Degradation of antibiotic contaminants from water by gas-liquid underwater discharge plasma
2023-03-15FuLU卢伏JianZHOU周建andZhengweiWU吴征威
Fu LU (卢伏), Jian ZHOU (周建) and Zhengwei WU (吴征威),2,*
1 School of Nuclear Science and Technology, University of Science and Technology of China, Hefei 230026, People’s Republic of China
2 Institute of Advanced Technology, University of Science and Technology of China, Hefei 230022,People’s Republic of China
Abstract Antibiotic contamination adversely affects human health and ecological balance.In this study,gasliquid underwater discharge plasma was employed to simultaneously degrade three antibiotics,sulfadiazine(SDZ),tetracycline(TC),and norfloxacin(NOR),to address the growing problem of antibiotic contaminants in water.The effects of various parameters on the antibiotic degradation efficiency were evaluated,including the discharge gas type and flow rate,the initial concentration and pH of the solution,and the discharge voltage.Under the optimum parameter configuration,the average removal rate of the three antibiotics was 54.0% and the energy yield was 8.9 g(kW·h)-1 after 5 min treatment; the removal efficiency was 96.5% and the corresponding energy yield was 4.0 g(kW·h)-1 after 20 min treatment.Reactive substance capture and determination experiments indicated that·OH and O3 played a vital role in the decomposition of SDZ and NOR,but the role of reactive substances in TC degradation was relatively less significant.
Keywords: antibiotic contamination, non-thermal plasma, gas-liquid underwater discharge,degradation efficiency, plasma reactive substances
1.Introduction
Since antibiotics with strong antibacterial effects were introduced in 1928, humans have been able to avoid suffering bacterial infections [1].However, the substantial increase in the use of antibiotics in human medicine, livestock husbandry, and aquaculture has increased the antibiotic content in the water body.Overabundant antibiotics in the water environment can accumulate in the body through the food chain, which has adverse effects on the bacterial flora in the human body [2], disrupting the colony dynamic balance and leading to drug-resistant bacteria.The World Health Organization has long recognized the proliferation of antibiotics as one of the challenges to human health [3].Therefore, it is necessary to degrade and remove antibiotic pollutants from the environment.
Many treatment technologies have been developed to treat antibiotic pollutants in the aqueous environment,such as absorption processes, advanced oxidation processes (AOPs),advanced biological treatment,and hybrid technologies[4-6].However, all of them have some problems; for example, the removal rate of adsorption processes is low, causing secondary pollution, and oxidation processes generally require additional oxidation reagents which are uneconomical [7].Thus, there is a need to develop a new technology to treat antibiotics in wastewater.
Non-thermal plasma, as one type of AOP, has been widely noticed in the field of environmental remediation.Non-thermal plasmas have various designs,such as dielectric barrier discharge (DBD), glow discharge, and corona discharge.In recent years, DBD as a non-thermal plasma technology has been widely used to degrade pollutants in the environment.DBD can evoke various chemical and physical effects in situ, like forming oxidation reactive substances(RSs) (O3, H2O2, ·OH, ·O), high-energy electrons, shock waves,ultraviolet(UV)radiation,electrohydraulic cavitation,etc.It has many advantages, such as high degradation efficiency, simple equipment, non-selective degradation of organics, and a mild treatment process.However, current plasma degradation technology faces difficulties such as poor energy efficiency and incomplete mineralization.Novel discharge reactors or combination with other degradation processes should be the route of the future [8, 9].

Table 1.Chemo-physical characteristics of antibiotics.
At present, the most common contaminants in water in China are tetracyclines, fluoroquinolones, and sulfonamides[10-12].Some researchers have reported using plasma to degrade antibiotics [13-15].However, most existing works using plasma to treat antibiotic contaminants have two shortcomings.On one hand, conventional experiments deal with a single antibiotic solution, which oversimplifies the problem of managing antibiotics in an aqueous environment.On the other hand, although it is effective to couple the plasma and catalysts to improve the degradation efficiency,secondary pollution of the catalysts cannot be avoided.We believe that it is difficult for existing experiments to support plasma technology application in practice.Therefore, we attempt to maximize the degradation capacity of the plasma itself to treat wastewater containing multiple antibiotics.We hope to facilitate the development of plasma technology towards practical applications.
