bags was clean- ed regularly to prevent it from affecting the flow of seawa- ter through the bag. The mesh bags were changed periodi- cally when the oysters grew. The density of oysters in the mesh bags was adjusted to avoid the influence of density on the experiment.
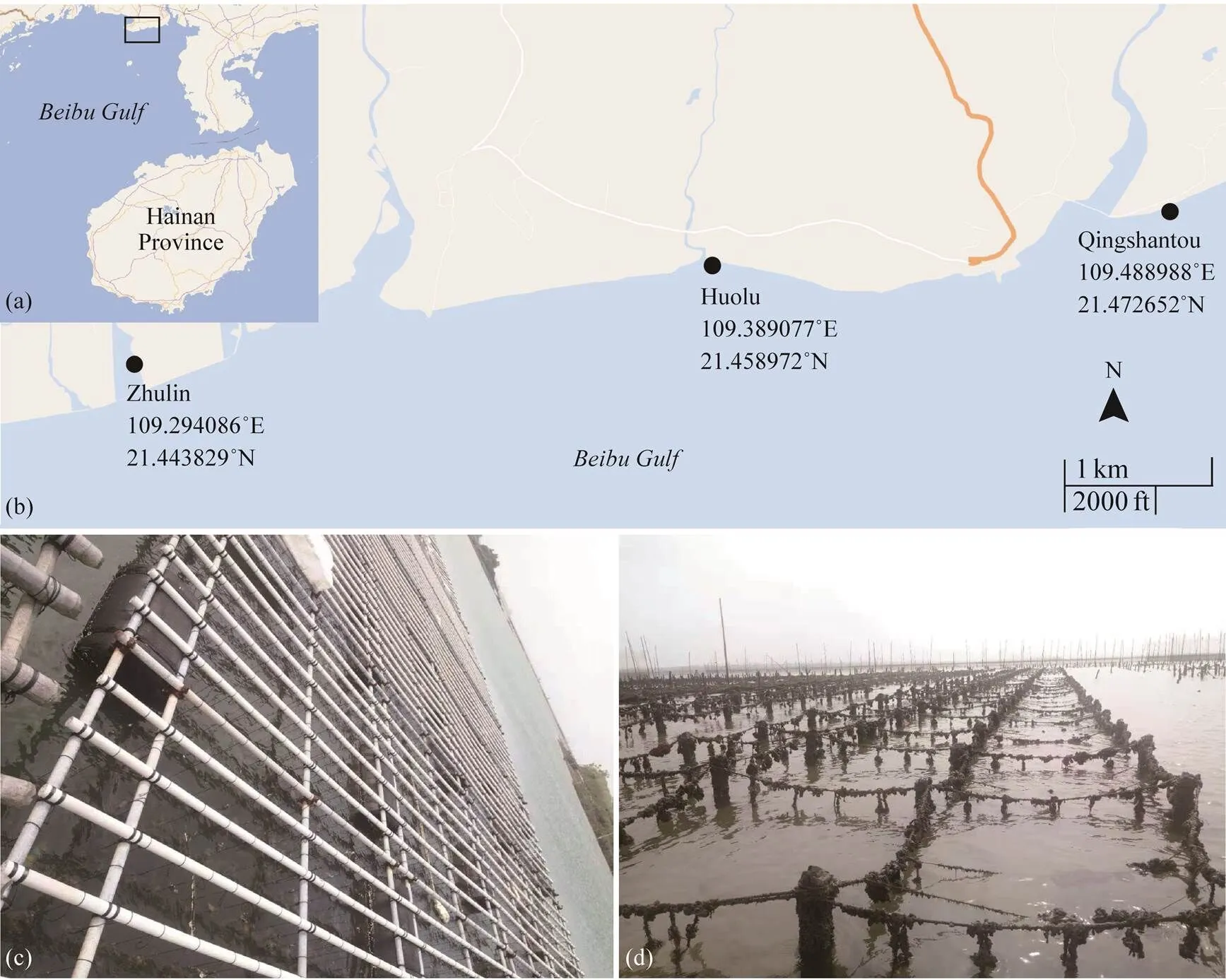
Fig.1Culture sites and methods. (a) and (b), the three culture sites used in this study; (c), oyster hanging culture at Zhulin; (d), oyster off-bottom cultures at Huolu and Qingshantou.
2.4 Evaluation of the Growth Performance
The fertilization rate (), hatching rate (), relative survival rate (), and growth parameters of each family were evaluated during the culture period. The equations and evaluation methods were as follows:
行为类主要是指在中国共产党的革命与建设过程中形成的一些纪念日与纪念行为,如建党纪念日、国庆节、五四青年节等,这些纪念日红色特征明显。因此也被称为红色节日。
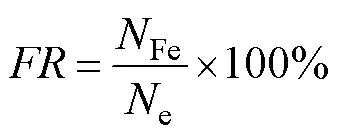
whereis the fertilization rate,Feis the number of fer- tilized eggs per unit volume, andeis the number of eggs per unit volume. The presence of the first polar body was adopted as an indicator of successful fertilization.
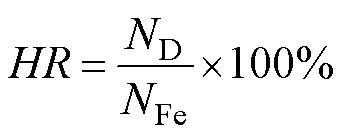
whereis the hatching rate,Dis the number of D- shaped larvae per unit volume.
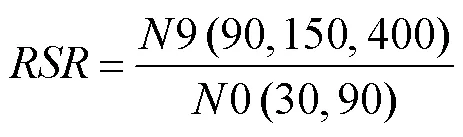
whereis the relative survival rate,9(90, 150, 400) is the number of 9- (90-, 150-, and 400-)-day-old surviv- ing individuals,0(30, 90) is the number of 0- (30-, 90-)- day-old surviving individuals. The relativewas calcu- lated according to the method of Allen and Downing (1986). Theof 9-day-old individuals was the number of 9- day-old surviving individuals as a percentage of the 0-day- old surviving individuals, the 90-day-oldwas the num- ber of 90-day-old surviving individuals as a percentage of the 30-day-old surviving individuals, and the 150-and 400- day-olds were the number of 150-and 400-day-old surviving individuals as a percentage of 90-day-old survi- ving individuals.
