一例具有可逆热诱导电荷转移行为的二维氰基桥联WⅤ⁃CoⅡ配合物
2023-02-27孟银杉
刘 丹 赵 亮 邵 震 孟银杉 刘 涛
(大连理工大学,精细化工国家重点实验室,大连 116024)
0 Introduction
Magnetic molecular switchable materials exhibit‑ing bistable chemical and physical properties associat‑ed with the electron movement and charge redistribu‑tion under external stimuli such as temperature[1‑2],light[3‑4],electric field[5],pressure[6],or guest mole‑cules[7],are attracting considerable interest for both fun‑damental interests and potential applications such as switching,display,sensors,information storage devic‑es[8‑14].Typical magnetic molecular switchable materi‑als have been demonstrated concerning variations in electron configuration,such as spin crossover(SCO)of 3d4‑3d7ions,valence tautomerism in a pair of metal ions,and ring‑opening/closing and cis‑trans isomeriza‑tion[15‑20]. Among them, ETCST (electron‑transfer‑coupled spin transition)compounds have emerged as the topic issue in this field because of their excellent adjustable bistable characters deriving from the inter‑converted metal‑to‑metal charge transfer(MMCT)between two energetically adjacent metal sites[21‑23].In the recent decade,the MMCT process has been utilized as the switchable unit to manipulate magnetic,electric,thermal expansion,and photochromic properties[24‑25].The most famous MMCT system is the cyano‑bridged bimetallic Fe‑Co Prussian blue analogues(PBAs)that were first observed by Hashimoto et al.and widely used to construct molecular‑based materials with a syn‑ergistic response to multiple functions[26‑31].Octacyano‑metalate‑based compounds have also been certified to be suitable systems to expand the family of ETCST materials,such as in CuⅡ‑MoⅣ,WⅤ‑CoⅡ,and WⅤ‑FeⅡcompounds involving the valence‑state conversion WⅣ/Ⅴand MoⅣ/Ⅴ[5,32‑37].
Moreover,octacyanometallate ions possessing more diffuse 4d/5d orbitals and large spin‑orbit cou‑pling constants can produce stronger magnetic interac‑tion with another adjacent metal ion,which will give benefit in constructing multi‑responsive molecular magnets with higher Curie temperature[3,38].In particu‑lar,the W ‑Co charge‑transfer compounds display appealing features in their photo‑induced magnetic states,such as huge magnetic hysteresis and site‑selective switching[39‑42].However,it is still a big chal‑lenge to construct W‑Co charge‑transfer compounds.Until now,only a few samples have been reported,and most of them fail to obtain well‑defined structures at different temperatures to confirm the occurrence of the charge‑transfer process.First,it is because[W(CN)8]3−unit has a more flexible way to connect with CoⅡwith its eight cyano groups.As a result,it is not easy to con‑trol the coordination sphere of CoⅡcenters and the dimensions of the overall structure.Second,the occur‑rence of charge transfer requires the constituent CoⅡcenter located at a suitable coordination environment to provide equivalent redox potential with adjacent WⅤions.Therefore,rational selection of the auxiliary ligands is important.Third,the thermal hysteresis of the charge‑transfer materials,which is crucial for practical application,needs suitable intermolecular interactions.With these concerns,we aim to assemble[W(CN)8]3−with CoⅡto obtain a novel WⅤ‑CoⅡcharge‑transfer compound,in which the auxiliary ligands 4‑(2‑naphthalene‑1‑yl)vinyl pyridine(4‑nvp)is selected to adjust the coordination sphere of the CoⅡcenter and provide intermolecular π ‑π interaction.Herein,a 2D reticular cyano‑bridge compound{[WⅤ(CN)8]2[CoⅡ(4‑nvp)4]3}·4CH3OH(1)is reported,which underwent incomplete MMCT in a temperature range of 90‑180 K with a 27 K‑width thermal hysteresis.
1 Experimental
1.1 Materials
All chemicals were purchased from commercial suppliers and used without further purification.The building blocks(Bu4N)3[W(CN)8]·2H2O(Bu4N=tetrabu‑tylammonium)were synthesized according to the litera‑ture.
