Network pharmacology and verification experiment-based prediction of active components and potential targets of Alpiniae Oxyphyllae Fructus-Saposhnikoviae Radix(Yizhiren-Fangfeng)for treatment of diabetic kidney disease
2023-02-17XianWangChangLiuHuanJiangBoCenChenXuYangManXiaoYiQiangXieKaiLi
Xian Wang,Chang Liu,Huan Jiang,Bo-Cen Chen,Xu Yang,Man Xiao,Yi-Qiang Xie*,Kai Li*
1School of Traditional Chinese Medicine, Hainan Medical University, Haikou 571199, China. 2School of Basic Medicine and the Life Sciences, Hainan Medical University,Haikou 571199,China.
Abstract Background: In this study, we analyzed the potential active components, related crucial targets and possible signaling pathway mechanisms of Alpiniae Oxyphyllae Fructus and Saposhnikoviae Radix(AOF‑SR) herb pairs in the treatment of diabetic kidney disease(DKD)using network pharmacology and verification experiments. Methods: The active compounds and potential targets of AOF‑SR were derived from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform, The Encyclopedia of Traditional Chinese Medicine,and PubChem databases,and the potential therapeutic targets of DKD were derived from the OMIM, Drugbank, and DisGeNET databases. The“compounds‑diseases‑targets”network was constructed using Cytoscape 3.6.0.ClusterMaker functionality in Cytoscape is being used to screen important targets for AOF‑SR treatment of DKD.Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analysis of important targets were performed using DAVID database. In addition, according to the predicted results of network pharmacology, HK‑2 cells were used to construct DKD model for verification experiment. HK‑2 cells were divided into control group, high glucose (HG)group and AOF‑SR (HG + AOF‑SR) group to detect survival rate and expression of key proteins in NF‑κB and PI3K/Akt signaling pathways.Results:A total of 38 compounds were selected from AOF‑SR, of which 23 were Alpiniae Oxyphyllae Fructus and 15 were Saposhnikoviae Radix. Through enrichment analysis of 82 important targets, 88 signaling pathways were identified; some of these pathways, such as the NF‑κB, PI3K‑Akt, IL‑17, and JAK/STAT signaling pathways, regulate the pathological process of DKD. In verification experiment, the HK‑2 cells survival rate was higher in the HG + AOF‑SR group than in the HG group (P < 0.05). Moreover, western blotting results showed that the expression levels of NF‑κB, p‑PI3K, and p‑Akt in HG + AOF‑SR group were significantly lower than those in HG group (P < 0.05). Conclusion: Overall, this study revealed the active compounds,important targets and possible mechanisms of AOF‑SR treatment for DKD, and conducted preliminary verification experiments on its correctness, provided novel insights into the treatment of DKD by AOF‑SR.
Keywords: network pharmacology; traditional Chinese medicine; Alpiniae Oxyphyllae Fructus; Saposhnikoviae Radix; HK‑2 cells; diabetic kidney disease
Highlights
The current study applied network pharmacology analysis and verification experiments to study the potential mechanisms ofAlpiniae Oxyphyllae FructusandSaposhnikoviae Radixherb pairs,an empirical formula derived from the clinical experience of Ziguang Guo,in the treatment of diabetic kidney disease.
Medical history of objective
Saposhnikoviae Radixis one of the earliest medicinal herbs utilized in ancient China that first appeared in the Chinese bookShennong’s Classic of Materia Medica(It is believed to have been written by Shennong and was written between approximately 200 C.E.and 250 C.E.).Alpiniae Oxyphyllae Fructusis one of the four famous southern herbs first recorded in the pharmaceutical bookKaibao Herbs(Revised by Han Liu,Zhi Ma,etc.,973 C.E.)during the Song Dynasty(960 C.E.to 1279 C.E.).In current studies on diabetic kidney disease,Alpiniae Oxyphyllae FructusandSaposhnikoviae Radixhave shown better therapeutic effects.However,the combined effect ofAlpiniae Oxyphyllae FructusandSaposhnikoviae Radixherb pairs on diabetic kidney disease remains unexplored and needs to be further elucidated.