Growth parameters: The shell length of each family on day 9, 90, 150, and 400 and the shell width on day 400 were measured respectively. The 9-day-old shell length wasmeasured with a micrometer under a microscope (10×), and the other parameters were measured by an electronic ver- nier caliper (accuracy 0.01mm).
2.5 Statistical Analyses
Differences in the fertilization rate, hatching rate, rela- tive survival rate, and growth parameters among the dif- ferent groups were analyzed by multiple comparisonsa two-way analysis of variance (ANOVA) of the means. The fertilization rates, hatching rates, and relative survival rates were transferred to arcsine values to stabilize the va- riances of errors while the growth index was transformed to logarithms to ensure the normality and homoscedasticity.All statistical analyses were performed using R software Version 3.6.3 for Windows. All analyses were set to a sig- nificance level of<0.05.
To evaluate the effects of the egg origin (genotype) and mating strategy (environmental factors) on the growth of, a two-factor ANOVA was used (Zhang., 2007) as follows:
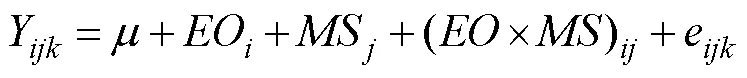
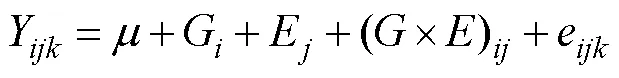
whereYis the mean shell length (or shell width during the grow-out stage) of thereplicate,egg origin (geno- type), andmating strategy (site);EO(G) is the effect of the egg origin (genotype) on the shell length (or shell width during the grow-out stage) (=1, 2, 3);S(E) is the ef- fect of the mating strategy (environmental) on the shell length (shell width during the grow-out stage) (=1, 2, 3); (×)((×)) is the interaction effect between the egg origin (genotype) and the mating strategy (environ- mental); andeis the random observation error (=1, 2, 3).
The formulas for the general combining abilities () and specific combining abilities () of shell length and width were obtained according to Griffing’s Method 1 (com- plete diallel set) (Griffing, 1956).
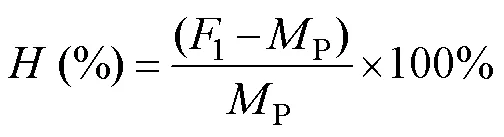
where1is the mean shell length (or shell widthduring the grow-out stage) of the hybrid cross andPis the mean shell length (or shell widthduring the grow-out stage) of both self-crosses.
3 Results
3.1 Fertilization and Hatching Rates of Different Groups
The fertilization and hatching rates of the different groupsare shown in Table 2. There were high fertilization and hat-ching rates for the different groups. The XY group had thehighest fertilization rate (92.37%±3.99%) in the hybrid line and the YY group had the highest fertilization rate (92.15%±1.99%) in the self-cross lines. There was no significant dif-ference in the fertilization rate between the XY and YY groups (>0.05). The highest hatching rate was observed in the YY group (95.41%±2.70%). There was no obvious difference in the fertilization and hatching rates between the self-cross and hybrid lines. This suggested that there were no sperm-egg recognition barriers among these three.

Table 2 Fertilization and hatching rates of each group
Notes: The different superscript letters in each column indicate significant differences between groups (<0.05).The results are presented as means±standard deviation.
3.2 Relative Survival Rates of the Different Groups
