Effects of Fe 3+and Ca 2+on sorption of phenanthrene by Humin in karst soil,Southwest China
2023-03-09XianjinAnWeiLiXinyueDiJiachengLan
Xianjin An · Wei Li · Xinyue Di · Jiacheng Lan
Abstract Humin (HM) is the main organic matter component to affect the migration and transformation of polycyclic aromatic hydrocarbons (PAHs) in soil.The study on influence of the morphology change of inorganic ions on the adsorption of PAHs in soil and its organic matter is still rare at microscopic scale.In this paper,yellow soil humin (YS-HM) and lime soil humin (LS-HM) were chosen as samples,then Fe 3+and Ca 2+were added into samples to facilitate the precipitation by changing the existing conditions of ions,and the mechanism by which inorganic precipitation changed adsorption capacity of karst soil was analyzed from the microscopic scale.The results showed that the adsorption capacity of HM reduced with the inorganic precipitation increasing.The precipitation of Ca 2+and Fe 3+both reduced the adsorption capacity of YS-HM and LS-HM by 61.71% and 71.83% on average,respectively.The results of scanning electron microscope-energy dispersive spectrometry (SEM-EDS) and pore analysis showed that the HM porosity decreased after formation of Ca 2+and Fe 3+precipitation.According to the value of Freundlich parameter n,it may be because the precipitation or colloid of Ca 2+and Fe 3+filled micropores and covers high-energy adsorption sites of the HM.This research provides theoretical support for studying the PAHs migration and bioavailability of Calcium-rich in karst soil.
Keywords Inorganic ions · Humin · Phenanthrene · Adsorption · Soil · Karst
1 Introduction
The fate of PAHs in the natural environment is mainly controlled by physicochemical processes such as adsorption/desorption (Ukalska-Jaruga et al.2020;Schneckenburger &Thiele-Bruhn 2020;Chen et al.2021;Wang et al.2021),and their environmental toxic effects are closely related to their bioavailability.However,the traditional organic pollution risk assessment is still mainly based on the observed concentrations of organic pollutants,which may lead to overestimation of the short-term effects of organic pollutants and underestimation of their long-term effects in studies (Alexander 2000;Louchart &Voltz 2007;Qian et al.2020a,b).A large number of studies have shown that soil organic matter (SOM) is an important component for the adsorption of toxic pollutants (Lamichhane et al.2016;An et al.2021).As a part of SOM,HM is the main component to control the adsorption of PAHs in soil.Studies have shown that HM is the momentous “hard carbon” in soil,which performs nonlinear adsorption of PAHs by kinds of ways such as surface high energy site and pore adsorption (Chen et al.2017).Many reports have been conducted on PAHs in karst ecology (Shao et al.2014;Yu et al.2018;Cheng et al.2021).Lan et al (2018) reported that the migration of PAHs in karst groundwater closely related to seasonal rainfall.Zhu et al.(2022) illustrated that high ring PAHs were more enriched in the soil of typical karst trough valleys in Chongqing,and PAHs of soil generally caused a low risk of carcinogenicity.Qian et al (2020) showed that PAHs of low molecular weight was mainly found in the karst cave sediment,and PAHs distribution had no significant relationship with the sediment particle size.Chen et al (2022) shown that particle-promoted transport with rapid water flow was pivotal for PAHs mobilization to sediments and significant correlations of PAHs compositions were observed in the multimedia environment of karst spring systems.However,these studies mainly focused on the environmental behavior of PAHs overall,and the migration of PAHs in karst soil is rarely reported from the microscale.The effect of HM on the PAHs adsorption is still rarely studied from the microscopic scale.In order to accurately investigate the effect of ion precipitation in high-calcium soils in karst areas on the adsorption of PAHs by hard carbon organic matter of soil-HM,HM was demineralized after extraction,and inorganic mineral ions (such as calcium and iron) were removed.
