Comparison of polysaccharides from 10 species of genera Paris,Trillium,Aspidistra,and Polygonatum
2023-02-17ChengChengZhaoPingZhaoYingWangYingWangXiaoXiaoGaoXiaLiWenYuanGao
Cheng-Cheng Zhao,Ping Zhao,Ying Wang,Ying Wang,Xiao-Xiao Gao,Xia Li*,Wen-Yuan Gao*
1Tianjin Key Laboratory for Modern Drug Delivery&High-Efficiency,School of Pharmaceutical Science and Technology,Tianjin University,Tianjin 300193,China.2School of Chinese Materia Medica,Tianjin University of Traditional Chinese Medicine,Tianjin 301617,China.
Abstract Background: The genera Paris, Trillium, Aspidistra, and Polygonatum originally belonged to the family Liliaceae; however, recently, in the APG system, the genera Polygonatum and Aspidistra were placed in the family Asparagaceae and the genera Paris and Trillium were placed in the family Melanthiaceae. Methods: To study the application of polysaccharide chemotaxonomy in Asparagaceae and Melanthiaceae, we extracted polysaccharides from the rhizomes of plants, including Paris polyphylla var. yunnanensis, Paris daliensis, Paris polyphylla var. chinensis, Trillium tschonoskii, Aspidistra elatior, Aspidistra sichuanensis,Aspidistra caespitosa, Polygonatum sibiricum, Polygonatum cyrtonema, and Polygonatum kingianum. Physicochemical and structural characterization of these polysaccharides was performed using molecular weight, monosaccharide composition, Fourier‑transform infrared spectroscopy, nuclear magnetic resonance spectroscopy, and scanning electron microscopy. The immunomodulatory activities were evaluated using the macrophage cell line, RAW264.7. Results: In this study, we found that the polysaccharides from the genus Polygonatum and Aspidistra were mainly comprised of fructose and glucose; the molecular weights were mostly concentrated between 4.6 –6.2 kDa. The polysaccharides from species of genus Polygonatum differed in that they contained acetyl groups, while polysaccharides from species of genus Aspidistra did not. Paris and Trillium species polysaccharides mainly consisted of glucose and mannose; the molecular weights of the two major peaks were 6.3–13 kDa and 1 – 1.5 kDa, respectively; the main differences between polysaccharides were the ratio of glucose and mannose and the degree of branching. These results suggest that fructans might be the main feature of the family Asparagaceae and fructans with acetyl groups might be the main feature of the genus Polygonatum which is consistent with the results of previous studies. Additionally, all polysaccharides from the 10 plants promoted phagocytic activity. Some of them exhibited strong activity, including nitrogen monoxide release and TNF‑α secretion by RAW264.7 macrophages, indicating that they could be used as potential immunomodulatory agents. Conclusion: The molecular weight distribution,monosaccharide composition, and surface morphology of the polysaccharides of the Polygonatum and Aspidistra plants were similar, while those of Paris and Trillium plants were similar. These results support the APG classification system, which placed the genera Polygonatum and Aspidistra in the Asparagaceae family and placed the genera Paris and Trillium in the Melanthiaceae family.
Keywords:Paris; Trillium;Aspidistra; Polygonatum; polysaccharides
Highlights
To study the application of polysaccharide chemotaxonomy in Asparagaceae and Melanthiaceae,ten polysaccharides were extracted fromParis,Trillium,Aspidistra,andPolygonatumspecies,all of which belong to the former family Liliaceae.By comparing analysis,thePolygonatumandAspidistraplants were similar,while those ofParisandTrilliumplants were similar.These results support the APG classification system,which placed the generaPolygonatumandAspidistrain the Asparagaceae family and placed the generaParisandTrilliumin the Melanthiaceae family.
Background
As naturally active components, plant polysaccharides have beneficial activities such as antioxidant, hypoglycemic, anti‑inflammatory, and anti‑tumor properties [1–4]. Polysaccharides have proven useful for chemotaxonomic studies. Studies have shown that the structural characteristics of polysaccharides differ to a certain extent, making them a good guiding significance for chemotaxonomy; for example,there are significant differences in the structural characteristics of polysaccharides among different genera of medicinal plants [5, 6].Specifically, the chemotypes of glucans and mannose‑containing heteropolysaccharides have been used as adjuncts to the classification and identification of lichenized fungi [7–11]. The structural diversity of fructans within the order poales and asparagales has also been proposed as a useful chemotaxonomic aid [12, 13]. The presence or absence of excreted polysaccharides in the mycelia of higher fungi is considered to be a beneficial guide to taxonomic classification and habitat [14]. The polysaccharides from different species of the same genus often have similar structure and function [10, 15]. In previous studies onPolygonatummedicinal plants with polysaccharide as a feature, our research team discovered that the results were generally consistent with the results of genetic analysis based on traditional morphological classification, DNA, and small molecular chemical components [16, 17].