The relative survival rates of different groups are shown in Fig.2. In general, the relative survival rates of the self- cross lines were higher than those of the hybrid lines. On day 9, the highest relative survival rate was 86.59%±1.21%in the KK group. The XX group had the highest relative sur-vival rate on day 90 and was significantly different fromthe other groups (<0.05). The relative survival rates weresignificantly affected by the environment. The relative sur- vival rate of the same groups at Qingshantou and Huolu was higher than that at Zhulin. In the hybrid lines, the XY group maintained a high relative survival rate throughout the experimental period. It had the highest relative survi- val rate on days 150 and 400 at Qingshantou, at 84.63%±1.20% and 68.32%±3.34%, respectively.
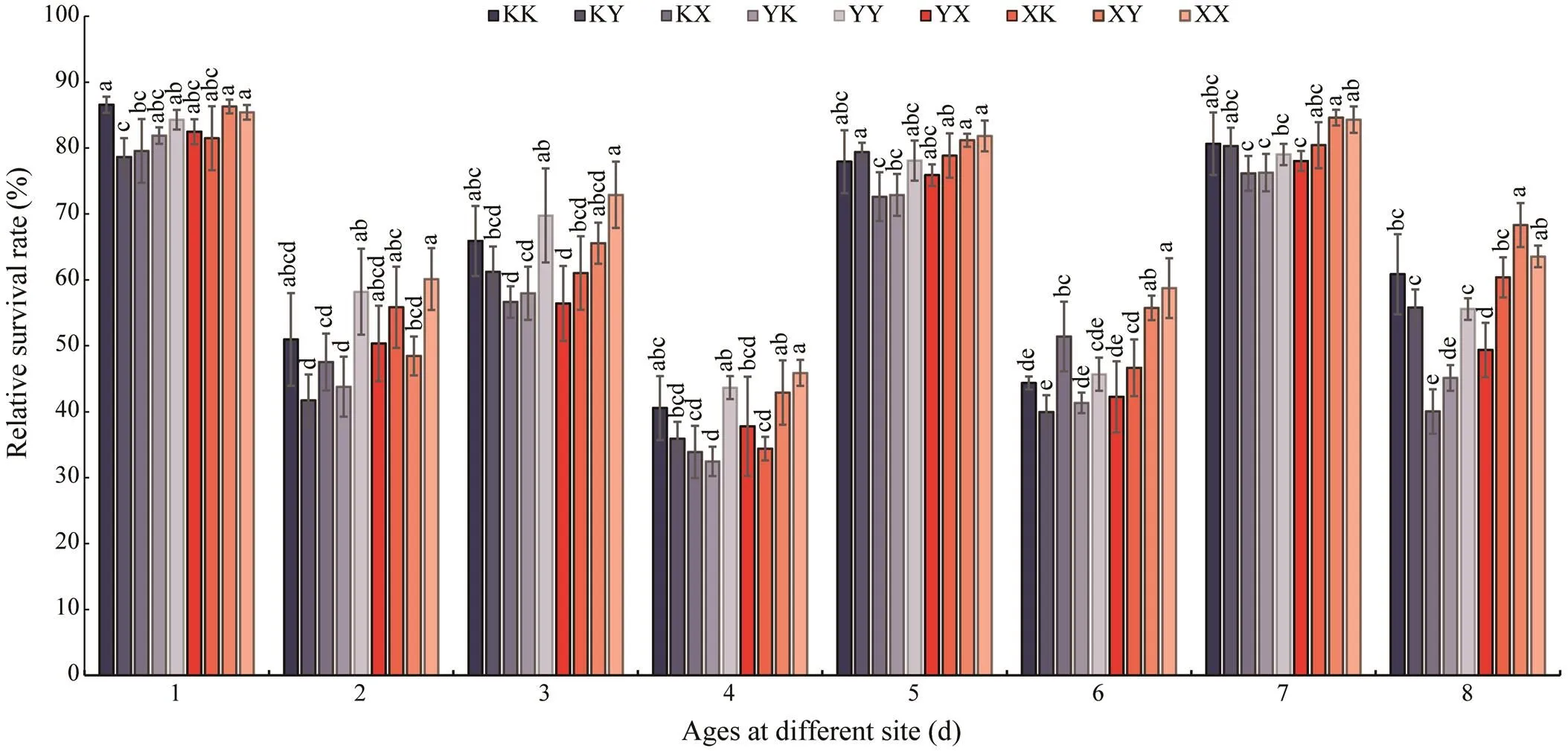
Fig.2 Relative survival rates of each group. 1 for day 9; 2 for day 90; 3 for day 150 at Zhulin; 4 for day 400 at Zhulin; 5 for day 150 at Huolu; 6 for day 400 at Huolu; 7 for day 150 at Qingshantou; 8 for day 400 at Qingshantou. The lower-case letters above each bar indicate significant differences among groups (P<0.05). The results are presented as means±stan- dard deviation.
3.3 Growth Parameters of the Different Groups
The growth parameters of the different groups are shown in Table 3. Results showed that a difference in shell lengthgrowth among the different groups was already apparent onday 9. The XY group had the largest shell length of 114.43μm±19.12μm, which was significantly different to those of the other groups (<0.05). However, the shell length of the XY group (2.46mm±0.47mm) was the smallest on day 90, which was significantly different to those of the other groups (<0.05).It was observed that the shell lengthon day 150 was significantly affected by the genotypes, en- vironmental factors, and the interaction between the geno- types and environmental factors (<0.001).The shell length on day 400 was significantly affected by the genotypes and the interaction between the genotypes and environmental factors (<0.001) (Table 4).The XK group had the larg- est shell length on day 400 in both Huolu and Qingshantou,with values of 55.93mm±6.32mm and 55.76mm±9.55mm, respectively.The shell width was also significantly affect- ed by the genotypes and the interaction between the geno- types and environmental factors on days 150 and 400 (<0.001) (Table 4). The largest mean shell widths at Zhulin, Huolu, and Qingshantou on day 400 were in the XX group (16.75mm±1.66mm), XK group (17.57mm±2.09mm), and KY (18.48mm±1.53mm), respectively (Figs.3a–c).
3.4 Combining Ability of the Different Groups
The variances of theandfor shell length and width are shown in Table 5. The variances were significant(<0.05), which was the result of both additive genetic va-riance and nonadditive genetic variance. Thefor shelllength and width is presented in Table 6. On day 9, theof the Y group was positive, while for the X and K groups it was negative for both the male and female parents.Thefor shell length and width on day 400 for the X group were positive for the female parent, while for the K group they were positive for both male and female parents.Thefor shell length and width is presented in Table 7.Thefor shell length in group XY reached a maximumof 6.67 on day 9. On day 150, thefor shell length and width for group XK reached maximum values of 2.68 and 0.94, respectively. On day 400, the hybrid line with the highestof shell length was YX (0.73), but the hybrid line with the highestfor shell width was YK (0.25).
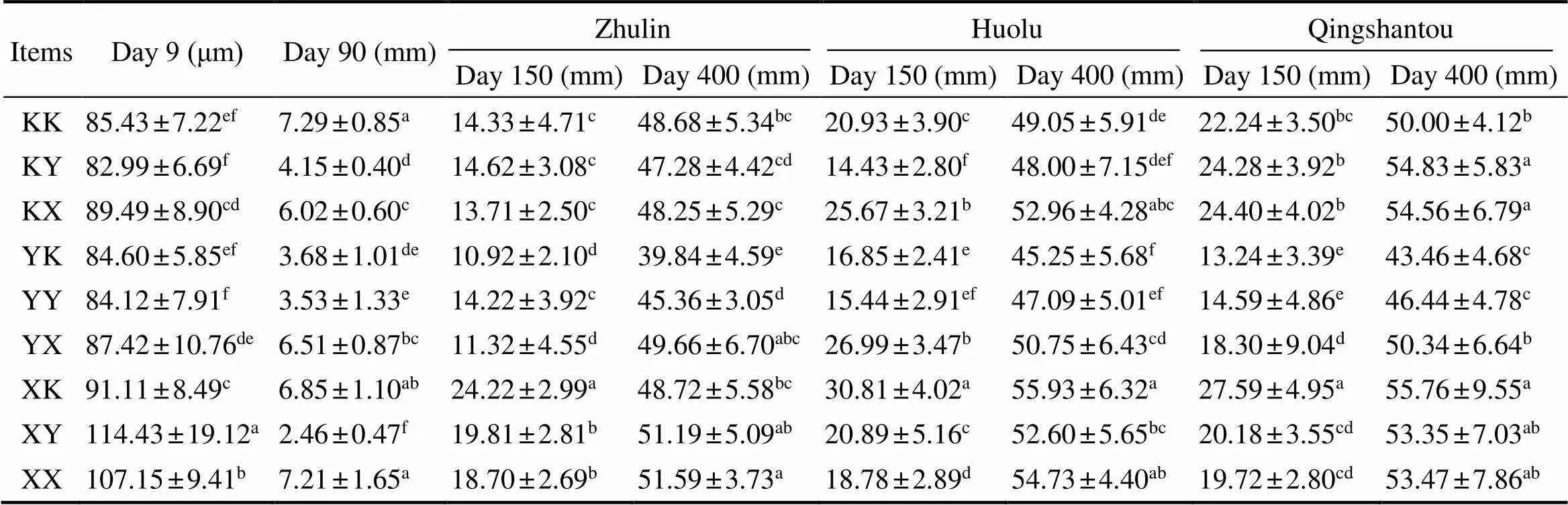
Table 3 Shell length of each group
Notes: The different superscript letters in each column indicate significant differences between groups (<0.05).The results are presented as means±standard deviation.