This study used plasma to simultaneously treat three common antibiotic pollutants,sulfadiazine(SDZ),norfloxacin(NOR), and tetracycline (TC).The effects of different operating parameters, such as the initial concentration of antibiotics,the initial pH of the antibiotic solution,the voltage of the discharge,the type of working gas, and the gas flow rate,on the removal efficiency of pollutants were determined.The critical roles of O3and ·OH in the degradation of SDZ and NOR were confirmed by RS scavenging experiments.In addition, the concentration of RSs was tested, further explaining that RSs produced by plasma play an important role in antibiotic degradation.
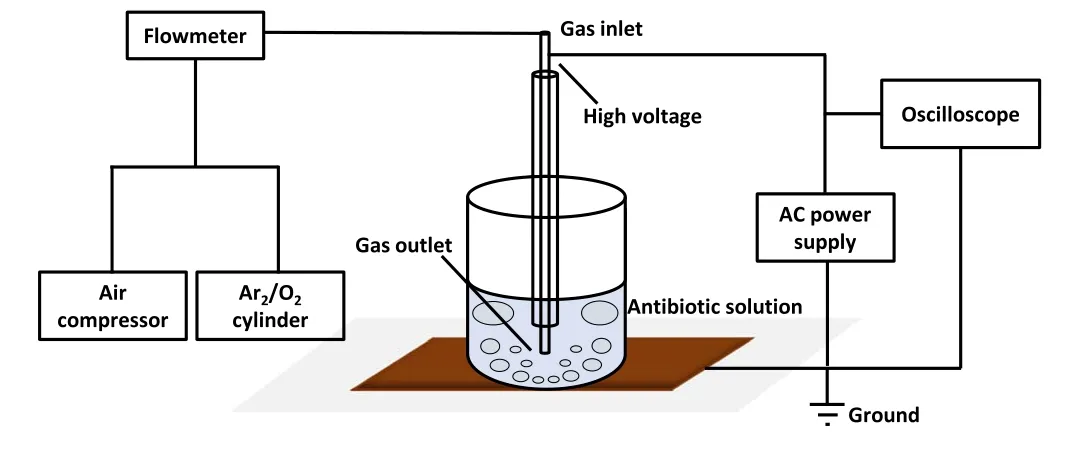
Figure 1.Overview of the plasma discharge treatment system used in antibiotic degradation experiments.
2.Experimental details
2.1.Materials and reagents
The antibiotics SDZ, NOR, and TC were purchased from Aladdin,China,and the chemical and physical characteristics are listed in table 1.They were above 96%purity and directly used in the experiments without further purification.Methanol(HPLC purity) and formic acid (HPLC purity) were also obtained from Aladdin,China.The antibiotics were dissolved in 1 l of deionized water to prepare three antibiotic stock solutions with a 100 mg l-1concentration.The stock solutions were stored at 4°C for further use.Analytical grade uric acid(UA),isopropanol(IPA),sodium pyruvate(SP),titanium sulfate (Ti(SO4)2), sodium indigo disulfonate, terephthalic acid (TA), 2-hydroxyterephthalic acid (HTA), sulfuric acid(H2SO4), phosphoric acid (H3PO4), hydrochloric acid (HCl),and sodium hydroxide (NaOH) were purchased from Macklin, China.
2.2.Plasma treatment apparatus
Figure 1 displays a schematic of the plasma treatment apparatus.The apparatus can be subdivided into the reaction container, the plasma generation, and the working gas input section.A 50 ml experimental beaker was used as the reaction vessel to contain the antibiotic solution.The plasma generation section comprised a power supply and a plasma nozzle.The high-voltage and high-frequency AC power supply featured the CTP-2000 K provided by Nanjing Suman Electronics Co.Ltd,China,with an adjustable output frequency of 10-45 kHz.A digital oscilloscope (GW Instek, GDS-2104) was used to monitor the voltage and current waveforms and the Lissajous figure.Figure 2 presents the typical voltage and current output waveforms during operation.The plasma nozzle was manufactured from cylindrical,coaxial quartz and copper tubes.The copper tube was embedded in the quartz tube and supplied with working gas at the inlet port, while the other port was inserted into the solution.The high-voltage electrode of the power supply was connected to the high-voltage port of the copper tube, and the low-voltage electrode was connected to the copper sheet at the bottom of the reaction vessel to generate a high-strength electric field in the copper tube.When the gas passed through the copper tube, it was excited by the electric field to generate plasma.The plasma was sprayed into the solution from the outlet and then interacted with the antibiotics.An oxygen or argon cylinder, or an air compressor (Jieba,JB12), and a flow meter constituted the gas input section.