The generaParis,Trillium,Aspidistra, andPolygonatumoriginally belonged to the family Liliaceae [18]; however, recently, in the angiosperm phylogeny group system, the generaPolygonatumandAspidistrawere placed in the family Asparagaceae and the generaParisandTrilliumwere placed in the family Melanthiaceae [19, 20]. The rhizomes of the species within these four genera are used in traditional Chinese medicine. The rhizomes ofPolygonatum sibiricum(PSP),Polygonatum kingianum(PKP),Polygonatum cyrtonema(PCP, as known “Huangjing”) are widely used for treating osteoporosis,feebleness, diabetes, fatigue and several other disorders [21]. The rhizomes ofAspidistra(known as “Zhizhubaodan”) are used to treat cough, abscess, traumatic injury, and pain[22]. The rhizomes ofParis polyphyllavar.yunnanensis(PPYP) andParis polyphyllavar.chinensis(PPCP, as known “Chonglou”) are used for treating sore throat, boils,carbuncles, traumatic pain, and venomous snake bite [23]. The rhizomes ofTrillium tschonoskii(TTP, known as the “Yanlingcao”) are used for easing pain, activating blood, eliminating carbuncles, and expelling rheumatism [24]. Polysaccharides play an important role in determining the efficacy of these plants. Polysaccharides ofPolygonatumspecies have attracted a great deal of attention because of their excellent immunoregulatory, neuroprotective, anti‑osteoporosis,and anti‑diabetic activities [21, 25]. However, reports on the characterization and bioactivity of polysaccharides from theAspidistra,Paris, andTrilliumplants are limited.
To uncover the distribution of active polysaccharides in the plants,we compared the characterization and immunomodulatory activity of these polysaccharides in threeParisspecies (Paris polyphyllavar.yunnanensis(Franch.) Hand.‑Mazz.,Paris daliensisH. Li & V. G.Soukup, andParis polyphyllavar.chinensis(Franch.) H. Hara), threePolygonatumspecies (Polygonatum sibiricumF. Delaroche,Polygonatum kingianumCollett and Hemsl., andPolygonatum cyrtonemaHua), threeAspidistraspecies (Aspidistra elatiorBlume,Aspidistra sichuanensisK.Y.Lang & Z. Y. Zhu, andAspidistra caespitosaC. Pei), and oneTrilliumspecies (Trillium tschonoskiiMaxim.). Physicochemical and structural characterization of these polysaccharides was performed using molecular weight, monosaccharide composition, Fourier‑transform infrared (FT‑IR) spectroscopy, nuclear magnetic resonance (NMR)spectroscopy, and scanning electron microscopy (SEM). The immunomodulatory activities were evaluated using the macrophage cell line, RAW264.7.
Materials and methods
Materials and chemicals
The rhizomes of ten species were collected from China (Table 1) in autumn of 2017, and all of them were identified by Professor Wen‑Yuan Gao (School of Pharmaceutical Science and Technology,Tianjin University). Water Milli‑Q was purchased via a Millipore system (Bedford, MA, USA). D‑mannose, D‑glucose, D‑xylose,L‑arabinose, D‑galactose and L‑rhamnose were obtained from the National Institute for Food and Drug Control (Beijing, China).Dextrans of different molecular weights and D‑fructose were bought from the Sigma Chemical Company (St. Louis, MO, USA). The RPMI‑1640 medium and fetal bovine serum used in cell culture were obtained from Gibco (Fort Worth, TX, USA). Neutral red staining solution was purchased from Beijing Solarbio Science & Technology Company (Beijing, China). Tumor necrosis factors (TNF)‑α and nitrogen monoxide (NO) assay kits were purchased from Wuhan Boster Biological Technology (Wuhan, China). And all other reagents and chemicals were analytical grade.
Extraction of the polysaccharides
All the rhizomes were fully dehydrated and pulverized into powders by the disintegrator (WND‑200 High Speed Traditional Chinese Medicine Grinder, Lanxi Weinengda Electric Appliance Co., Ltd.,Lanxi,China). Then the dried powder(200 g)was defatted by sopping in 95% ethanol under stirring at room temperature for 2 days. After the filtration, residues were extracted by three times for 2 h with boiling distilled water. These supernatants were concentrated by rotary evaporator (RE‑200A Rotary Evaporator, Shanghai Yarong Biochemical Instrument Factory,Shanghai,China),and precipitated at 4 °C overnight with 5 times volume of ethanol. Then, the precipitates were collected via centrifugation (SC‑3614 Low Speed Automatic Balancing Centrifuge, Anhui Zhongke Zhongjia Scientific Instrument Co., Ltd., Hefei, China), washed with ethanol and acetone sequentially. These precipitates were redissolved in the distilled water, and then added papain with concentration of 0.5% that incubated to remove protein at 60 °C for 2 h preliminarily. The reactions were terminated in the boiling water bath and the deactivated protease was removed by centrifugation. These precipitations were further deproteinized by the Sevag reagent that n‑butanol/chloroform, v/v = 1:4, for at least 10 times. The solutions were precipitated, then these precipitates were dialyzed with deionized water for 48 h(cutoff Mw 3.5 kDa)and lyophilized(Bell Jar Vacuum Freeze Dryer, Beijing Boyekang Experimental Instrument Co.,Ltd., Beijing,China). The total carbohydrate contents were measuredby the phenol‑sulfuric acid method [26]. Protein content was measured by using the Bradford’s method [27].

Table 1 The collecting locations of the plants and the code of their polysaccharides
Characterization of the polysaccharides
Determination of molecular weight.The molecular weights of polysaccharides were analyzed by the high‑performance gel permeation chromatography technique, that were determined by the high performance liquid chromatography (HPLC) system (Ultimate 3000, Dionex, Germering, Germany) with the TSKgel GMPWXL column (7.8 mm × 300 mm, Tosoh Corp., Chonan, Japan) and evaporative light scattering detector (ELSD 2000ES, Alltech, Chicago,Illinois, USA). It was purged with deionized water at the flow rate of 0.5 mL/min and kept at 40 °C. The evaporator temperature for the evaporative light scattering detector was 110 °C with the nebulizing gas flow rate of 3.0 mL/min. Dextrans with different the molecular weights (1.0, 5.0, 12.0, 50.0, 150.0, 210.0, 410.0 kDa) were used as standard to obtain molecular weight calibration curve, that by plotting the retention time against the logarithm of the molecular weight respectively.