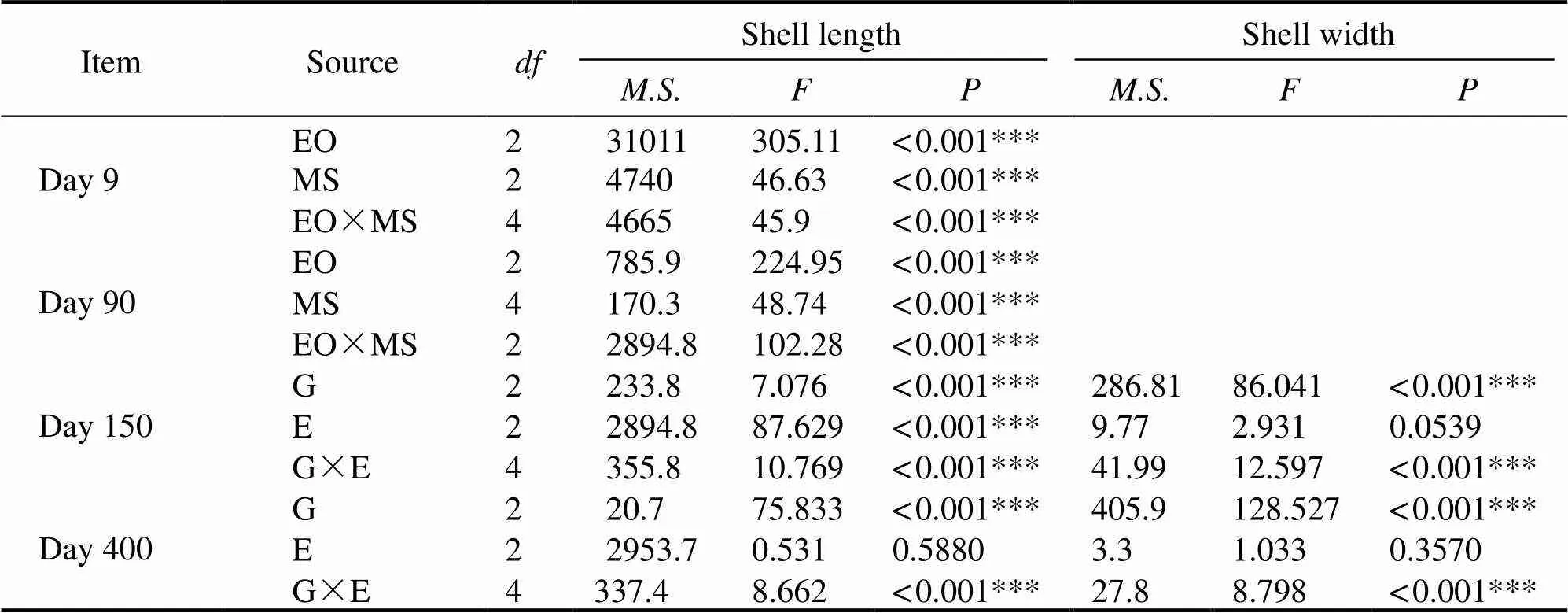
Table 4 Effects of egg origin (EO) or genetic type (G) and mating strategy (MS)or environment factor (E) on growth
Notes:, mean square; *** indicates<0.001.
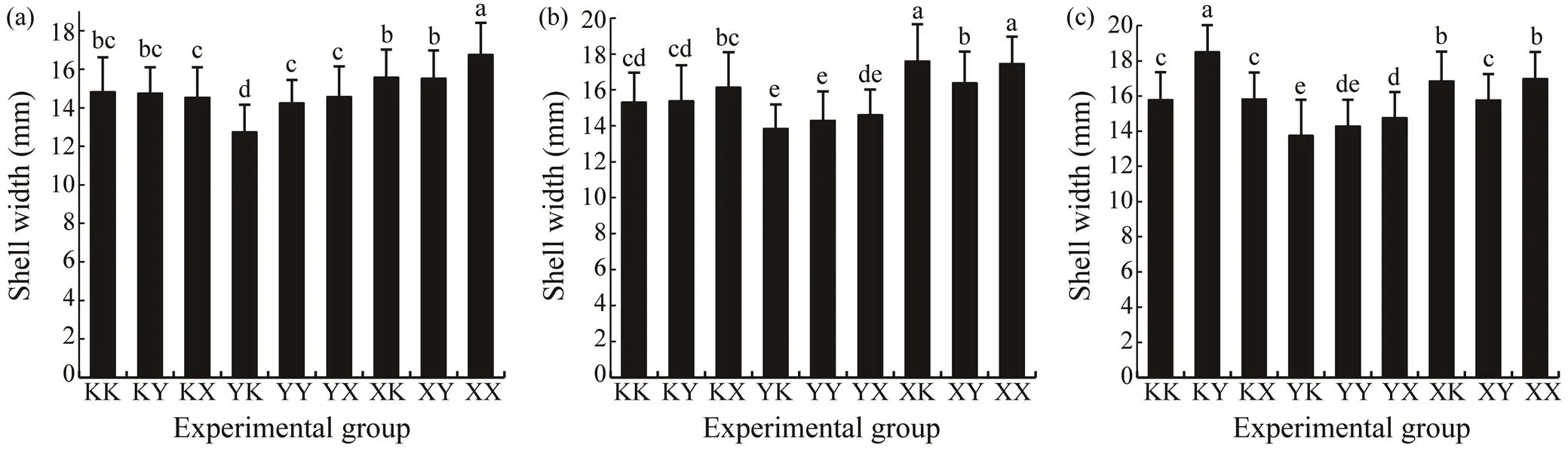
Fig.3 Shell width of 400-day-old P. martensiiin different experimental group. (a), Zhulin; (b), Huolu; (c), Qingshantou. In each panel, the lower-case letters above each bar indicate significant differences among groups (P<0.05).