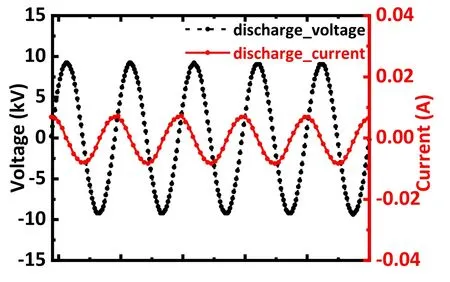
Figure 2.Waveforms of typical discharge voltage and current recorded by the oscilloscope during discharge.The input discharge voltage was 15 kV.
2.3.Plasma treatment and analytical methods
The reaction vessel was filled with 20 ml of antibiotic solution each time, diluted to the appropriate concentration, and the initial pH was adjusted by 0.1 mol l-1HCl and 0.1 mol l-1NaOH.The initial concentration, pH, discharge voltage, type of pumped gas, and flow rate were varied to determine the optimal degradation parameters.
After plasma treatment, the antibiotic levels in the water were determined using an ultra-high-performance liquid chromatograph (Water, ACQUITY UPLC H-class) equipped with a UV detector and a T3 column (2.1 mm × 100 mm,1.8 μm).The mobile phases were 0.1% formic acid aqueous solution (Phase A) and methanol (Phase B).Table 2 shows the mobile phase gradient settings,with the mobile phase flow rate maintained at 0.3 ml min-1, which allowed the simultaneous determination of the three antibiotics from one sample.The sample volume was 10 μl, and the retention time was 10 min.The column temperature was maintained at room temperature, and the concentrations of SDZ, NOR, and TC were detected at 265, 278, and 355 nm, respectively.
The removal efficiency (DE) of antibiotics treated was calculated by equation (1) [16, 17]:
where C0represents the concentration of antibiotics before degradation, and Ctrepresents the concentration after t min.The energy yield(EY)of plasma treatment was calculated byequation (2):
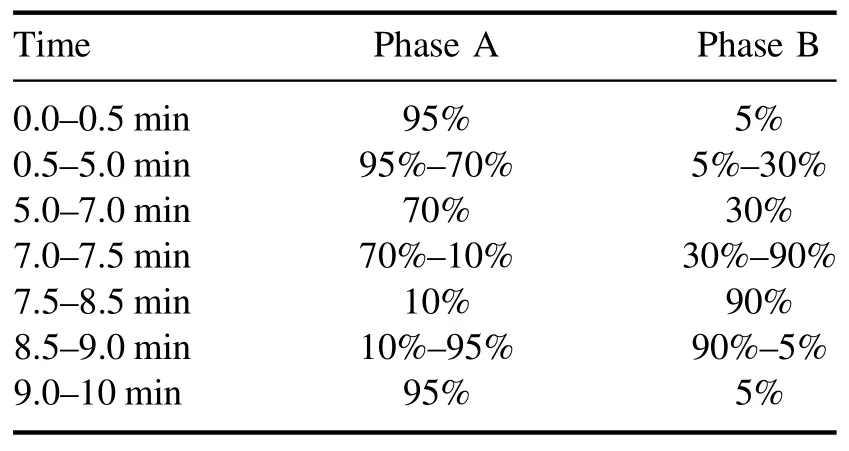
Table 2.Mobile phase establishment for detecting residual antibiotic concentrations in aqueous solutions via UPLC.
where C0refers to the initial concentration of antibiotics,V is the volume of solution,DE is the removal efficiency,and P is the output power of the power supply, which could be calculated by the Lissajous figure(figure S1),and t is the time of plasma treatment.
where U is the output voltage,dUc/dtis the current of the sampling capacitor,f is the discharge frequency,C=0.47 μF is the sampling capacitance of the power supply,k=1000 is the voltage sampling ratio,and S is the area of the Lissajous figure.