Monosaccharide composition analysis.The polysaccharides from the genusPolygonatumandAspidistraplants were hydrolyzed in the sealed ampules with the 0.05 mol/L sulfuric acid at 80 °C for 40 min,that neutralized by using barium carbonate. Then the solutions were filtered by centrifugation. Polysaccharides from the genusParisandTrilliumwere hydrolyzed in the sealed ampules with the 2.0 mol/L trifluoroacetic acid at 110°C for 4 h,and dried with the nitrogen flow.The acid was removed by methanol then drying for three times.Before monosaccharide composition analysis,the hydrolysates were analyzed by molecular weight method to detect these polysaccharides had been hydrolyzed completely or not.
The hydrolysates of these polysaccharides were measured by using HPLC with LC‑2030 C Prominence‑i System (Shimadzu, Kyoto, Japan)and RID‑20A refractive index detector. Waters XBridge Amide column(4.6 × 250 mm, 3.5 μm) was used with setting at 30 °C. The mobile phase was acetonitrile‑water = 75:25 with the 0.2% triethylamine,and flow rate was 0.1 mL/min. Identification of the monosaccharides in the hydrolysates of these polysaccharides was analyzed by comparing the retention times with those of standard monosaccharides.
FT-IR spectra analysis.The FT‑IR spectra of polysaccharides were purchased on FT‑IR instrument (Tensor 27, Bruker, Ettlingen,Germany)with potassium bromide pellets,the range is set from 400 to 4,000 cm‑1.
NMR analysis.The 30 mg of polysaccharides were add to 5 mm NMR tube, and dissolved in 1.0 mL of deuterium (D2O, 99.9%).1H NMR spectra was obtained at 298 K on an AVANCE III 600 MHz NMR spectrometer (Bruker Biospin, Rheinstetten, Germany), equipped with the 5 mm broadband observe probe, and it used the water suppression sequence(Bruker pulse sequence noesyprld) [28].
SEM analysis.The surface morphology of polysaccharide was observed with the Phenom G2 Pro desktop scanning electron microscope (Eindhoven, Netherlands), and the accelerating voltage was set to 5 kV.
Immunomodulatory activity analysis in the RAW264.7 cell line
The murine macrophage cell line RAW264.7 was purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai,China). Cells were cultured in the Dulbecco’s modified eagle medium with 10% fetal bovine serum, 100 mg/mL streptomycin and 100 units/mL penicillin,and cultured in the humidified incubator with 5%CO2atmosphere at 37 °C. To measure immunomodulatory activity,cells were incubated with different concentration of the samples(12.5,50, 200 μg/mL) and LPS (200 μg/mL).
Phagocytes assay.According to the previous description, the phagocytic activity of RAW264.7 cells was evaluated by the neutral red phagocytosis assay[29].
Measurement of NO.NO level in the macrophage RAW264.7 cells was evaluated according to the reported method [30].
Assays of TNF-α level.According to the manufacturer’s protocol, the concentrations of TNF‑α released from cells were conducted by using the enzyme‑linked immunosorbent assay kits.
Statistical analysis.The experiments were conducted in triplicate.The results were regarded as means ± standard deviation. The statistical significance was analyzed by using SPSS software version 20(Chicago, Illinois, USA), the one‑way analysis of variance and with Fisher’s least significant difference test.P‑value<0.05 was expressed as statistically significant.
Results and discussion
Characterization of the polysaccharides
Ten crude polysaccharides were obtained from PSP, PCP, PKP,Aspidistra elatior(AEP),Aspidistra caespitosa(ACP),Aspidistra sichuanensis(ASP), PPCP, PPYP,Paris daliensis(PDP), and TTP. The total carbohydrate contents in these polysaccharides were 73.5%,85.2%, 77.0%, 80.6%, 94.4%, 90.4%, 87.6%, 90.0%, 89.3%, and 91.8%, respectively, which was measured using the phenol‑sulfuric acid method. The Bradford assay revealed no protein in the polysaccharides.
Determination of molecular weight.High‑performance gel permeation chromatography was used to detect the molecular weights of the ten polysaccharides. As shown in Figure 1, more than 60% of the molecular weights (MWs) of PSP, PCP, PKP (Figure 1A,Polygonatum), AEP,ACP, and ASP (Figure 1B,Aspidistra) had the average MWs of 5.0, 4.9, 4.6, 4.7, 4.9, and 6.2 kDa, respectively. The molecular weight distributions of PPYP, PPCP, PDP, and TTP had a wide margin and two major peaks were observed.The average MWs of the former peak of PPYP, PPCP, PDP (Figure 1C,Paris), and TTP(Figure 1D,Trillium) were 6.3, 13.0, 6.0, and 11.0 kDa, respectively,and that of their latter peak were 1.3, 1.5, 1.2, and 1.0 kDa,respectively.