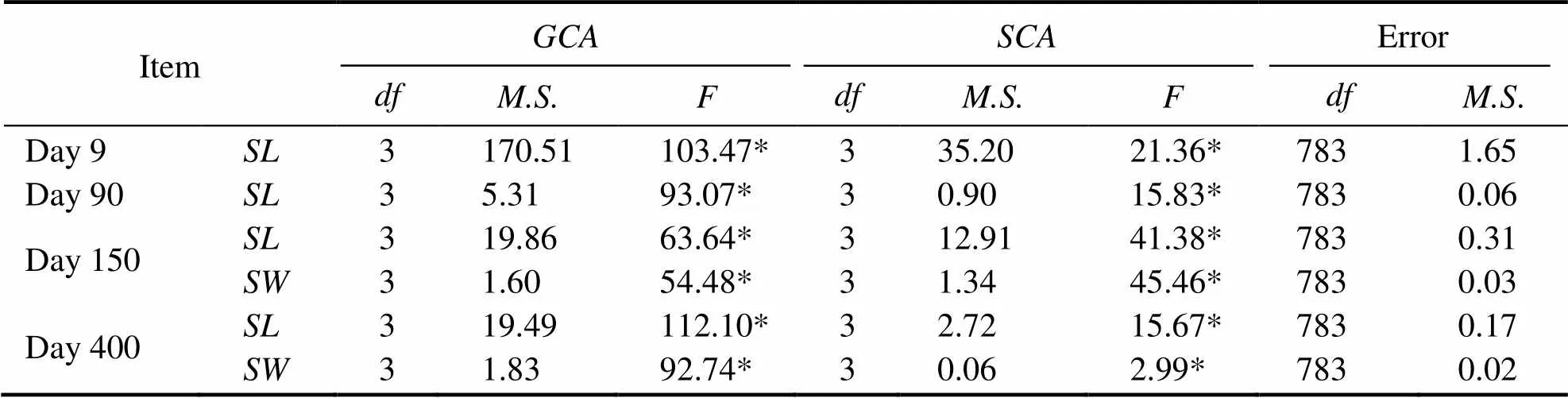
Table 5 Analysis of variance of the GCA and SCA for shell length (SL) and shell width (SW) in the different groups
Notes:, mean square;*indicates that the variance was significant (<0.05).
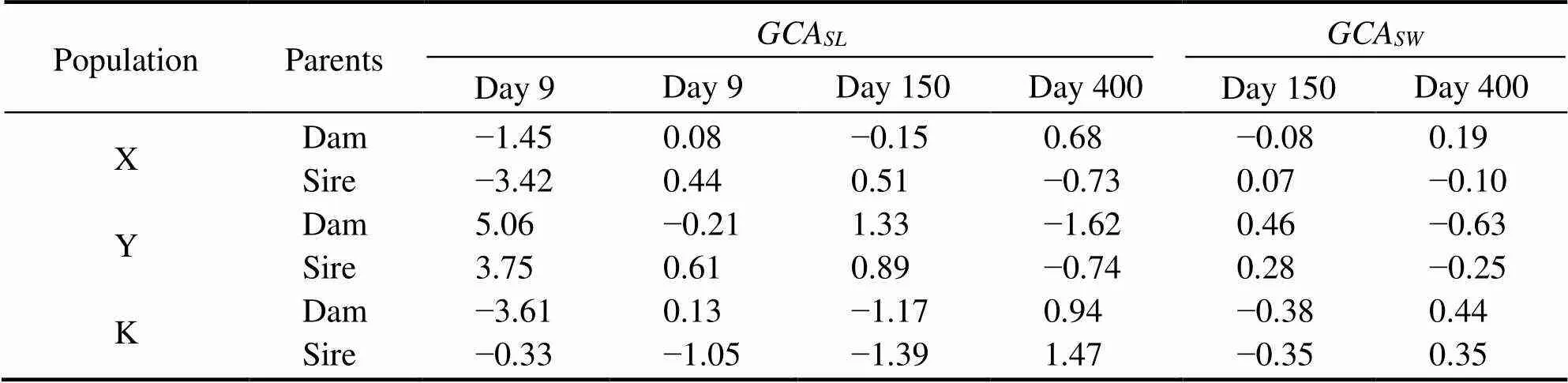
Table 6 Estimates of the GCA for shell length (SL) and shell width (SW)
3.5 Heterosis
The heterosis of the hybrid combinations ofin terms of shell length and width is shown in Table 8. The results showed that only the combination of Y and K was positive on day 9. On day 90, the three combinations were all negative. At the grow-out stage, the heterosis of Y and X in terms shell length at Zhulin increased while the other combinations both decreased. The heterosis of Y and X in terms of shell width was opposite to that of shell lengthon day 400, while the heterosis of X and K in terms of shell length and width was positive, which suggested that the re-ciprocal hybrid of X and K was an appropriate combination.