The content of Ca2+in the soil of karst areas is abnormal,which is 2-3 times that of the same type of soil in other areas (Gao et al.2019).Under the complex environmental conditions of karst,the Ca2+and Fe3+are precipitated after saturation and transformed into precipitates or colloids due to repeated drying and wetting,freeze-thaw cycles,etc.The inner and outer surfaces and pores of particulate organic matter such as HM may be covered and filled by these inorganic precipitates due to changes in climate and temperature.However,there are few researches considering the influence of inorganic ions on the the migration of PAHs due to the soil organic matter change.It has been reported that the freeze-thaw cycle of the samples may affect the extraction of phenanthrene in the soil,mainly because phenanthrene enters the deeper soil pores due to the aging effect (Zhao et al.2009).The author’s previous study also showed that the supergenous process had a significant hysteresis effect on the desorption of PAHs,mainly because PAHs were trapped,resulting in the amount of desorption decrease (An et al.2021).
In this study,two types of karst soil (yellow soil,YS;lime soil,LS) and its humin fraction (YS-HM and LS-HM) were selected and inorganic ions (Ca2+and Fe3+) were loaded in the soil adsorption experiment by adjusting the pH adsorption background solution to change the presence form of two ions,in order to verify how the inorganic ion precipitation at the micro scale affect the soil organic matter components,and affect PAHs migration behavior and bioavailability in the karst soil.The study provided more accurate data to support the migration and bioavailability of PAHs in karst soil matrix.
2 Materials and methods
Two types of typical HM fraction were obtained after karst YS and LS being separated and demineralized in this research.Both soils were obtained from uncultivated karst areas in southwest China (Fig.1).All soils were collected at 0~ 12 cm from the surface,which were air-dried naturally and then screened through a 2 mm sieve,and PAHs weren’t detected.The separation and demineralization of HM components were carried out according to the extraction method by Di et al.(2019).
The main physical and chemical properties of soil and its components were shown in Table 1.The pH determination: The soil was dispersed in an aqueous solution at a ratio of 1:2.5 and measured by a pH meter.C,H,N,O elements of soil were measured by Vario EL III elemental analyzer (Elementar type),13 C-nuclear magnetic resonance (NMR) (Bruker AscendTM600WB type) spectrum was used to analyze the main carbon groups of HM (Fig.2),and NMR spectrum of different wavelength bands was used to calculate the hydrophobicity degree (ration of hydrophilic carbon and hydrophobic carbon,HB/HI) of HM (Savarese et al.2021).

Fig.2 13C-NMR spectrum curve of HM fractions in YS and LS
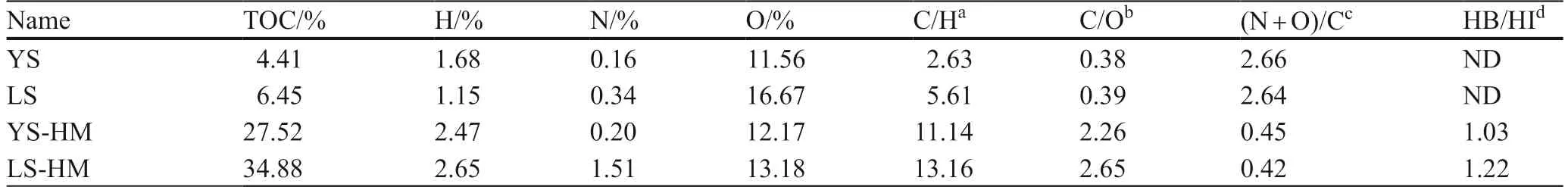
Table 1 Properties of soil and its fraction
2.1 Selection and characteristics of chemicals
In the study,phenanthrene was selected as the target organic pollutant (pure grade in high-performance liquid chromatography),which was a common pollutant in the environment and also a common by-product in industrial and agricultural production (Lv et al.2022).The adsorption/desorption of phenanthrene in soil and sediments had been extensively studied.Phenanthrene was purchased from Sigma Chemical Company,with the purity > 98%,the molecular weight of 178.23 g/mol,the density of 1.063 g/cm3,the solubility of 1.29 mg/L,log Kowof 4.57 and a molar volume of 167.6 cm3/mol.
2.2 Properties and preparation of solutions
The background solution for the adsorption test included 0.005 mol/L CaCl2as the main mineral component,100 mg/L NaN3to control the microorganism growth,and 5 mg/L NaHCO3to stabilize pH of the solution about to 7.A methanol stock solution of 1000 mg/L phenanthrene was prepared in advance,then diluted to stock solution with a series of concentrations and stored at a low temperature of 4 °C.The stock solution and the background solution were mixed in a certain proportion to obtain the initial concentration C0of the experiment.In order to avoid the methanol-assisted dissolution effect (Lu &Pignatello 2002),the methanol content was kept below 0.2% in this process.