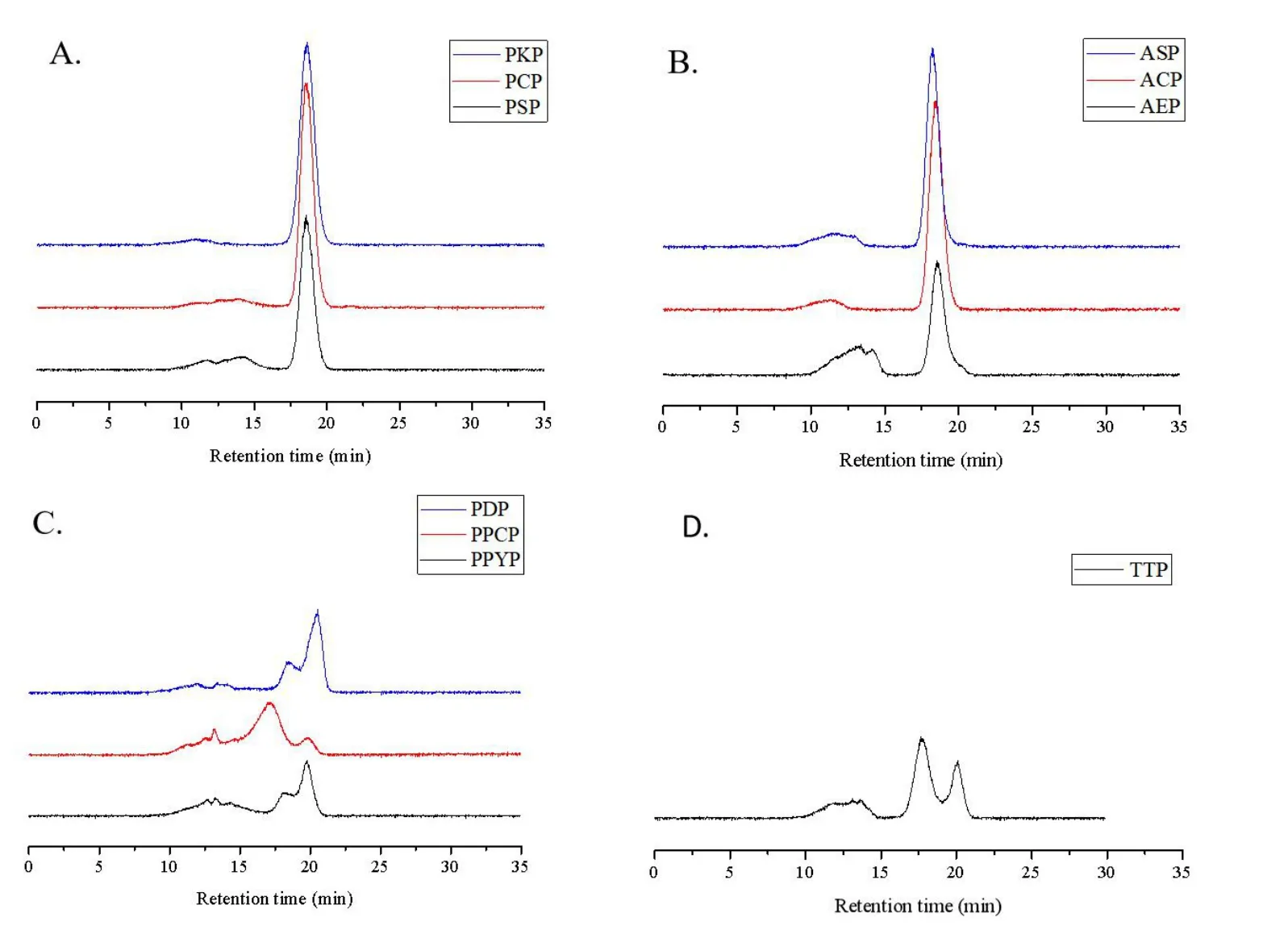
Figure 1 Molecular weight distributions of the polysaccharides from genus Polygonatum (A), Aspidistra (B), Paris (C) and Trillium (D)plants. PSP, Polygonatum sibiricum; PCP, Polygonatum cyrtonema; PKP, Polygonatum kingianum; AEP, Aspidistra elatior; ACP, Aspidistra caespitosa;ASP, Aspidistra sichuanensis; PPCP, Paris polyphylla var. chinensis; PPYP, Paris polyphylla var. yunnanensis; PDP, Paris daliensis; TTP, Trillium tschonoskii.
Monosaccharide composition analysis.As fructose is converted into mannose and glucose when hydrolyzed with strong acid, PSP, PCP,PKP, AEP, ASP, and ACP were treated with a diluted acid [31]. PPYP,PPCP, PDP, and TTP were hydrolyzed with a strong acid, and all the hydrolysates were analyzed by the molecular weight determination method before monosaccharide composition analysis to ensure complete hydrolysis of polysaccharides. Additionally, fructose could be converted into glucitol and mannitol derivatives during the derivatization process; therefore, monosaccharide composition analysis was conducted using the HPLC‑refractive index detector method,and the samples were injected directly after being hydrolyzed without derivatization. As shown in Figure 2, PSP, PCP, PKP (Figure 2A,Polygonatum), AEP,ASP, and ACP(Figure 2B,Aspidistra)consisted of fructose and glucose in molar ratios of 29:1, 32:1, 32:1, 40:1, 33:1,and 48:1, respectively, whereas PPYP, PPCP, PDP (Figure 2C,Paris),and TTP (Figure 2D,Trillium) consisted of glucose and mannose in molar ratios of 3:1, 5:1, 8:1, and 42:1, respectively.