2.4.Reactive substance capture and measurement experiments
In order to understand the contribution of RSs to the degradation, RS scavengers UA, IPA, and SP were used to demonstrate the roles of O3[16], ·OH (reactions (4) and (5))[18], and H2O2[19] in the degradation process, respectively.
The selective indigo method was used to determine the O3level after plasma treatment [20].The amount of H2O2was detected by titanium sulfate photometry [21].The ·OH can react with TA to produce HTA,which emits fluorescence at 450 nm when excited by light at a wavelength of 315 nm.The content of ·OH in the solution can be quantified as the fluorescence intensity of HTA, which was detected by a fluorescent microplate reader (Molecular Devices, SpectraMax iD5) [15].
3.Results and discussion
3.1.Effect of working gas types and flow rates on removal efficiency
Argon,oxygen,and air were used to excite plasma to observe the effect of gas type on the degradation efficiency.The mixed solutions with a concentration of 20 mg l-1were treated with plasma generated by different gases, and the treatment time ranged from 0 to 25 min.The voltage used was maintained at 15 kV.The results show that the degradation efficiency continues to increase with the extension of treatment time regardless of the gas used (figure 3).After 5 min plasma treatment, 96.5% SDZ and 93.6% NOR removal could be achieved using O2.However, when air was used as the feed gas,the degradation efficiency dropped to 60.7%and 51.7%, respectively.When argon was used, the removal efficiency further declined to 10.1% and 7.8%.The result implies that for SDZ and NOR, the type of gas significantly affects the degradation efficiency.This phenomenon is attributed to the fact that the plasma removal ability of antibiotics is dependent on the oxidation of RSs in water.The more RSs, the more efficient the degradation of antibiotics.Using O2as the filling gas generates more reactive oxygen substances in water, explaining the strongest scavenging effect of the O2plasma [16, 17, 22, 23].
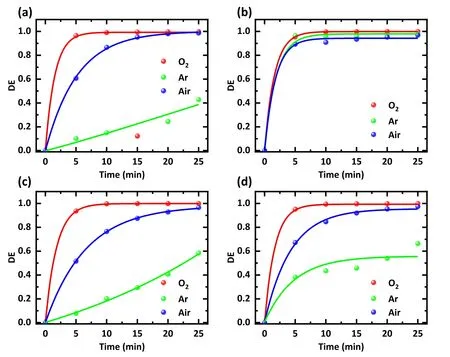
Figure 3.Effect of different working gas types on the degradation efficiency of different antibiotics.Treatments were performed with(a)an SDZ solution, (b) a TC solution, (c) a NOR solution, and (d) a mixture of SDZ, TC, and NOR solution.The solution concentration was 20 mg l-1, the discharge voltage was 15 kV, and the treatment time was 0-25 min.Working gases were O2, argon, and air, respectively.
Filling with different working gases has much less effect on TC degradation efficiency (figure 3(b)).It is possible because its structure is more unstable and susceptible to other factors, such as the photodegradation of TC by the light emitted by the plasma.Figure 3(d) depicts the average degradation efficiency by different input gases, showing that O2had the fastest degradation rate.After 10 min,the average degradation efficiency of the antibiotics was 99.5%using O2.Air took 20 min to remove ~95%of antibiotics.Only 66.4%of the total antibiotics were removed using argon after 25 min.Considering the use of air as the working gas, a satisfactory treatment effect with low cost was achieved, so the air was used as the working gas for subsequent experiments.
The flow rate of gas can affect the degradation of antibiotics in two ways.It can affect the direct interaction between plasma and antibiotics, like the direct collision between high-energy electrons and antibiotic molecules.It can also change the content of RSs in water and affect the indirect interaction.After identical treatment time, with an increase in the air filling speed,the treatment effects of SDZ,TC, and NOR increase first and then decrease, and the treatment effect is best at 3 l min-1(figure 4).Corresponding to the air filling speeds of 1, 3, and 5 l min-1, the average degradation efficiency after 25 min is 82.7%, 96.5%, and 94.9%, respectively (figure 4(d)).When the air velocity is small, a larger air velocity is conducive to bringing the exciting RSs into the solution, thus playing a better role in degrading antibiotics [24, 25].However, too fast an air velocity may cause air molecules to be discharged before being converted into RSs, resulting in reduced RSs.It is also possible to generate larger bubbles in the water and reduce the contact between RSs and antibiotic molecules.As a result,an excessive gas flow rate reduces the degradation efficiency[26-28].In conclusion, 3 l min-1of air flow is the most suitable for degrading antibiotics.