2.3 Fe 3+and Ca 2+loaded in HM
Fe3+and Ca2+with three different concentrations were added to YS-HM and LS-HM to simulate the effect of natural environment in karst areas on the structure of organic matter itself.The main steps in the experiment of adsorbent treatment by Fe (OH)3were as follows.Firstly,a 100 mL centrifuge tube was washed and dried at high temperature.FeCl3solution of different concentrations was added into the centrifuge tube pre-placed with 0.5 g HM samples,then the centrifuge tube shaken on a constant temperature shaker for 24 h.1 mol/L NaOH was added into the corresponding centrifuge tube and shaken on a constant temperature shaker for 48 h to fully mix the inorganic precipitate and the adsorbent sample.Secondly,the shaken centrifuge tube was withdrawn,centrifuged in a high-speed centrifuge at 10,000 rap/min for 10 min to remove the supernatant.The mili-Q water was injected at the same time,and centrifuged again to remove the excess inorganic precipitate on the surface of the HM.This process was repeated 3 times.Subsequently,the treated samples were freeze-dried.The experimental procedure for the treatment of inorganic precipitated CaCO3was similar to that of Fe(OH)3,the difference was that the FeCl3solution was replaced by CaCl2,and the NaOH solution was replaced by Na2CO3solution.The specific ion addition amount was shown in Table 2.

Table 2 Treated HM sorbents by different ions

Table 3 Frundlich sorption isotherm parameters and calculated concentration-dependent Koc values
2.4 Adsorption experiment
A certain amount of phenanthrene was accurately weighed and dissolved in methanol to prepare a standard solution of 1000 mg/L.Before the experiment,the standard solution of phenanthrene was taken out and added to the background solution in proportion.The phenanthrene was prepared into solutions with different concentrations of 1~ 1000 mg/L.Through preliminary experiments,we selected 21 d as the adsorption equilibrium time,and 50 mL brown glass bottles with a Teflon septum lined lid were used as the adsorption reaction bottle.The preliminary experiment proved that the reaction bottles could effectively prevent the volatilization of phenanthrene.The experimental steps were as follows.Soil or HM fraction and treated samples were added to 50 mL brown glass bottles,then about 50 mL of phenanthrene solutions of different concentrations were added and the bottles were fastened with a Teflon septum lined lid.Later,the bottles were shaken at a shaker for constant temperature (21 °C) for 21 d.During this period,the bottles were taken out and manually shaken for 5 min at an interval of 3 d to prevent the samples deposition at the top of bottle.After the experiment was equilibrated for 21 d,the samples were taken out and placed in a dark room for 3 d to make the samples in the ampoule settle naturally.Huang et al.(1997) found that there was no obvious difference for solidliquid separation between natural stillness and centrifugal separation.Then,about 2 mL supernatant was taken out and added into a 5 mL vial prefilled with about 3 mL of methanol.The phenanthrene concentration was determined by ultra-high-performance liquid chromatography (UPLC).The original concentration was calculated by density-based dilution factor,and the phenanthrene concentration in the equilibrated solution phase was calculated by mass balance.A blank control group was set up in the experiment.The rate of solute loss in the reaction vial was evaluated by control experiments.The study found that the evaluated loss rate of this experiment was lower than the initial concentration of 4%,so the effect of the experimental consumables on the adsorption could be neglected when calculating the adsorption data.