Figure 2 HPLC-RID profiles of mixed monosaccharide standards and the polysaccharides from genus Polygonatum(A),Aspidistra(B),Paris(C) and Trillium (D) plants. HPLC‑RID, high performance liquid chromatography‑refractive index detector; PSP, Polygonatum sibiricum; PCP,Polygonatum cyrtonema; PKP, Polygonatum kingianum; AEP, Aspidistra elatior; ACP, Aspidistra caespitosa; ASP, Aspidistra sichuanensis; PPCP, Paris polyphylla var. chinensis; PPYP, Paris polyphylla var. yunnanensis;PDP, Paris daliensis; TTP, Trillium tschonoskii.
FT-IR analysis.In the FT‑IR spectra of these ten polysaccharides(Figure 3), the strong absorption peaks at 3,400 cm‑1and the weaker peaks at 2,930 cm‑1were assigned to the stretching vibrations of O‑H and C‑H, respectively. The peaks at approximately 1,730 cm‑1were assigned to the valence vibration of C=O,and the peaks at 1,634 cm‑1were assigned to the bound water [32, 33]. The FT‑IR spectra of PSP,PKP, PCP, AEP, ASP, and ACP exhibited similar characteristic absorption peaks between 1,200–400 cm‑1, and those of PPYP, PPCP,PDP, and TTP also had similar characteristic absorption peaks in this range. The strong peaks at 1,200–1,020 cm‑1represented the stretching vibration of the C‑O bond, which was split into two peaks(1,124 and 1,028 cm‑1) in the FT‑IR spectra of PSP, PKP, PCP, AEP,ASP, and ACP, indicating the presence of furanose, whereas it was split into three peaks (1151, 1083, and 1024 cm‑1) in the spectra of PPYP, PPCP, PDP, and TTP, indicating the presence of pyranose.Moreover, peaks at 933, 877, and 815 cm‑1for PSP, PCP, PKP, AEP,ASP, and ACP were markers for the presence of β‑fructofuranose,while the peaks at 852 cm‑1for PPYP, PPCP, PDP, and TTP were markers for the α‑configuration [34–36]. Moreover, the peaks at 1,375 and 1,246 cm‑1were due to the symmetric C‑H bending vibration of the methyl group and C‑O vibration of O‑acetyl group,respectively [37]. Except for AEP, ASP, and ACP, all other polysaccharides had absorption peaks at approximately 1,375 and 1,246 cm‑1, indicating the existence of O‑acetyl groups. Overall, the results showed that PSP, PCP, PKP, AEP, ASP, and ACP contained β‑fructofuranose (Fruf) units, and PPYP, PPCP, PDP, and TTP contained α‑pyranose units. Except for AEP, ASP, and ACP, the other polysaccharides contained O‑acetyl groups.
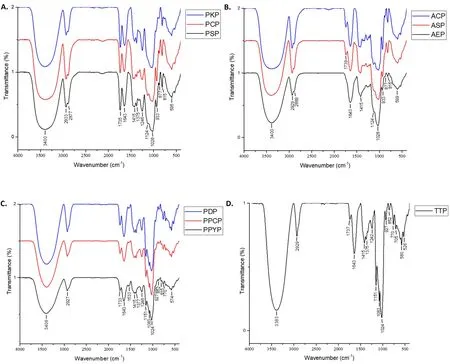
Figure 3 FT-IR spectra of the polysaccharides from genus Polygonatum (A), Aspidistra (B), Paris (C) and Trillium (D) plants. FT‑IR,Fourier‑transform infrared; PSP, Polygonatum sibiricum; PCP, Polygonatum cyrtonema; PKP, Polygonatum kingianum; AEP, Aspidistra elatior; ACP,Aspidistra caespitosa; ASP, Aspidistra sichuanensis; PPCP, Paris polyphylla var. chinensis; PPYP, Paris polyphylla var. yunnanensis; PDP, Paris daliensis;TTP, Trillium tschonoskii.
1H NMR analysis.The structural features of these ten polysaccharides were further examined by NMR spectral analysis and compared with literature data. In1H NMR spectra of PSP, PKP, PCP, AEP,ASP, and ACP (Figure 4A, Figure 4B), small signal at 5.35 ppm was assigned to H‑1 ofα‑D‑Glcp, and complex signals between 4.1 and 3.4 ppm were ascribed to the β‑D‑Fruf residues and H‑2 to H‑6 of α‑D‑Glcp[38–40].In1H NMR spectra of PPYP, PPCP, PDP, and TTP (Figure 4C, Figure 4D), high intensity signal at 5.36 ppm could be attributed to H‑1 of 1,4‑linked‑α‑D‑Glcp and 1,2‑linked‑α‑D‑mannopyranose[41–47].The small signals at 5.20 and 4.92 ppm could be attributed to H‑1 of terminal and 1,4,6‑linked‑α‑D‑Glcp, respectively [41–43].Additionally, except for AEP, ASP, and ACP, all other polysaccharides had signals at approximately 2.1 ppm, which confirmed the existence of O‑acetyl groups.