1.2 Synthesis of 4⁃nvp
In a 250 mL three‑necked flask,reaction mixtures of 1‑bromonaphthalene(2.25 g,10.937 mmol),4‑vinyl pyridine(1.2 mL,11.288 mmol),tri(o‑tolyl)phosphine(0.6 g,1.970 mmol),Pd(OAc)2(21 mg,0.093 5 mmol),and triethylamine(60 mL)in dry DMF(30 mL)were thoroughly mixed under argon.The reaction mixture was then degassed by free‑pump‑thaw five times before heating at 85℃for 12 h.After this time,the reaction mixture cooled to room temperature,and the triethyl‑amine was removed by rotary evaporation,water(50 mL)was added.The mixture was extracted with CH2Cl2(3×50 mL).Then the organic phase was extracted with H2O(3×50 mL)and saturated NaHCO3(3×50 mL).The solution was dried over anhydrous MgSO4and concen‑trated under a vacuum.The crude product was purified by column chromatography(silica gel,ethyl acetate/petroleum ether,2∶1,V/V)and a yellow product(2.115 g,69%)was got.
1.3 Synthesis of compound 1
Compound 1 was synthesized by diffusion method in a test tube.The aqueous solution(1.0 mL)of Co(ClO4)2·6H2O(1.83 mg,0.005 0 mmol)was slowly add‑ed dropwise to the bottom of the test tube.Then a mix‑ture of methanol/water(1∶1,V/V,3 mL)was layered as the middle buffer.Finally,1.0 mL methanol solution of(Bu4N)3[W(CN)8](0.010 mmol)and 4‑nvp(0.010 mmol)was carefully added as the third layer.After a few weeks,black red crystals were collected(Yield:1.23 mg,19% based on Co(ClO4)2·6H2O).Anal.Calcd.for C224H172Co3W2N28O4(%):C 69.73,H 4.46,N 10.17;Found(%):C 69.81,H 4.45,N 10.20.
1.4 Physical measurement
The single‑crystal X‑ray diffraction data for 1 were collected on a Bruker D8 Venture CMOS‑based diffractometer(Mo Kα radiation,λ=0.071 073 nm)using the SMART and SAINT programs.Final unit cell parameters were based on all observed reflections from the integration of all frame data.The structures were solved with the ShelXT structure solution program using Intrinsic Phasing and refined with the ShelXL refinement package using Least Squares minimization that was implanted in Olex2.The powder X‑ray diffrac‑tion(PXRD)patterns were collected on a Rigaku Smartlab 9 kW X‑ray diffractometer(Cu Kα radiation,λ=0.154 178 nm,U=45 kV,I=200 mA)in a range of 5°‑50°at a rate of 5(°)·min−1.Variable‑temperature infrared spectra were measured on KBr pellet samples using a Nicolet iS10 FT‑IR spectrometer equipped with a Bruker cryostat(Optistat CF2).UV‑Vis absorption spectra were recorded on a HITACHI UH‑4150 UV‑Vis spectrophotometer.Magnetic measurement of the sample was performed on a PPMS magnetometer.Data were corrected for the diamagnetic contribution calcu‑lated from Pascal constants.The sample(12.85 mg)was measured under a DC field of 1 000 Oe.The variable‑temperature magnetization data were collected within a temperature range of 2‑300 K at a rate of 2 K·min−1.The elemental analysis was performed by Ele‑mentar Vario EL Ⅲ(Germany).Thermogravimetric analysis was performed under an N2atmosphere at 10 K·min−1using a TG/DTA STD ‑Q600 system(TA Instruments,the United States).