2.5 Determination of compounds
UPLC was used for the determination and analysis of phenanthrene in methanol-water mixed solution (ODS,5 μm,2.1×250 mm column,Agilent 1290 model).The chromatography had a diode detection array UV detector and a fluorescence detector.In this study,the concentration of PAHs was determined using a UV detector (DAD) with detection range of 0.01~ 1000 mg/L at the state key laboratory of environmental geochemistry.The standard determination curve (R2=0.99) of phenanthrene was established in preliminary experiment at 0.01-1000 mg/L.The UPLC determination conditions were as follows: the mobile phase was acetonitrile and mili-Q water,with a volume ratio of 78:22 and a flow rate of 0.4 mL/min.The detector wavelength was 254 nm,and the retention time was 2.3 min.The solute concentrationsCeandC0of the aqueous phase were calculated by the dilution factor (Weber &Huang 1996).The solid phase adsorbate concentration was derived from the two-phase mass balance as follows:
Where,qewas the concentration of the solid phase adsorbent,mg/kg;C0,Cewere the concentrations of pollutants in the liquid phase at the initial time and at equilibrium,respectively,mg/L;Vwas the volume of the reaction system solution,L;mwas the adsorbent mass,kg.
3 Results and discussion
3.1 Morphology and characteristics of HM
The microstructure of HM samples and treated samples was observed under scanning electron microscopy (SEM),revealing many microscopic pores.A large number of previous studies have shown that the adsorption of organic pollutants by adsorbents such as soil and sediment closely related with their specific surface area and micropores (Luo et al.2012;Sun et al.2020;Dai et al.2022;Jesus et al.2022).Microstructure of original HM samples was observed under the SEM (Fig.2).It can be seen from the figure that the HM samples contain a unique microporous structure with different surface morphological structures,which may relate to the plant origin and its humification process.At the same time,when the treated samples were observed under the SEM,it was found that the surface area of the HM samples was covered and the micropores of the HM samples were also blocked or filled by inorganic precipitates (Fig.3).EDS can qualitatively and quantitatively analyze the elemental composition and content of the observed sample,,and therefore,the EDS spectrum and elemental data could better illustrate the treated results for different samples (Fig.4).The specific values of the inorganic ions in the HM samples were shown in Fig.5.

Fig.3 Micpore structure of HM fraction

Fig.4 Surface of HM fraction being covered and filled
It showed that there weren’t peaks of Fe3+and Ca2+in Fig.5 (a and b) which indicated that most of the inorganic ions had been removed during the HM extraction process,but all were lower than the instrument detection value.the peak of Fe3+and Ca2+were obvious in figures c,d and e,f.It showed that this treatment experiment had obvious effect on HM.Because the excess inorganic precipitate on the HM surface was removed by repeated washing with deionized water,with most of them forming a binding state with HM.As can be seen from Fig.6,as the content of artificially added inorganic ions increased,the content of the two ions also increased.Therefore,this experiment was valid to change the morphology of HM.

Fig.5 Spectrum for original soil and HM fractions (a,b refer to YS-HM and LS-HM;c,d refer to Fe 3+treated YS-HM and LS-HM;e,f refer to Ca 2+treated YS-HM and LS-HM)

Fig.6 Content of inorganic ions in different aging samples tested by EDS
At the same time,the pore distribution was determined before and after inorganic precipitation treatment of HM.The results showed that HM had higher porosity.After inorganic precipitation treatment,soil porosity decreased significantly (Fig.7),and YS-HM had significantly higher pore number and size.The possible reason is that vegetation coverage is significantly higher in YS in karst area than in LS,and the former has significantly higher proportion of plant roots in soil (Liu et al.2021).Meanwhile,due to less vegetation in LS,insufficient amount of organic matter was secreted by plant roots,and the microbial content was lower than that in YS.Decreased soil microbial activity also affects the pore content and size.

Fig.7 Pore distribution of HM and treated HM (a refers to YS-HM with preloaded Ca 2+and Fe 3+;b refers to LS-HM with preloaded Ca 2+and Fe 3+)
3.2 Nonlinear adsorption isotherms and adsorption properties
The experimental results showed that all the data regarding PAHs adsorption by adsorbent samples could be well fitted by the Freundlich adsorption model.
Where,qewas the pollutant concentration in the solid phase (μg/kg);Cewas the liquid phase concentration (μg/L) at the equilibrium adsorption of pollutants;Kfwas the adsorption coefficient ((μg/kg)/(μg/ L)n);nwas the adsorption linearity index.