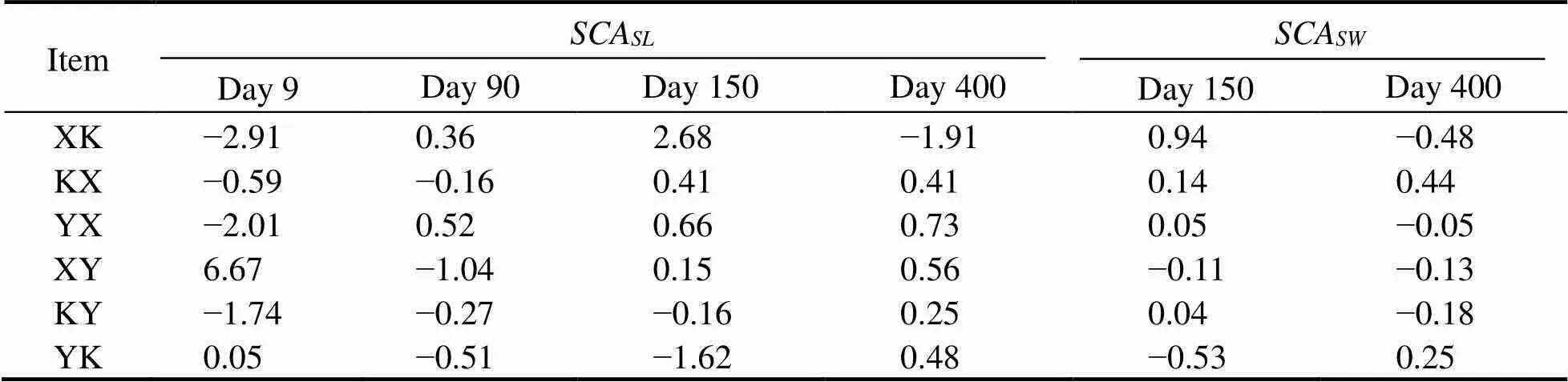
Table 7 Estimates of the SCA for shell length (SL) and shell width (SW)
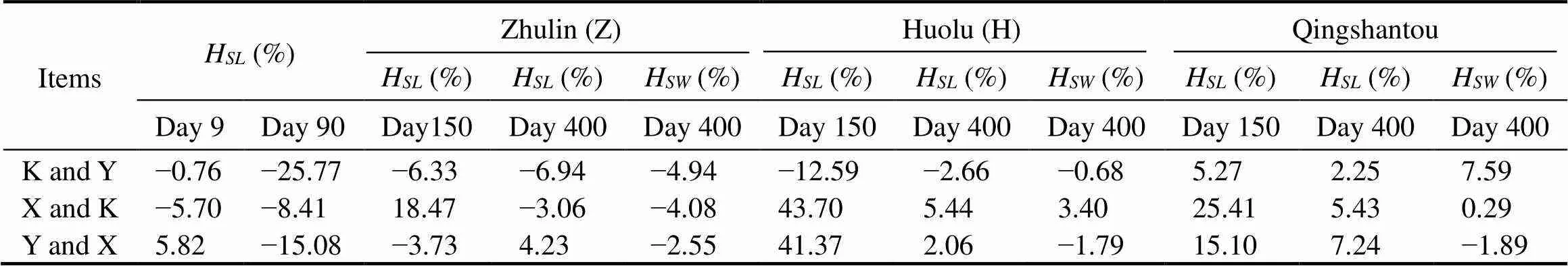
Table 8 Estimates of heterosis (H) in terms of shell length (SL) and shell width (SW)
4 Discussion
Before the shellfish farming moves from offshore into theopen ocean (Stevens., 2008), future expansion will be constrained by competition for alternative uses of coastal areas including residential areas, as well as by the degrada- tion of coastal and estuarine habitats. As a result, shellfishaquaculture may need to become more efficient with moreproducts in a smaller area. Genetic improvement and domes-tication are proven routes for increasing agricultural produc- tivity (Hedgecock, 2011). Hedgecock (2011) speculated that the hybridization of inbred lines derived from the natural populations of shellfish would provide considerable scope for genetic improvements in such species. Many studies havesubsequently confirmed this speculation. Hybridization be- tween different geographical populations or inbred parent lines can obtain the additive component of the genetic va- riance of quantitative traits, which has become an importanttool in shellfish breeding (Newkirk., 1977; Mallet and Haley, 1983; Hedgecock and Davis, 2007).The long-term repetitive breeding ofin closed environments has caused a serious inbreeding depression and loss of gene-tic diversity (Miyake., 2016;He., 2019, 2020; Liu., 2020). BothHaixuan No. 1 and Nan- ke No. 1 were bred through successive generations from wild populations, andHaiyou No. 1 has also been selected over successive generations. Therefore, it is of practical significance to use the hybridization among the threevarieties for genetic improvement.
The growth trait is one of the main determinants of aqua- culture productivity, and it is the most common target trait in shellfish breeding, among which the most important eva- luation method is the measurement of shell size (Rose and Baker, 1994). The size of the pearl oyster, especially the shell width, is an important criteria used to predict the sizeof the implanted pearl nucleus. Before selection, populations or strains with superior characteristics should be selected as base populations because they may have superior genetic value for commercial traits or may have a high degree of genetic diversity (Jerry., 2002). In this study, the ba- sic population was three new varieties of, which had been bred for several generations and had growth ad- vantages that were suitable for cultivating pearls. Therefore,their offspring were more likely than the other populations to have obvious growth advantages. We measured the shell length and width of the offspring of the self-cross and hy- brid lines established by three new varieties of, which reflected the differences in the growth ability of the different groups. There was little difference in the growth of the larval stage among the different groups, and the hy- brid lines did not display an obvious growth advantage. These results indicate that the larvae of each group display an obvious maternal effect whereby the main nutrition for early growth is from the eggs. Meanwhile mating strategiesaccount for early growth differences. In addition to geneticbackground, environmental conditions affect the later growthdifferences. Similar phenomena have been reported in(Huo., 2015),(Zhang., 2017), and(Deng., 2010). In this experiment, the growth of each line on day 90 may have been affected by the maternal parent effect, but it mayalso have been due to mitochondrial heredity because mostmitochondria are passed on to the offspring through the egg(Cundiff, 1972).
The growth of each group was different at the three sites. In general, the conditions at commercial aquaculture sites vary due to local environmental factors, diet composition and abundance, and the degree of fouling (Cruz., 1998; Southgate and Beer, 2000). On day 150, the shell length of all groups at Zhulin was smaller than those at the other twosites. This may be because Zhulin was a large farming pond,and the infrequent water exchange led to insufficient diet abundance. In addition, long-term aquaculture may cause a certain degree of fouling at the bottom of the pond, which is also an important factor affecting the growth of shellfish. The sites at Huolu and Qingshantou are located in interti- dal areas, where the flow exchange caused by the tide re- sult in an abundance of plankton for shellfish growth if thereis not extreme climate change. On day 400, the shell lengthof all groups at Zhulin was still smaller than at the other twosites, but the differences clearly reduced over time. During this experiment, the water temperature in the South China Sea was gradually rising and plankton were abundant be-fore day 400. This indicates that the shellfish experience rapid growth during this period. At this time, the concen- tration of plankton may increase, and the sudden abundanceof plankton may enable the shellfish to grow at a faster rate. Although the growth performance of each group was dif- ferent at the different sites, the fast-growing groups were the same at all three sites. Therefore, in this experiment, en- vironmental differences were of minor consequence, and the growth potential of fast-growing lines could be realized. Ingeneral, culture ponds are only suitable for short-term farm- ing.