2 Results and discussion
2.1 Crystal structure
Compound 1 was synthesized by the diffusion method through the reaction of Co(ClO4)2·6H2O,4‑nvp,and(Bu4N)3[W(CN)8]·2H2O in a methanol/water mix‑ture,and the crystals were obtained after a few weeks.Single‑crystal X ‑ray diffraction analysis at 120 K revealed that 1 crystallizes in a monoclinic space group P21/n(Table S1,Supporting information).The unit cell consists of two[W(CN)8]3−units and three[Co(4‑nvp)4]2+units,forming a wavy‑like layer connected by CN−bond along the a‑axis and c‑axis(Fig.S1‑S3).The layer is constituted with alternately WⅤand CoⅡions through cyano groups,presenting a hexagonal grid structure arranged in order(Fig.1).In the layers,each[W(CN)8]3−unit bridges three CoⅡions through three of its eight CN−groups,and each CoⅡion is coordinated to two nitrogen atoms from the cyanide ligand in the apical positions and four nitrogen atoms from the 4‑nvp ligand in the equatorial positions.The coordination geometry of the W and CoⅡsite were square antiprism(D4d)and octahedron(Oh),respectively.Two free water molecules were located around[W(CN)8]3−,forming hydrogen bonds with the uncoordinated CN−ligands as hydrogen bond lengths of 0.192 1 and 0.201 6 nm(H…O),respectively(Fig.S4).The nearest metal‑metal dis‑tance between the two layers is 1.763 9 nm.
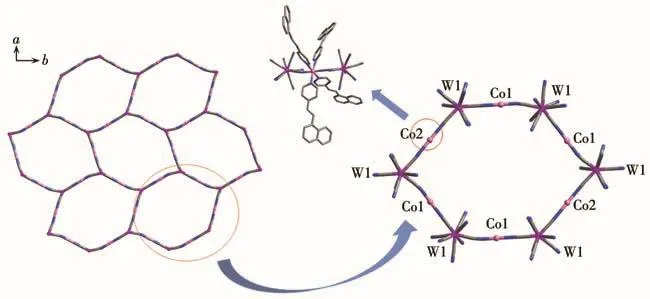
Fig.1 Arrangement of 1 in the ab plane(left)and detailed structural linkage(right)
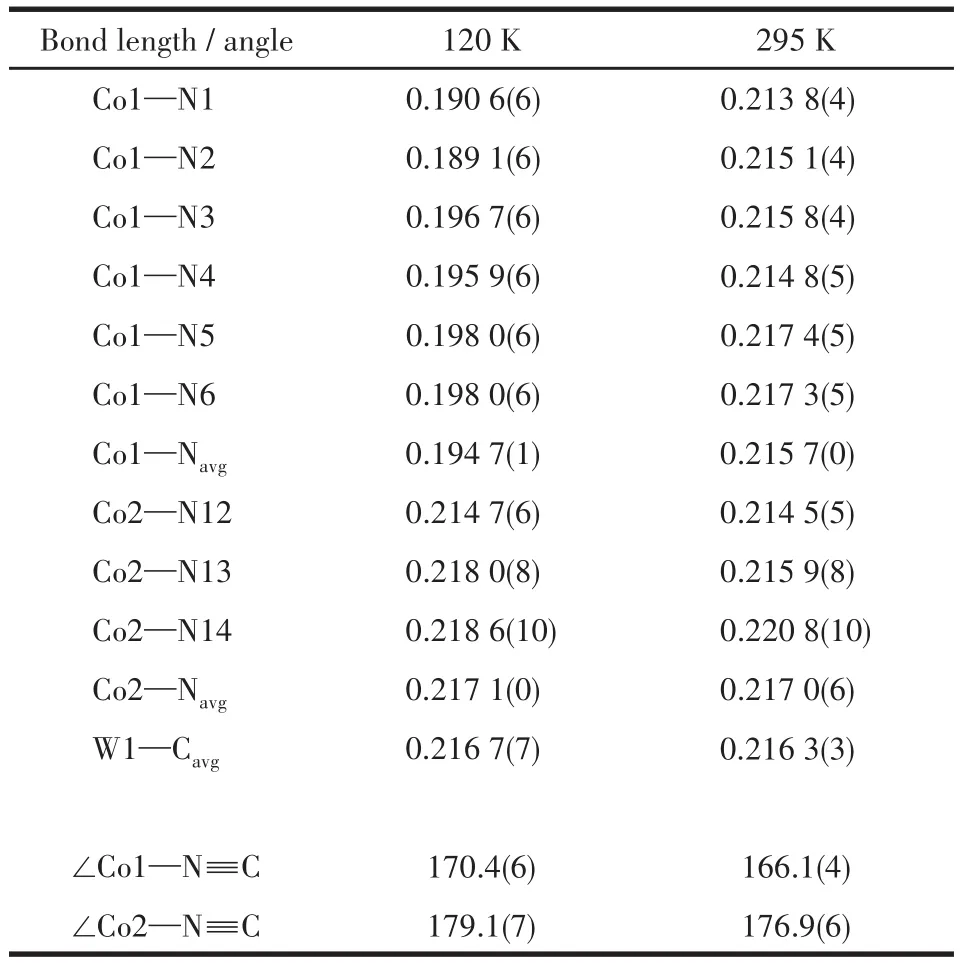
Table 1 Selected bond lengths(nm)and bond angles(°)of 1 at 120 and 295 K,respectively
At 295 K,the Co—Ncyanideand Co—N4‑nvpbond distances are 0.213 8(4)‑0.215 1(4)and 0.214 8(5)‑0.217 4(5)nm,respectively,which are characteristic of the high spin(HS)CoⅡions.The angles of Co—N≡C are 166.1(4)°‑176.9(6)°,departing from linearity slightly.The average distances of the W—C bond are 0.216 3(3)nm,and the angles of W—N≡C are close to 180°.Three independent Co—W—Co angles are 137.0(2)°,136.7(3)°,and 81.5(3)°,respectively.When the temperature declined to 120 K,Co1—Ncyanideand Co1—N4‑nvpbond distances are shortened to 0.189 1(6)‑0.190 6(6)and 0.195 9(6)‑0.198 0(6)nm,respectively,whereas Co2—Ncyanideand Co2—N4‑nvpbond lengths remain unchanged,which indicate Co1ⅡHSions change into Co1ⅢLS(LS=low spin).Moreover,Co1—N≡C and Co2—N≡C angles decrease by 4.3(2)°and 2.2(1)°to 170.4(6)°and 179.1(7)°,respectively(Table S1 ‑S4).These structural characteristics variations and charge compensation indicate the occurrence of the charge transfer between Co1Ⅱand WⅤions,and 1 underwent a metal‑to‑metal charge‑transfer from the paramagnetic WⅤ—CN—Co1ⅡHSlinkage to the diamagnetic WⅣ—CN—Co1ⅢLSlinkage.