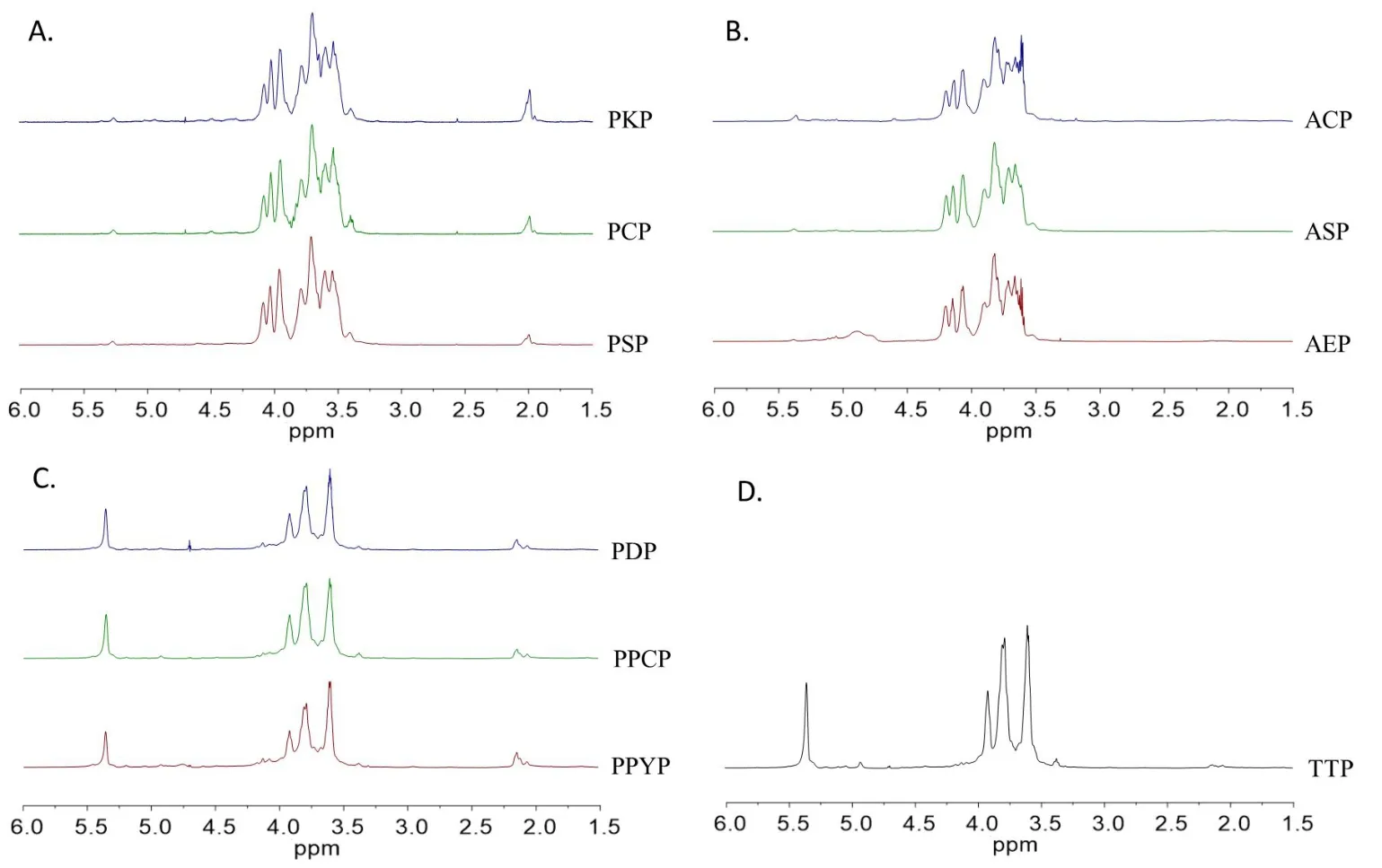
Figure 41H NMR spectra of the polysaccharides from genus Polygonatum(A),Aspidistra(B),Paris(C)and Trillium(D)plants.NMR,nuclear magnetic resonance; PSP, Polygonatum sibiricum; PCP, Polygonatum cyrtonema; PKP, Polygonatum kingianum; AEP, Aspidistra elatior; ACP, Aspidistra caespitosa; ASP, Aspidistra sichuanensis; PPCP, Paris polyphylla var. chinensis; PPYP, Paris polyphylla var. yunnanensis; PDP, Paris daliensis; TTP,Trillium tschonoskii.
According to a previous report, polysaccharides from PCP contain a(2→1)‑linked β‑D‑Fruf backbone and (2→6)‑linked β‑D‑Fruf side chains with internal α‑D‑Glcp in neokestose form [48]. These polysaccharides were similar to those fromAgave tequilana,Agave Americana,Agave durangensis,Urginea maritima,Cordyline australis,Muscari szovitsianum, andOphiopogon japonicus[39, 40, 49–53]. All these plants belonged to the Asparagaceae family, and the degree of polymerization values for these fructans ranged from 10–29. This shows that the characteristics of the polysaccharides fromPolygonatumandAspidistraplants were in accordance with those of the species from Asparagaceae. This demonstrates that this type of highly branched fructan with an internal glucose containing both β-(2→1)‑and β‑(2→6)‑linkages might be the chemotaxonomic feature of Asparagaceae. Additionally, the difference betweenPolygonatumandAspidistraspecies was that the polysaccharides fromPolygonatumspecies possessed O‑acetyl groups, whereas those fromAspidistraspecies did not. Acetyl groups were also not found in the polysaccharides of the other species of Asparagaceae. Thus, the acetyl group contained in fructans may be a chemotaxonomic characteristic of the genusPolygonatum.