Theandare measures of additive and non- additive genetic action, respectively (Deng., 2010). The success of crossbreeding practice depends on the combin- ing ability of parents and cannot be judged only by the per- formance of the parents (Bhateria., 2006). A popula- tion with a higheris likely to obtain a better hybrid strain, and theof two populations is one of the bases used to select a good hybrid combination (Sheng and Chen, 1999). Yield generally increased with theof the self- cross lines, which is a measure of additive genetic variance.This was expected from the results of family selection re- ported by Langdon. (2003). In this study, theandfor the shell length and width of each line indi- cated that additive and non-additive genetic variance con- tributed to these traits.On day 400, thewas the high-est when Nanke No. 1 was employed as parents regardless of shell length or width, followed by thewhen Hai- xuan No. 1 as the female parent. This suggests that the pro- geny of the hybrid with Haixuan No. 1 as the female pa- rent and Nanke No. 1 as the male parent may have more growth advantages during crossing.
Heterosis is a very common but very complex biological phenomenon. However, it may not be fully expressed in theearly life stage because of the existence of a maternal ef- fect. Heterosis then manifests itself as the maternal effect weakens or disappears (Solemdal, 1997). Hybrids of the self-cross lines are expected to increase heterozygosity and reduce the effects of inbreeding depression, resulting in he- terosis (Hedgecock and Davis, 2007;Deng., 2010). However, this heterosis may be associated with levels of inbreeding depression (Evans., 2004). The results ofthis study showed that each group had advantages and dis- advantages in their heterosis at different growth stages or different sites. Therefore, the level of inbreeding depressionin these three new varieties may not be high. However, he-terosis in terms of shell length and width was obvious in thecross of Haixuan No. 1 and Nanke No. 1 on day 400. It wastherefore predicted that the cross of Haixuan No. 1 and Nan- ke No. 1 would be an ideal hatchery method to improve the growth ofseed.
The aim of this study was to determine the possibility of further improving the growth performance ofthrough hybridization. We evaluated the growth, survival, combining ability, and heterosis of the progeny in each line,which were established by three new varieties of. The results showed that these three newvarieties still had a strong growth performance, and the hybridization among the new varieties improved the growth performance of. Moving the same population from one area to another, as in this experiment, is a form of translocation. However, translocations are problematic for disease and other ecological reasons (Hégaret., 2008).Therefore, prior to translocation and crossbreeding, it is ne-cessary to consider the protection of habitats firstly. Atten-tion should also be given to separating culture stocks from natural stocks to avoid accidental hybridization.
Acknowledgements
This project was funded by the NationalNaturalScienceFoundation ofChina (No. 911221680). We would like to thank Hainan University, Guangdong Ocean University, and the South China Sea Institute of Oceanology, Chinese Aca- demy of Sciences for breeding the new varieties of. We thank Beihai Xiaoyuzhou Cooperative, and Guang- xi Jinggong Marine Science and Technology Co., Ltd. for providing experimental sites.
Allen, S. K., and Downing, S. L., 1986. Performance of triploid Pacific oysters,(Thunberg). I. Survival, growth, glycogen content, and sexual maturation in yearlings., 102:197-208.
Bhateria, S., Sood, S. P., and Pathania, A., 2006. Genetic analy- sis of quantitative traits across environments in linseed (L.).,150: 185-194.
Cruz, P., Ramirez, J. L., Garcia, G. A., and Ibarra, A. M., 1998. Genetic differences between two populations of catarina scal- lop () for adaptations for growth and sur- vival in a stressful environment., 1663: 321-335.
Cundiff, L. V., 1972. The role of maternal effects in animal breed-ing: VIII. Comparative aspects of maternal effects., 35: 1335-1337.
Deng, Y. W., and Du, X. D., 2009. Selection for fast growth in the Chinese pearl oyster,: Response of the first generation line.,40: 843-847.
Du, X. D., Deng, Y. W., Wang, Q. H, Xie, S. H., and Liu, D., 2015. Haixuan No 1 stock of pearl oyster., 10:53-55 (in Chinese with English abstract).
Evans, F., Matson, S., Brake, J., and Langdon, C., 2004. The ef- fects of inbreeding on performance traits of adult Pacific oys- ters ()., 230: 89-98.
Fan, S. G., Wang, J. X., Huang, G. J., Liu, B. S., Guo, Y. H., and Yu, D. H., 2016. Analysis of genetic diversity of nine families of., 12: 90-96 (in Chinese with English abstract).
Griffing, B., 1956. Concept of general and specific combining abi-lity in relation to diallel crossing systems., 9: 463-493.
Gu, Z. F., Wang, Q. Y., Fang, J. G., Ye, N. H., Mao, Y. Z., Shi, Y. H.,., 2009. Growth of cultured pearl oyster () in Li’an Lagoon, Hainan Island, China., 28:465-470.
Gu, Z. F., Shi, Y. H., Wang, Y., and Wang, A. M., 2011. Heri- table characteristics in the pearl oyster: Comparisons of growth and shell morphology of Chinese and Indian populations, and reciprocal crosses., 30: 241-246.
He, J. J., Liang, H. Y., Zhu, J. P., and Fang, X. C., 2019. Separa- tion, identification and gene expression analysis of PmAMP-1 from., 92: 728-735.
He, J., Shen, C. H., Liang, H. Y., Fang, X. C., and Lu, J. Z., 2020. Antimicrobial properties and immune-related gene expression of a C-type lectin isolated from., 105: 330-340.
He, M. X., Lin, J. S., Liu, W. G., and Xie, Y. X., 2016. Nanke No 1 stock of pearl oyster., 11:78-81 (in Chinese with English abstract).
Hedgecock, D., 2011.Wiley, Chichester, West Sussex,339-357.
Hedgecock, D., and Davis, J. P., 2007. Heterosis for yield and crossbreeding of the Pacific oyster., 272:17-29.