All adsorption data were processed by origin 2021.Kf,nandR2and the number of observations were listed in Table 3.In order to eliminate the difference in adsorption performance due to the different organic carbon contents in different adsorbent-adsorbate systems,the single-point organic carbon normalization coefficientKoc=[(qe/Ce)/foc] at Ce=0.005,0.05 and 0.5Sw(qeandCewere the solid and liquid phase concentrations at the adsorption equilibrium of pollutants,respectively) (Hawthorne et al.2006).focwas the organic carbon percentage of the adsorbent;Sw(the water solubility of the phenanthrene) was introduced to calculate the adsorption performance of the adsorbent.Koccan directly compare the adsorption capacity among different adsorbents,which can directly reveal the adsorption capacity of the adsorbent under determinednvalue andCe.The adsorption isotherms data of all adsorbents were shown in Fig.8.
Adsorption behavior was an important factor to affect the bioavailability and attribution of organic pollutants in soil.The adsorption isotherms of different types of soil HM and treated HM by different treated methods of phenanthrene were shown in Fig.8.It showed that the adsorption curves of different HMs with respect to phenanthrene basically present the same upward trend with the increase of the liquid phenanthrene concentration.The adsorption of phenanthrene by the original HM and the inorganic ion-treated HM well fitted the Freundlich model which described well the behavior of PAHs adsorption by HM (Sharma et al.2020),so the adsorption of phenanthrene by HM was dominated by molecular layer adsorption.Analysis from the adsorbent properties,YS-HM has higher polarity than LS-HM could be effect phenanthrene adsorption behavior.Some studies have pointed out that the polarity and aromatic carbon content of soil components are closely correlated with the adsorption capacity and nonlinearity,while the polarity is inversely proportional to nonlinearity indexn(Wen et al.2007),which is consistent with our experimental result.The data in Table 3 showed that the adsorption isotherms of 16 adsorbents with respect to phenanthrene were all nonlinear,withnvalues ranging from 0.60 to 0.78.Thenvalue of the original YS-HM was 0.66,and thenvalue of the original LS-HM was 0.64.Comparison with theKfandKocvaluesof soil and its HM fraction showed that the later had much higher adsorption capacity than original soil,which indicated that HM fraction had a significantly high adsorption affinity for PAHs.HM fraction was a hard carbon organic matter in soil,its adsorption of PAHs was mainly nonlinear,and there was an obvious desorption hysteresis effect (Yang et al.2010).The results showed that YS-HM had lower adsorption capacity than LS-HM,which may be because LS-HM had higher TOC content than YS-HM.The HB/HI value in Table 1 showed that LS-HM had higher degree of hydrophobicity than YS-HM,which can more easily adsorb PAHs from the liquid phase.LS-HM has higher C/H ratio and lower polarity index [(N+O)/C] than YS-HM.Previous studies have shown that high C/H and low N+O/C suggest more aromatic structure which may increase the adsorption capacity of PAHs (Savarese et al.2021).From the value ofn,it can be seen that HM fractions exhibited obvious nonlinearity.Some studies from a microscopic point of view have pointed out that,the adsorption of organic pollutants by organic matter is mainly dominated by the dual mode model (DMM) (Xing &Pignatello 1997) and the dual reactivity model (DRM) (Weber et al.1992).By referring to the relevant mechanism of high molecular polymers,the study pointed out that soil organic matter had two phases with different physicochemical properties,one of which was a “soft carbon " (rubber state or amorphous carbon) with a relatively loose structure and high degree of spatial freedom.While the other phase was opposite,namely a “hard carbon " (glassy state or condensed state) with less spatial freedom and many micropores.The adsorption of organic pollutants

Fig.8 Adsorption isotherm curves for soil,HM,and treatment sample.Fe 3+treated YS-HM (a);Ca 2+treated of YS-HM (b);Fe 3+treated of LS-HM (c);Ca 2+treated of LS-HM (d).
on soft carbon involves a linear partition and dissolution mechanism,while the adsorption on hard carbon was a result of combined effect of dissolution process and pore filling.From SEM and pore distributions analysis,it can be considered that the adsorption of organic pollutants phenanthrene by HM was mainly the result of the combined action of pore filling effect of hard carbon,condensed carbon and surface distribution mechanism.It has been reported that when carbonaceous adsorbent adsorbs tetrabrominated diphenyl ether,at low concentration (Ce=0.005Sw),it involved the pore filling mechanism dominated by surface micropores.When the concentration increases,the adsorption is closely related to surface functional groups of the carbonaceous adsorbent (Xin et al.2013).The results of this study also showed that for a single adsorbent-adsorbate system,when the HM fraction samples were at a low equilibrium concentration,theKocvalue was higher due to the adsorption of many micropores.As the equilibrium concentration increased,adsorption of the surface functional groups played a dominating role,resulting in decreased adsorption capacity,which was also consistent with the determination results of HM pores.