The π‑π interaction is crucial not only in control‑ling the assembly or packing of the structure but also in manipulating the properties of the compound.The usual π‑π interaction is an offset or slipped stacking of the benzene rings or aromatic nitrogen heterocycles,and the effective distance is 0.330 0‑0.380 0 nm.In the layer of compound 1,two kinds of the π‑π interac‑tion are observed between the naphthalene ring of 4‑nvp molecules belonging to the Co1 and Co1 site,and Co1 and Co2 site,with the distance of 0.344 8 and 0.329 9 nm,respectively(Fig.S5).The distances between the ligands among adjacent layers are in a range of 0.363 8 to 0.371 8 nm(Fig.S6),which is in the normal range of the π‑π interaction.Those observed π‑π interactions directly affect the coordination mode of W—C≡N—Co.The sum of the angles of the three independent Co—W—Co angles is 355.2°,close to 360°.As a result,the plane only fluctuates slightly.For the PXRD analysis,the comparison of the powdered sample of 1 with the simulated pattern calculated by the single crystal structure proves that the single crys‑tal and powder samples had the same crystallographic structure,so we used the polycrystalline sample for the next test(Fig.S6).
2.2 Spectroscopic analysis
The solid‑state FT‑IR spectroscopy provides fur‑ther evidence for thermo‑induced charge transfer.The spectrum of 1 was recorded in a range of 80‑300 K,showing an explicit temperature dependence(Fig.2a).IR peaks caused by CN−stretching patterns were observed at 2 000 ‑2 300 cm−1.At low temperatures,four peaks due to the cyanide stretching vibrations were observed:2 166,2 160,2 140,and 2 120 cm−1,where the peaks centered at 2 120 and 2 140 cm−1can be attributed to[WⅣ(CN)8]4−unit.As the temperature gradually rose,these peaks gradually diminished and finally disappeared,thus confirming the thermally‑induced conversion of WⅣto WⅤ.Variable‑tempera‑ture solid‑state UV‑Vis‑NIR absorption spectra of 1 were also performed in the temperature interval of 80‑300 K to further investigated the charge‑transfer pro‑cess.As the temperature decreased,the broad absorp‑tion bands in the region of 800‑1 000 nm for the char‑acteristic band of the WⅣ→CoⅢMMCT gradually increased,which demonstrates the occurrence of the charge transfer from WⅤions to CoⅡions(Fig.2b and S7).