Hégaret, H., Shumway, S. E., Wikfors, G. H., Pate, S., and Burk-holder, J. M., 2008. Potential transport of harmful algae via re- location of bivalve molluscs., 361: 169-179.
Huo, Z. M., Yan, X. W., Zhao, L. Q., Liang, J., Yang, F., and Zhang, G. F., 2015. Larval and juvenile growth performance of Ma- nila clam hybrids of two full-sib families., 14: 564-568.
Jerry, D.R., Purvis, I.W., and Piper, L.R., 2002. Genetic diffe- rences in growth among wild populations of the yabby,(Clark).,33: 917-923.
Langdon, C., Evans, F., Jacobson, D., and Blouin, M., 2003. Im- proved family yields of Pacific oystersThun- berg derived from selected parents., 220: 227-244.
Liu, H. R., Zhang, H., Pan, X. L., Xu, M., Huang, J., and He, M. X., 2020. A high density genetic map by whole-genome rese- quencing for QTL fine-mapping and dissecting candidate genes for growth or sex traits in the pearl oyster ()., 519: 734839.
Mallet, A. L., and Haley, L. E., 1983. Growth rate and survival in pure population matings and crosses of the oyster., 40: 948-954.
Miyake, T., Isowa, K., Ishikawa, T., Komaru, A., and Kawamu- ra, K., 2016. Evaluation of genetic characteristics of wild and cultured populations of the Japanese pearl oysterby using AFLP markers., 24: 537-548.
Newkirk, G. F., 1980. Review of the genetics and the potential for selective breeding of commercially important bivalves., 19:209-228.
Newkirk, G. F., Waugh, D. L., and Haley, L. E., 1977. Genetics of larval tolerance to reduced salinities in two populations of oysters,., 34: 384-387.
Ohta, H., Kawamoto, T., Isowa, K., Aoki, H., Hayashi, M., Nari- ta, T.,., 2007. Motility of spermatozoa obtained from testes of Japanese pearl oyster., 73: 107-111.
Peng, Z. M., Tao, H. Q., Liu, G., and Liu, Z. G., 2015. Recearch on family selection and evaluation ofwith red adductor muscle., 35(4): 37-45 (in Chinese with English abstract).
Qiu, Y., Huang, X. Z., Lu, H., Shi, Y. H., Wang, A. M., and Wang, Y., 2013. Development of EST-derived microsatellite markers in the pearl oyster(Dunker) for genetic re- source monitoring., 5: 401-403.
Rawson, P., and Feindel, S., 2012. Growth and survival for gene- tically improved lines of Eastern oysters () and interline hybrids in Maine, USA., 326-329: 61-67.
Rose, R. A., and Baker, S. B., 1994. Larval and spat culture of theWestern Australian silver- or goldlip pearl oyster,Jameson (Mollusca: Pteriidae)., 126: 35-50.
Sheng, Z. L., and Chen, Y. S., 1999.. Sci- ence Press, Beijing, 444pp.
Shi, Y. H., Kui, H., Guo, X. M., Gu, Z. F., Wang, Y., and Wang, A. M., 2009. Genetic linkage map of the pearl oyster,(Dunker): Genetic map of., 41: 35-44.
Solemdal, P., 1997. Maternal effects–A link between the past and the future., 37: 213-227.
Southgate, P. C., and Beer, A. C., 2000. Growth of blacklip pearl oyster () juveniles using different nursery culture techniques., 187: 97-104.
Stevens, C., Plew, D., Hartstein, N., and Fredriksson, D., 2008. The physics of open-water shellfish aquaculture., 38: 145-160.
Wang, A. M., Shi, Y. H., Gu, Z. F., and Wang, Y., 2006. A breed- ing method of high quality pearl oyster for cultivating seawa- ter pearls. Patent, CN200610003813.1, 2006-11-15.
Wang, H., Du, X. D., Lü, W. G., and Liu, Z. G., 2010. Estimat- ing the heritability for growth-related traits in the pearl oyster,(Dunker)., 42(1): 57-64.
Wang, Q. H., Deng, Y. W., Du, X. D., Fu, S., and Lu, Y. Z., 2011. Realized heritability and genetic gains of three generation for superior growth in the pearl oyster., 31(2): 108-111.
Yang, J. M., Cai, W. Y., Luo, S. J., Wang, Q. H., Jiao, Y., Huang,R. L.,., 2018. Variation analysis of DNA methylation in Inbred and hybrid families of pearl oyster ()., 37(5): 1926-1932.
Yin, X. S., and Hedgecock, D., 2019. Bayesian hierarchical mo- deling of yield in incomplete diallel crosses of the Pacific oys-ter., 510:43-50.
Zhang, H. B., Liu, X., Zhang, G. F., and Wang, C. D., 2007. Growth and survival of reciprocal crosses between two bay scallops,Say andLamarck., 272: S88-S93.
Zhang, X. K., 2016. Study on the improvement of the strains of shell color and shell shape infor different ge- nerations. Master thesis. Dalian Ocean University.
Zhang, Y. H., Su, J. Q., Li, J., Zhang, Y., Xiao, S., and Yu, Z. N., 2017. Survival and growth of reciprocal crosses between two stocks of the Hong Kong oyster(Lam & Morton, 2003) in southern China., 48: 2344-2354.
(June 7, 2021; revised September 14, 2021; accepted March 22, 2022)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
#The two authors contributed equally to this work.
Corresponding author. E-mail: zpwang@ouc.edu.cn
(Edited by Qiu Yantao)
猜你喜欢
杂志排行
Journal of Ocean University of China的其它文章
- Quality Changes and Safety Evaluation of Ready-to-Eat Roasted Antarctic Krill (Euphausia superba) During Storage at Room Temperature (25℃)
- The Influence of Sea Sprays on Drag Coefficient at High Wind Speed
- Highly Efficient Heavy-Metal-Ion Removal from Shellfish Processing Liquid with Low Protein and Polysaccharide Loss by Hybrid Mesoporous Silica Diol-APDC-SBA15
- Ship Weather Routing Based on Hybrid Genetic Algorithm Under Complicated Sea Conditions
- L-Band Analysis of the Effects of Oil Slicks on Sea Wave Characteristics
- A Method for Reducing Ocean Wave-Induced Magnetic Noises in Shallow-Water MT Data Using a Complex Adaptive Filter