3.2.Effect of initial concentration and pH of antibiotics solution
The concentration of antibiotics significantly impacts the degradation efficiency.When the discharge voltage was immobilized at 15 kV, air at 3 l min-1was used as the discharge gas.The quantities of RSs produced by the plasma discharge over a period of time are limited.If the concentration of antibiotics in the water is too low, the RSs will be underutilized, resulting in a waste of energy.Conversely,too high a concentration of antibiotics will result in a large number of byproducts.Therefore, the competition between the byproducts and the target antibiotic molecules will accentuate the scarcity of RSs, resulting in inadequate degradation of the antibiotics [29-31].
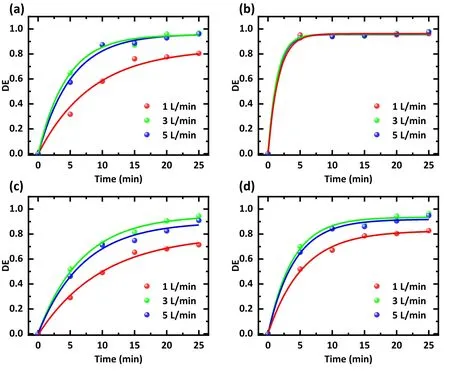
Figure 4.Effect of the gas flow rate on the degradation efficiency of different antibiotics.Treatments were conducted with (a) an SDZ solution,(b)a TC solution,(c)a NOR solution,and(d)a mixture of SDZ,TC,and NOR solution.The solution concentration was 20 mg l-1,the working gas was air, the discharge voltage was 15 kV, and the treatment time was 0-25 min.Flow rates were 1, 3, and 5 l min-1,respectively.
The results of different initial concentrations of antibiotic treatment are displayed in figure 5,and figure 5(d)graphs the evolution of the average degradation efficiency with treatment time for the mixed solution.The lower initial concentrations increase the treatment efficiency.Following a treatment time of 25 min,a treatment efficiency of 95.4%was achieved using an initial concentration of 10 mg l-1.When the concentration was 20 mg l-1, the treatment efficiency decreased to 94.8%,and further increasing the concentration to 30 mg l-1only removed 92.9% of the antibiotic.
Nevertheless, this does not mean that lower initial concentrations are desired.Figure 6 presents the energy efficiency when treating different initial concentrations of antibiotics, showing that the energy efficiency tendency decreased over time, as most antibiotics were removed in the first 10 min.The energy yield of the plasma decreases as the concentration of the antibiotic decreases.In broad terms, an initial concentration of 20 mg l-1is appropriate to obtain an acceptable degradation and energy efficiency.
In addition, experiments with different initial pH values were conducted to investigate the effect of pH on degradation efficiency.The output voltage was 15 kV, the air rate was 3 l min-1,and the initial concentration of antibiotics was also fixed at 20 mg l-1.The initial pH values varied from 3 to 11,and the experimental results are exhibited in figure 7.SDZ is more readily degraded in acidic solutions (figure 7(a)).In contrast, an alkaline environment is more favorable for the degradation of TC(figure 7(b))and NOR(figure 7(c)),which is consistent with the experimental results of He D et al [32]and Tang S et al[28].After 5 min,the degradation efficiency of TC in solution at pH value of 11 was 1.9 and 2.2 times higher than those at pH values of 7 and 3,and the degradation efficiency of NOR in solution at pH value of 11 was 2.8 and 4.9 times as effective as those at pH values of 7 and 3,respectively.