3.3 Effect of inorganic ions on the adsorption of organic pollutants by HM components
From the adsorption data of the treated HM samples with respect to the pollutant phenanthrene,it can be seen that YS-HM and LS-HM exhibit greater nonlinearity than the other inorganic ion treated samples.In Table 3,it showedthat in the YS-HM samples,with the concentrations increase of Ca2+and Fe3+,theKfvalue decreased,and thenvalue increased,and theKfvalue of YS-HM-1 to YS-HM-6 was in the range of 5631.20-2245.68.TheKfof YS-HM treated by Ca2+decreased by 52.48%~76.60%,with an average value of 62.93%,and theKfof YS-HM treated by Fe3+decreased by 41.32%~73.18%,with an average value of 59.49%.The n value was between 0.61 and 0.73.After treatment with 40 mmol/L Ca2+and Fe3+,YS-HM samples exhibited a larger n value,indicating that most of the region affecting the nonlinear adsorption of YS-HM may be wrapped or filled,and the adsorption of PAHs was the partition mechanism.For LS-HM treated with Ca2+,theKfdecreased by 58.02%~83.64%,with an average value of 71.26%;For LS-HM treated with Fe3+,theKfdecreased by 57.37%~83.99%,with an average value of 72.41%.For LS-HM,thenvalues of samples wrapped by Fe3+was greater and exhibited more linear adsorption characteristics,with n values ranging from 0.71 to 0.78 for LS-HM-4 to LS-HM-6;the n value of samples wrapped by Ca2+was lower than that of Fe3+samples,which also fully demonstrated that the adsorption mechanisms of LS-HM treated with Ca2+not only included a distribution mechanism,but also surface adsorption and pore filling mechanisms.Previous studies also pointed out that the filling of LS pores in karst areas may affect the desorption behavior and bioavailability of PAHs (An et al.2021).In order to investigate the influence of different samples,the calculation data of theKocwas studied,which obviously shows that: (1) For each adsorbent-adsorbate system,theKocvalue decreased with the increase of theCevalue;(2) For both soils,at lowerCevalue,the HM samples of the original soil exhibited greater adsorption performance than the other samples;(3) At a givenCe,Kocvalues of the 12 treated samples all decreased with the increase of inorganic ion concentration,which showed that adsorption capacity of the treated samples was lower than the original HM samples.At the concentrationCe=0.005Sw,the two original HM had the highestKocvalues,YS-HM (3.89×103) and LS-HM (18.66×103),and the mean value of LogKocwas 3.93.The mean value of LogKocwas higher than the LogKoc(3.78) calculated by the previous empirical formula logKoc=0.72×log Kow+0.49 (Schwarzenbach &Westall 1981),which may be because the concentration in the experiment was higher than the actual concentration,resulting in a higherKocvalue.
Figure 6 also showed that the Ca2+-coated treated samples had greater nonlinear adsorption and lower adsorption capacity than the original HM samples.It may be because high-energy adsorption sites for PAHs increase after sample processing (An et al.2021),resulting in greater non-linear adsorption capacity.At the same time,because part of the surface area and pores of the HM sample were wrapped and filled by inorganic precipitates,respectively,and besides the specific surface area of the adsorbed PAHs decreased,thus a lower adsorption capacity (Fig.9).Xiao et al.(2004) indicated that organic matter associated with soil sediment aggregates exhibited lower adsorption capacity,while the extracted organic matter had high adsorption capacity.According to them,this may be because the particulate organic matter was as an aggregate in soil or sediment.The coverage and trapping of inorganic and organic matter may significantly reduce the exposure of the inner and outer surface areas of organic matter to the aqueous phase,so a relatively low adsorption capacity is exhibited compared to original HM.Soil and sediment form a dynamic environmental system,which would change the adsorbent structure under different physicochemical conditions (such as freezethaw cycle,dry-wet cycle,and organic matter encapsulation,etc.) (Liu &Fan 2022).At the same time,inorganic ions undergo repeated dissolution and precipitation,thus covering the surface of soil organic matter (An et al.2022),and ultimately affecting the migration behavior of PAHs in soil media.Therefore,the action of inorganic ion precipitation studying was helpful for the migration of PAHs in karst soil and its bioavailability increase.