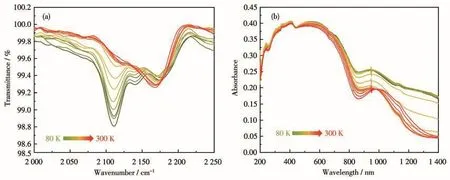
Fig.2 (a)Variable‑temperature solid‑state FT‑IR spectra for 1;(b)Variable‑temperature solid‑state UV‑Vis‑NIR absorption spectra for 1
2.3 Magnetic property
The temperature‑dependent magnetic susceptibili‑ty measurement of 1 was measured from 2 to 300 K under the direct current(DC)field of 1 000 Oe(Fig.3).At 300 K,the χMT per[Co3W2]unit was 10.03 cm3·mol−1·K (χMis the molar magnetic susceptibility),which was higher than the expected spin‑only value for two isolated WⅤ(S=1/2,g=2.04)and three CoⅡ(S=3/2,g=2.04)due to the orbital contributions of CoⅡions.Upon cooling,the χMT remained nearly constant until 180 K and then gradually decreased to 8.16 cm3·mol−1·K at 90 K with T1/2↓=127 K.When heating the sam‑ple,the χMT could return to the initial value with T1/2↑=154 K,which represents a reversible,thermally induced charge‑transfer process with a 27 K‑wide ther‑mal hysteresis.The transfer ratio was about 26.4%,which is probably attributed to the limited deformation of the coordination configuration of Co ions caused by the weak intra‑and intermolecular π‑π interactions of 1.Further cooling,the χMT gradually increased and got a sharp maximum of 161.35 cm3·mol−1·K at 9 K,which confirms the strong ferromagnetic interaction between WⅤand CoⅡcenter.Subsequently,the χMT dropped sharply to 58.25 cm3·mol−1·K at 3.7 K due to zero‑field splitting and/or antiferromagnetic interactions.This magnetic behavior determines a reversible charge‑transfer process that involves conversion between the high‑temperature(HT)phase with WⅤ(S=1/2)‑CoⅡHS(S=3/2)linkage and the low‑temperature(LT)phase with diamagnetic WⅣ(S=0)‑CoⅢLS(S=0)linkage.Tak‑ing into account the change in the χMT values,only one pair of WⅤ‑CoⅡHSlinkage converted to WⅣ‑CoⅢLS,in accordance with the result of the crystallographic anal‑ysis.Therefore,the transformation could be expressed as{[WⅤ(CN)8]2[CoⅡHS(4‑nvp)4]3}·4CH3OH→{[WⅤ(CN)8][WIⅤ(CN)8][CoⅡHS(4‑nvp)4]2[CoⅢLS(4‑nvp)4]}·4CH3OH.