On one hand, the pH of antibiotic-contaminated wastewater can play an essential role in producing RSs.Alkalinity or neutrality is preferable for converting O3to ·OH [21, 33-35].Among the RSs, ·OH has the most oxidizing power with the highest oxidation potential,implying that more·OH will cause faster degradation [36].On the other hand, the TC molecule will convert from the cationic state to the more unstable anionic form when shifting from acidic to alkaline solutions[32].These factors explain the accelerated removal efficiency of TC and NOR with increasing pH value.In contrast, SDZ was more easily removed in an acidic environment.The reason for this is probably that an acidic environment enables more powerful oxidation of ·OH [26, 31, 37-39], and O3is more easily concentrated in acidic conditions [29, 40].Overall,regarding the overall antibiotic degradation level(figure 7(d)),antibiotic degradation at alkaline pH conditions was deemed more beneficial in our experiments.
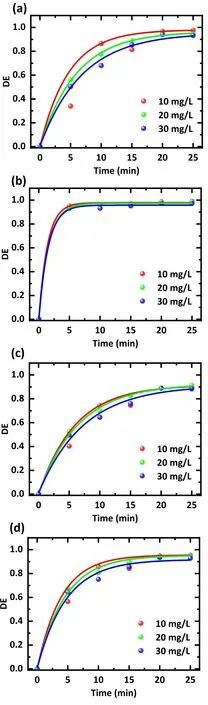
Figure 5.Effect of the initial concentration on the degradation efficiency of different antibiotics.Treatment was applied with (a) an SDZ solution, (b) a TC solution, (c) a NOR solution, and (d) a mixture of SDZ, TC, and NOR solution.The working gas was air,the flow rate was 3 l min-1,the discharge voltage was 15 kV,and the treatment time was 0-25 min.The solution concentrations were 10,20, and 30 mg l-1, respectively.
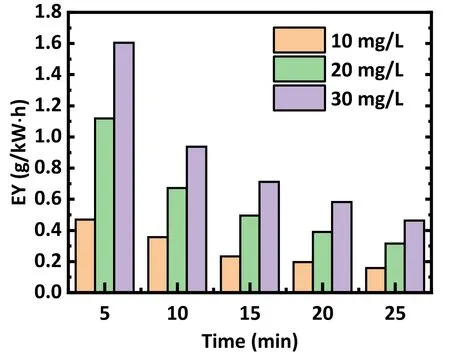
Figure 6.Influence of the concentration of antibiotic solution on the energy utilization efficiency (EY) with the same other parameters.Air was used as the working gas, the flow rate was set at 3 l min-1,and the discharge voltage was 15 kV.The solution concentrations were 10, 20, and 30 mg l-1, respectively.
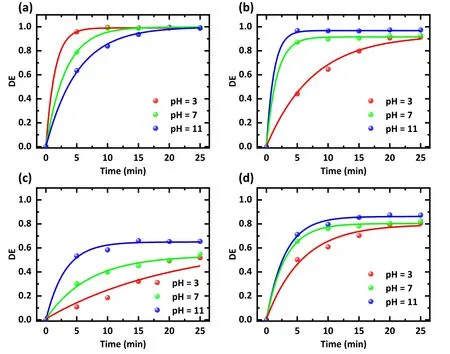
Figure 7.Effect of the solution pH on the degradation efficiency of different antibiotics.Treatment was conducted with(a)an SDZ solution,(b)a TC solution,(c)a NOR solution,and(d)a mixture of SDZ,TC,and NOR solution.The solution concentration was 20 mg l-1,the working gas was air,the flow rate was 3 l min-1,the discharge voltage was 15 kV,and the treatment time was 0-25 min.The initial pH settings were 3,7,and 11,respectively.
3.3.Effect of applied voltage
The effect of discharge voltages on the degradation was investigated.The results are depicted in figure 8.The initial concentration of 20 mg l-1,the air flow rate of 3 l min-1,and the initial pH of 12 were fixed, while different discharge voltages of 15 kV, 18 kV, and 21 kV were used.Figure 8(a)shows that after 10 min of treatment,the average degradation efficiency increases from 71.7% with 15 kV to 86.8% with 18 kV and subsequently to 88.5% with 21 kV.Extending the treatment time to 25 min increases the degradation efficiency from 96.4%at 15 kV to 96.6%at 18 kV and then to 97.4%at 21 kV.The results indicate that the degradation efficiency will enhance as the discharge voltage increases.The physicochemical effects in the discharge were enhanced with increased input energy.In addition to more RSs, higher voltages accelerate the electrons in the plasma, emitting more intense UV light and stronger shock waves, all of which increase the degradation efficiency of antibiotics [16, 41].