Fig.9 Mechanism of interaction relation between soil and Ca 2+(Fe 3+was similar to Ca 2+)
4 Conclusion
The HM fraction of the two types of karst soil was signifi-cantly stronger adsorption capacity than the original soil samples.After the addition of inorganic ions of Ca2+and Fe3+,the precipitation and colloid formed by the change of environmental conditions would significantly affect the adsorption capacity of HM.After the adding of Ca2+and Fe3+,theKfvalue of YS-HM decreased from 9597.21 to 2574.29;and theKfvalue of LS-HM decreased from 52599.13 to 8416.87.The precipitation of Ca2+and Fe3+reduced the adsorption capacity of YS-HM and the LS-HM by 61.71% and 71.83% respectively on average.It may be because the inorganic ion precipitation covered the inner and outer surface areas and filled the micropores of the HM sample.Meanwhile it may also be caused by inorganic precipitation which occupied high-energy sites of adsorbing organic pollutants.Therefore,when discussion the migration of PAHs in the complex karst environment,the structure and characteristics of the adsorbent and adsorbates should be both considered,meanwhile,the changes of the adsorbent-adsorbate system in the natural environment should be concerned with.Otherwise,the bioavailability of PAHs in karst soils could be change significantly.
AcknowledgementsThe authors thank two anonymous reviews for their revised comments on improving the quality of the article.This work was supported the National Natural Science Foundations of China under Grant (No.41761091);The first-class discipline group of geography of Guizhou Province under Grant (No.[2019]125);Youth Science and Technology Talents Growth Fund of Education Department of Guizhou Province,China (No.KY[2022] 001);Scientific Research Funds of Guiyang University,China (No.GYUKY-[2022]);The Joint Foundation of Guizhou Province under Grant (LH[2017]7348);The Doctor Foundation of Guizhou Normal University] under Grant (GZNUD [2017]10).The authors thank the support of Open Project Fund of school of Mountain Research of Guizhou Education University.
Authors’ contributionsAll authors contributed to the study conception and design.Material preparation,data collection and analysis were performed by [Xianjin An,Wei Li,Xinyue Di],[Xianjin An,Wei Li] and [Xianjin An,Wei Li,Xinyue Di],respectively.The first draft of the manuscript was written by [Xianjin An,Wei Li,Jiacheng Lan],and all authors commented on previous versions of the manuscript.All authors read and approved the final manuscript.
FundingThe national natural science Foundations of China under Grant (41761091);The first-class discipline group of geography of Guizhou Province under Grant (N0.[2019]125);Youth Science and Technology Talents Growth Fund of Education Department of Guizhou Province,China (No.KY[2022] 001);Scientific Research Funds of Guiyang University,China (No.GYU-KY-[2022]);The Joint Foundation of Guizhou Province under Grant (LH [2017]7348);The Doctor Foundation of Guizhou Normal University] under Grant (GZNUD [2017]10).
Data AvailabilityThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Competing interestsThe authors declare that they have no competing interests.
杂志排行
Acta Geochimica的其它文章
- The discovery of TiO2-II,the α-PbO2-structured high-pressure polymorph of rutile,in the Suizhou L6 chondrite
- Geochemical studies of hybrid granite from Madugulapalli area,Eastern Dharwar Craton,Southern India: Implications for crustal mixing
- How to estimate isotope fractionations of a Rayleigh-like but diffusion-limited disequilibrium process?
- Source rock potential assessment of the Huai Hin Lat Formation,Sap Phlu Basin,Nakhon Ratchasima Province,northeastern Thailand
- Distribution and geochemical significance of trace elements in kerogens from Ediacaran-Lower Cambrian strata in South China
- Fate and toxicity of nanoparticles in aquatic systems