Reports on polysaccharides from Melanthiaceae are very limited,and only two oligosaccharides came from the rhizomes of PPYP which are composed of glucose and mannose [54]. In this study, we found that the polysaccharides from the generaParisandTrilliumwere mainly composed of glucose and mannose, but as we experimented,we further found that their ratios and degrees of branching were different. The mannose ratio (%) was much lower inTrilliumplants(2%) than inParisplants (11%–25%). An iodine‑potassium iodide reagent was used to detect the degree of branching. The results showed that the reaction solution of PPYP and PDP appeared blue,that of PPCP was purple, and that of TTP was orange. This indicates that the degree of branching was PPYP–PDP < PPCP. Furthermore,from the 1H NMR spectra, the signals of H‑1 of branched Glcp were higher inTrilliumplants than inParisplants, suggesting that the degree of branching in the polysaccharides ofTrilliumplant was higher than that ofParisplants. From the1H NMR spectra, it was deduced that the content of O‑acetyl groups in the polysaccharides ofParisplant was higher than that inTrilliumplants.
SEM analysis.SEM measurements were used to qualitatively analyze the surface morphology of the polysaccharides. As shown in Figure 5,the surface morphologies of PSP, PCP, PKP, AEP,ASP, and ACP were quite similar, exhibiting a smooth surface and block shape, whereas those of PPYP, PPCP, PDP, and TTP were similar, exhibiting a rough surface and loose crumb structure.
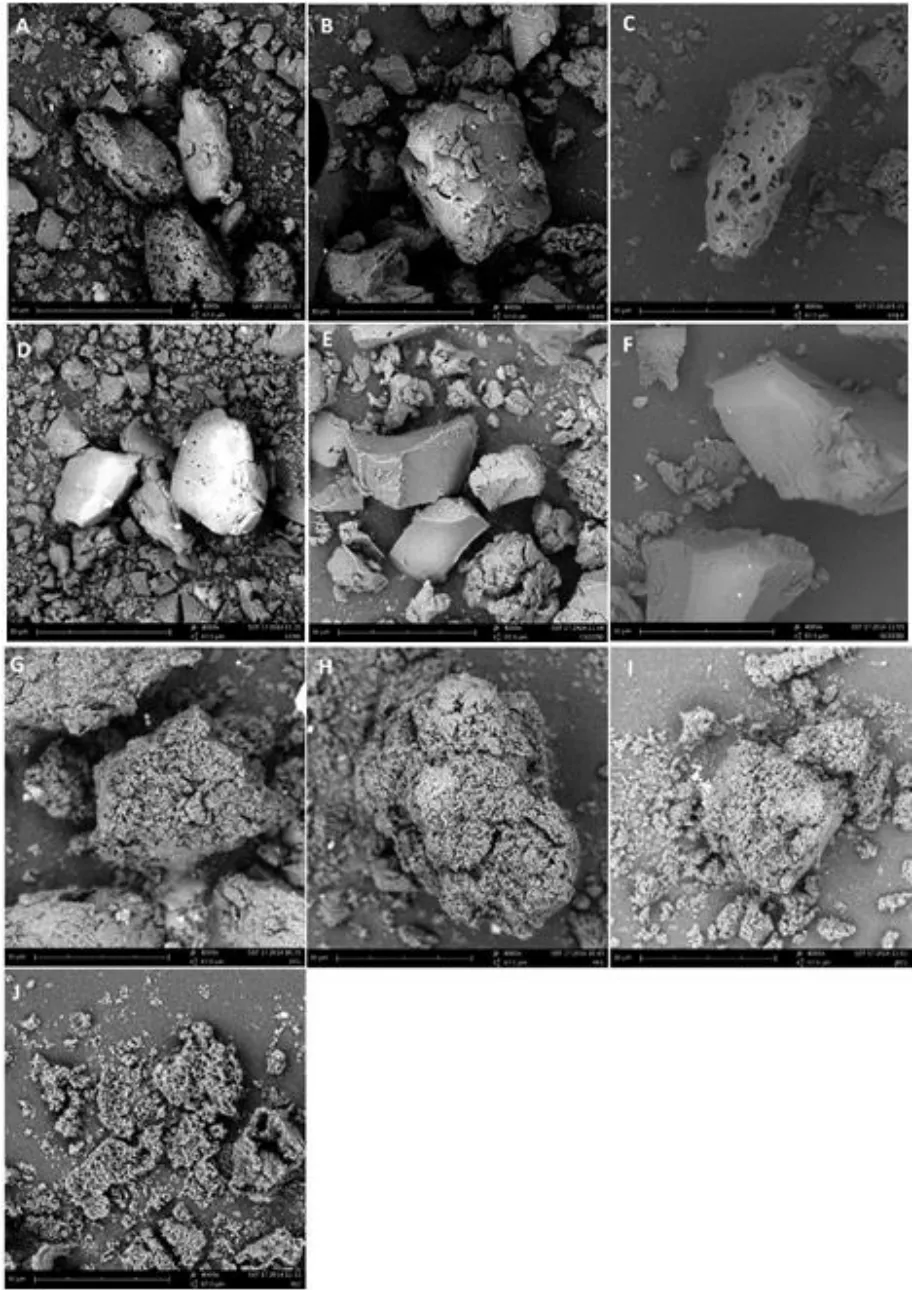
Figure 5 The SEM images of PSP(A), PCP(B), PKP(C), AEP(D),ASP(E),ACP(F),PPYP (G),PPCP(H),PDP (I) and TTP(J) at 4000×.SEM,scanning electron microscopy; PSP, Polygonatum sibiricum; PCP, Polygonatum cyrtonema; PKP, Polygonatum kingianum; AEP, Aspidistra elatior; ACP,Aspidistra caespitosa; ASP, Aspidistra sichuanensis; PPCP, Paris polyphylla var. chinensis; PPYP, Paris polyphylla var. yunnanensis; PDP, Paris daliensis;TTP, Trillium tschonoskii.