Figure 8.Impact of discharge voltage on the (a) degradation efficiency (DE) and (b) energy utilization efficiency (EY) of the antibiotic mixture.The solution was a mixture of SDZ,TC,and NOR at a concentration of 20 mg l-1,the pH value was set to 12,and 3 l min-1 of air was used as the working gas.
Figure 8(b)illustrates the energy efficiency with different discharge voltages.Because more than half of the antibiotics were removed within the first 5 min,the proportion of energy effectively utilized fell as the treatment time progressed regardless of the discharge voltage.Therefore, continuously increasing the discharge voltage is not recommended.Furthermore, as the discharge time increased to 25 min, the degradation efficiency at 21 kV was only 1%higher than that at 15 kV.A minimum operating voltage of 15 kV was considered to achieve the appropriate degradation efficiency and the best energy consumption and economy.
3.4.Reactive substance trapping agent experiment
During the gas-liquid discharge plasma, RSs such as H2O2,O3,and·OH are initiated through reactions(6)-(12)[42,43].These RSs often have strong oxidation potentials and react non-selectively with antibiotic molecules, eventually giving rise to their decomposition.SP, UA, and IPA were added to the antibiotic solution as H2O2,O3,and·OH trapping agents,respectively, to determine the role of RSs in the degradation.These agents reacted with the RSs in the solution while competing with the antibiotic molecules.
Figure 9 illustrates the effect of adding trapping agents at 1, 5, and 10 mmol l-1on the treatment of the SDZ(figure 9(a)), TC (figure 9(b)), and NOR (figure 9(c)).The results show that for SDZ and NOR,the addition of IPA and UA significantly decreased the degradation, and the addition of 1 mmol l-1UA and IPA decreased the degradation efficiency by 9.0%and 13.6%for SDZ and 15.6%and 21.4%for NOR.The degradation efficiency of antibiotics decreased as the number of added scavengers increased.The degradation rate of NOR decreased by 15.6%after adding 1 mmol l-1UA,and the addition of UA at concentrations of 5 and 10 mmol l-1corresponded to 30.9% and 53.5% decrease,respectively.Notably, the inhibitory effect of UA was stronger than that of IPA when the dosages of UA and IPA were higher, probably because UA also scavenged ·OH from the water.In addition, the addition of SP had a negligible effect on the degradation efficiency.These indicate that·OH and O3are the major degradation RSs.Figure 9(b) illustrates the degradation of TC with the addition of scavengers and shows that their impact on the degradation of TC is negligible.Other effects in the plasma discharge might play a more significant role in the degradation of TC, analogous to that discussed in section 3.1.
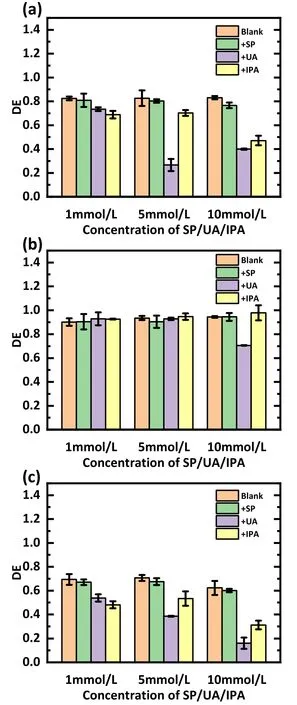
Figure 9.Outcomes of the RS capture experiments carried out with(a)an SDZ solution,(b)a TC solution,and(c)a NOR solution.The discharge voltage was 15 kV, 3 l min-1 of air was used as the working gas, the initial concentration was set at 20 mg l-1, and the pH value was 12.
In overview,O3and·OH influence the decomposition of the antibiotic SDZ and NOR, while H2O2plays a minor role.The role of RSs in TC decomposition is not obvious.