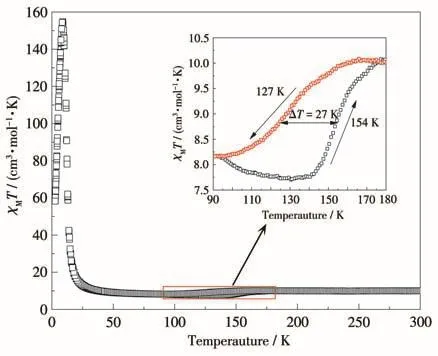
Fig.3 Temperature‑dependent magnetic susceptibilities of 1 in a temperature range of 2‑300 K under an applied field of 1 000 Oe
The field‑dependent magnetization of 1 was mea‑sured up to 50 kOe at 2 K(Fig.S8).The magnetization in the low field region increased rapidly to 4Nβ at 150 Oe,then gradually increased to 6.6Nβ at 50 kOe with‑out saturation.To further investigate the change of sus‑ceptibility with the applied magnetic field,a complete hysteresis loop was recorded at 2 K,in which a subtle hysteresis was observed with a remnant magnetization(Mr)of 4.15Nβ and a coercive field(Hc)of 250 Oe.Zero‑field‑cooled(ZFC)and field‑cooled(FC)magnetization of 1 were measured at 10 Oe in a temperature range of 2‑20 K to investigate the phase transformation at low temperatures(Fig.S9).The ZFC and FC curves were irreversible at 9 K,which indicates the presence of spontaneous magnetization of 1 below 9 K.According to all the magnetic analysis mentioned above,we can conclude that only one WⅤ‑CoⅡHSlinkage in the[Co3W2]unit undergo charge‑transfer to convert to WⅣ‑CoⅢLSand the remanent WⅤ‑CoⅡHSlinkage exhibit strong ferromagnetic coupling.However,no obvious photo‑respond magnetic behavior of 1 has been observed at low temperatures because less distortion of the inner coordination sphere of the CoⅢLScenter results in the fast relaxation speed from the photo‑induced excited state to the ground state.
To date,the report of WⅤ‑CoⅡcharge‑transfer compounds is very limited.Here,we summarize four cases of related WⅤ‑CoⅡcharge‑transfer compounds in the literature to discover some instructive laws for fur‑ther investigation.As shown in Table 2,firstly,both 2D and 3D compounds exhibited obvious thermal hystere‑sis,whereas zero‑dimensional(0D)clusters did not.Moreover,the width of hysteresis in 3D compounds was far broad than that of 2D ones.First,in general,ther‑mal hysteresis is attributed to intermolecular interac‑tions(interactions at transition sites)such as hydrogen bonding interaction and π‑π interaction.Therefore,to construct bistable molecules with hysteresis,the inter‑molecular interaction sites must be appropriately increased.Second,the cyanide‑bridged WⅤ‑CoⅡcom‑pounds with 2D and 3D configurations show large mag‑netic hysteresis than 0D ones,attributed to strong ferro‑magnetic interaction and axis anisotropy of the com‑pound.In a multidimensional structure,numerous metal sites could support stronger interactions and sys‑tematic axial anisotropy,resulting in magnetic hystere‑sis.Third,according to the∑Coand CShMCobefore and after spin transition in the well‑defined structure,we inferred that the ideal octahedral coordination sphere of the CoⅡcan stabilize the CoⅢLSstate in the WⅤ‑CoⅡcharge‑transfer system.In summary,to constitute a WⅤ‑CoⅡcharge‑transfer material with large thermal and magnetic hysteresis,the auxiliary ligands are important.According to the literature,the smallest pyr‑idine‑based auxiliary ligands with π‑π interaction site are preferred not only for their ability to release the spatial resistance of the CoⅡcenter to achieve the ideal octahedral coordination sphere of the CoⅡbut also to increase the magnetic dimension and intermolecular in‑teraction of the compound.