Effe cts on the immunoregulatory activity of RAW264.7 cells
Macrophages play an important role in adaptive and innate immune responses by phagocytosis or are involved in inflammatory reactions to prevent pathogen infection [55, 56]. Phagocytic activity is the first and most important defense mechanism of the immune response system and is one of the most essential parameters of macrophages[57].Cytokines,which are important for the functions of macrophages,can affect the unleashing of an effective immune response, influence the macrophage microenvironment, and link innate and adaptive immunity [58]. NO is an important signaling molecule that plays a key role in the course of infection [59]. NO and TNF‑α levels were utilized to assess immunomodulatory activity, with higher concentrations indicating better immunomodulatory activity [60]. As shown in Figure 6, all the polysaccharides significantly promoted phagocytosis and enhanced the secretion of TNF‑α of RAW264.7 macrophages at a concentration of 12.5–200 μg/mL in a dose‑dependent manner (P< 0.01). Likewise, all these polysaccharides could remarkably promote NO release from RAW264.7 macrophages, compared to untreated cells, at a concentration of 50–200 μg/mL in a dose‑dependent manner (P<0.01).

Figure 6 Effects of the polysaccharides from Polygonatum, Aspidistra, Paris and Trillium plants on the phagocytosis index (A), NO production (B) and TNF-α (C) secretion of RAW264.7 cells.*P < 0.05,**P < 0.01, compared to blank control group. TNF, tumor necrosis factors; NO, nitrogen monoxide; LPS, lipopolysaccharide; PSP, Polygonatum sibiricum; PCP, Polygonatum cyrtonema; PKP, Polygonatum kingianum;AEP, Aspidistra elatior; ACP, Aspidistra caespitosa; ASP, Aspidistra sichuanensis; PPCP, Paris polyphylla var. chinensis; PPYP, Paris polyphylla var.yunnanensis; PDP, Paris daliensis; TTP, Trillium tschonoskii.
PPYP at 200 μg/mL exhibited the strongest stimulatory effect among these ten polysaccharides on phagocytic activity, NO release,and TNF‑α secretion by RAW264.7 macrophages.Saponins from PPYP exhibits anticancer activity in rats and mice [61–63]. The immunoregulatory activities of PPYP in this study further confirmed and explained that the rhizomes of PPYP may be a potential agent for cancer therapy because of its anticancer and immunomodulatory effects.
The immunomodulatory activity is affected by polysaccharide structural characteristics, including monosaccharide composition and glycosidic bonds [64, 65]. Polysaccharides were composed of mannose and glucose,and the α‑(1→ 3)‑Man, α‑(1 → 4)‑Glc glycosidic bonds of polysaccharides were bound up with the immunomodulatory activity [64, 66, 67]. Acetyl groups have been reported to affect bioactivities by changing the solubility, conformation, and charge density of polysaccharides [68–70]. In this study, no obvious differences in immunoregulatory activity were observed betweenPolygonatumspecies (which had an acetyl group) andAspidistraspecies (which did not have an acetyl group). However, whether the acetyl group can affect other bioactivities still needs to be investigated.
Conclusion
Ten polysaccharides were extracted fromParis,Trillium,Aspidistra,andPolygonatumspecies, all of which belong to the former family Liliaceae. The molecular weight distribution, monosaccharide composition, and surface morphology of the polysaccharides of thePolygonatumandAspidistraplants were similar, while those ofParisandTrilliumplants were similar. These results support the angiosperm phylogeny group classification system, which placed the generaPolygonatumandAspidistrain the Asparagaceae family and placed the generaParisandTrilliumin the Melanthiaceae family. Fructans might be a chemotaxonomic characteristic of the Asparagaceae family,while fructans with acetyl groups might be characteristic of the genusPolygonatum. The polysaccharides from the generaParisandTrilliumwere mainly composed of glucose and mannose and contained acetyl groups. The main differences between the polysaccharides from the generaParisandTrilliumwere the ratio of glucose and mannose and the degree of branching. Due to the above structural characteristics,including monosaccharide composition and glycosidic bonds, PPYP exhibited the strongest immunoregulatory activity among the ten polysaccharides on phagocytosis activity, NO release, and TNF‑α secretion by RAW264.7 macrophages, suggesting that it could be used as a potential immunomodulatory agent.
杂志排行
Traditional Medicine Research的其它文章
- Theanine combined with cisplatin inhibits the proliferation and metastasis of TNBC cells through Akt signaling pathway
- Network pharmacology and verification experiment-based prediction of active components and potential targets of Alpiniae Oxyphyllae Fructus-Saposhnikoviae Radix(Yizhiren-Fangfeng)for treatment of diabetic kidney disease
- Mechanism of Baihu Renshen decoction on T2DM rats based on mitochondrial autophagy mediated by PINK1/Parkin
- Research progress of artesunate in diabetes and its complications
- Exploring the compatibility theory of traditional Chinese medicine formulae: the disassembled prescriptions study