3.5.Quantity of reactive substances in the antibiotic solution
The discharge voltage was 15 kV, the feeding air rate was 3 l min-1, the initial concentration of antibiotics was 20 mg l-1, and the initial pH was 12.The concentrations of O3, H2O2, and ·OH in the antibiotic solutions and the blank deionized water were analyzed after 5, 10, and 20 min, as demonstrated in figure 10.For the same plasma discharge time, more O3and ·OH were produced than H2O2.For example, when the underwater plasma was discharged for 5 min, the concentrations of O3and ·OH in the blank water group were 2.8 μmol l-1and 3.1 μmol l-1, while the concentration of H2O2was 9.0 nmol l-1.The ·OH content we measured was close to that measured by Duan L et al [44],but different from that reported by Guo H [26] and Shang K et al [45].This is attributable to the transition of O3to ·OH(reactions (13)-(15)).Research has shown that an alkaline environment will be more favorable for converting O3to·OH[21, 35].

Figure 10.Concentrations of (a) O3, (b) H2O2, and (c) ·OH in the antibiotic solutions and blank deionized water.The discharge voltage was 15 kV,3 l min-1 of air was used as the working gas,the initial concentration was set at 20 mg l-1, and the pH value was 12.
In addition, the concentration of these RSs increased with the discharge time.The concentration of O3in the treated blank deionized water changed from 2.8 μmol l-1at 5 min to 3.2 μmol l-1at 10 min, then to 4.5 μmol l-1when treated for 20 min(figure 10(a)).Moreover, as represented by O3, the 5 min treatment produced 2.8 μmol l-1of O3in the blank group,while the O3concentration was reduced to 2.6 μmol l-1in the SDZ solution,1.8 μmol l-1in the NOR solution,and 1.5 μmol l-1in the mixture solution.The concentration of RSs in the antibiotic solution was reduced compared to that within the blank group,supporting the involvement of RSs in antibiotic degradation.
4.Conclusion
In this study, we successfully removed three antibiotics, SDZ,TC, and NOR, simultaneously through gas-liquid underwater plasma discharge in water.Antibiotic degradation was differently affected based on the type and flow rate of the working gas, the initial concentration, the pH value, and the discharge voltage.O2exhibited the most effective degradation,and high or low gas flow rates reduced degradation efficiency.The lower the initial concentration, the higher the degradation efficiency but the lower the energy efficiency.TC and NOR preferred rapid degradation in alkaline solutions,whereas an acidic environment promoted SDZ degradation.A higher discharge voltage promoted degradation, but energy utilization efficiency decreased with increasing voltage.The most desirable degradation conditions were selected as: discharge voltage of 15 kV, air injection rate of 3 l min-1, pH value of 12, and initial concentration of 20 m l-1.Under such conditions,the average removal rate of the three antibiotics was 54.0% and the energy efficiency was 8.9 g(kW·h)-1for 5 min;and for 20 min of processing time,the removal efficiency was 96.5% and the corresponding energy utilization efficiency was 4.0 g(kW·h)-1.Experiments on the capture and detection of RSs demonstrated that O3and ·OH contribute to dissociating antibiotic molecules, but the role of H2O2was negligible.The study suggested that the role of RSs in TC degradation is unclear, probably because other effects influenced its degradation.The study showed that using underwater plasma to treat antibiotic contaminants possesses many merits and attractive prospects.Future research will focus on exploring the detailed evolution of antibiotic disintegration.Improving the experimental set-up and changing the discharge form will increase its efficiency while keeping it cost-effective.
Acknowledgments
This work was supported by the Key R&D Plan of Anhui Province (No.201904a07020013), Collaborative Innovation Program of Hefei Science Center,CAS(No.CX2140000018),and the Funding for Joint Lab of Applied Plasma Technology(No.JL06120001H).
杂志排行
Plasma Science and Technology的其它文章
- Application of integrated simulation environment SIEMNED to the analysis of the MEPHIST-0 tokamak operation
- Improvement of a temperature response function for divertor heat flux monitoring in fusion devices
- Degradation of sulfamethoxazole in water by dielectric barrier discharge plasma jet:influencing parameters, degradation pathway, toxicity evaluation
- Experimental investigation of dynamic stall flow control using a microsecond-pulsed plasma actuator
- Laser-assisted ablation and plasma formation of titanium explored by LIBS,QCM, optical microscopy and SEM analyses along with mechanical modifications under different pressures of Ar and Ne
- Study on the effect of focal position change on the expansion velocity and propagation mechanism of plasma generated by millisecond pulsed laser-induced fused silica