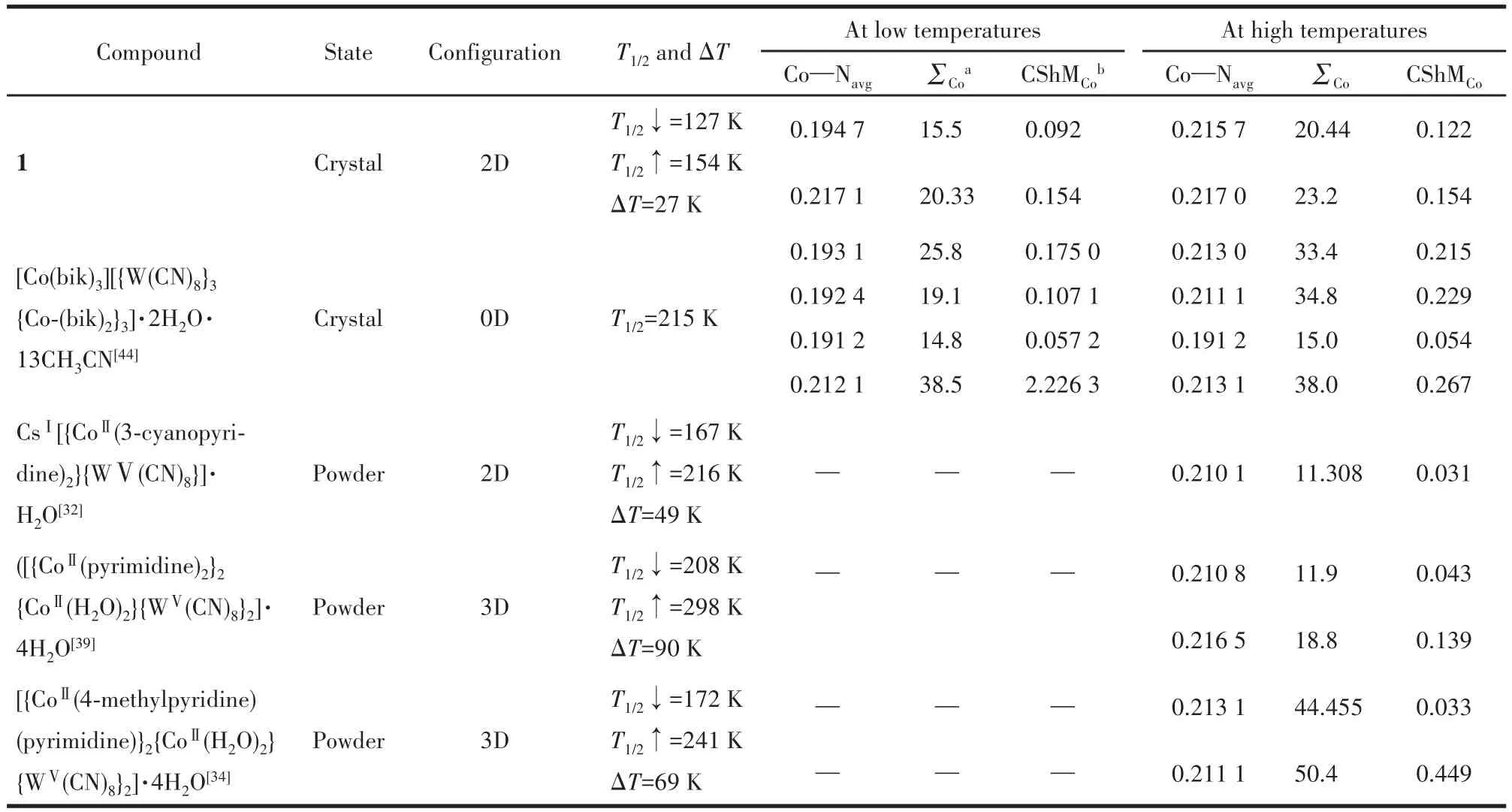
Table 2 W⁃Co metal⁃metal charge transfer compounds and related structure parameters
3 Conclusions
We synthesized a cyano‑bridged 2D layer WⅤ‑CoⅡcompound{[WⅤ(CN)8]2[CoⅡ(4‑nvp)4]3}·4CH3OH(1)by using 4‑(2‑(naphthalene‑1‑yl)vinyl)pyridine(4‑nvp)as the auxiliary ligands.Due to the intra‑and intermolecular interaction,compound 1 shows a wave‑like configuration.Magnetic and spectroscopic studies manifest that the 2D network displays incomplete reversible charge‑transfer behaviors with a 27 K width of thermal hysteresis associated with the intermolecu‑lar π‑π interaction.In addition,we summarize and an‑alyze the published WⅤ‑CoⅡcharge‑transfer com‑pounds and gave rough guidance to construct the new charge‑transfer materials,which will help expand the number of magnetic molecular switchable materials.
Acknowledgments:This work was financially supported by the National Natural Science Foudation of China(Grants No.22103009,22025101,22222103,22173015,21871039,21801037,91961114,and 22071017).Liu Tao thanks the finan‑cial support by the Fundamental Research Funds for the Central Universities of China(Grants No.PA2021GDSK0063 and PA2020GDJQ0028).
Supporting information is available at http://www.wjhxxb.cn