Background
In 2021, the number of people living with diabetes worldwide was approximately 537 million; by 2045, the number will exceed 783 million[1].As one of the most important microvascular complications of diabetes, diabetic kidney disease (DKD) is the main cause of end‑stage renal disease [2]. DKD is an important complication of diabetes mellitus, with an incidence of about 20–50% [3]. The mortality rate of DKD is gradually increasing in chronic kidney disease, and DKD has become the leading cause of end‑stage renal disease [4]. The pathophysiology of DKD includes glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, and ultimately end‑stage renal disease [5]. DKD is strongly associated with metabolic changes in diabetes, leading to glomerular hypertrophy, glomerulosclerosis, tubulointerstitial inflammation, and fibrosis [6]. Although intensive management of diabetes, such as controlling blood glucose and blood pressure, can reduce the incidence of DKD and slow its progression [7, 8], there is an unmet need for innovative treatment strategies to prevent and reverse DKD. Therefore, more innovative treatment strategies are needed to improve health outcomes in patients with DKD.Therapeutic research targeting specific disease mechanisms, such as inflammation and fibrosis, can effectively achieve this goal [9, 10].
Traditional Chinese medicine (TCM) has received increasing attention in the prevention and treatment of DKD to improve the survival rate of patients [11, 12].Alpiniae Oxyphyllae Fructusis the dried ripe fruit ofAlpinia oxyphyllaMiq. used in the clinical treatment of DKD. Flavonoids, terpenes, alkaloids, and diphenyl heptanes are potential bioactive compounds inAlpiniae Oxyphyllae Fructus[13, 14].We conducted relevant research on theAlpiniae Oxyphyllae Fructustreatment of DKD.Alpiniae Oxyphyllae Fructuseffectively reduced renal injury (glomerular and tubular atrophy) in DKD mice [15].Saposhnikoviae Radix(the dried root ofSaposhnikovia divaricata(Turcz.) Schischk.) is a traditional Chinese herb commonly used in clinical treatment of DKD. Its main components include 5‑O‑methylvisamminol, anomalin, divaricatol, and ledebouriellol[16]. Some doctors advocate the use ofSaposhnikoviae Radixin the treatment of chronic kidney disease [17]. Several animal experiments have shown thatSaposhnikoviae Radixat therapeutic doses does not cause kidney damage in rats and can reduce uric acid to relieve renal failure [18]. TCM doctors often useAlpiniae Oxyphyllae FructusandSaposhnikoviae Radix(AOF‑SR)herb pairs in combination.AOF‑SR has a good therapeutic effect on DKD; however, no studies have examined the effect of AOF‑SR combination on DKD. Therefore, further studies on the mechanism of AOF‑SR in DKD treatment are urgently required.
As a traditional Chinese herb commonly used for the treatment of DKD, the complex composition and pharmacological mechanism of AOF‑SR are difficult to define, often limiting its application in the clinical treatment of DKD. Fortunately, network pharmacology, a bioinformatics approach that integrates biology and pharmacology,has emerged. This is considered a cost‑effective and promising approach for drug development [19]. Network pharmacology is primarily used to study “multitarget, multicomponent” disease therapies, which are more effective and less toxic than traditional single‑target drugs [20]. This finding is consistent with the holistic and systematic concepts of TCM. Therefore, many researchers have used the method of network pharmacology to preliminarily reveal the possible role of traditional Chinese herb in clinical treatment. Luo et al. used network pharmacology analyzed and studied the possible mechanisms of Fushengong decoction for treating chronic renal failure and confirmed the prediction results through experimental verification [21]. Network pharmacology, an emerging field that integrates biological and pharmacological analyses, was used to analyze the synergistic mechanisms of traditional Chinese herb in clinical disease, providing insights into active compound screening and putative target prediction for AOF‑SR [22]. In this study, the potential active components, related crucial targets and possible signaling mechanisms of AOF‑SR in the treatment of DKD were analyzed through network pharmacology. At the same time, the results of network pharmacology were simply verified by validation experiment. Figure 1 shows a brief flowchart of the study.
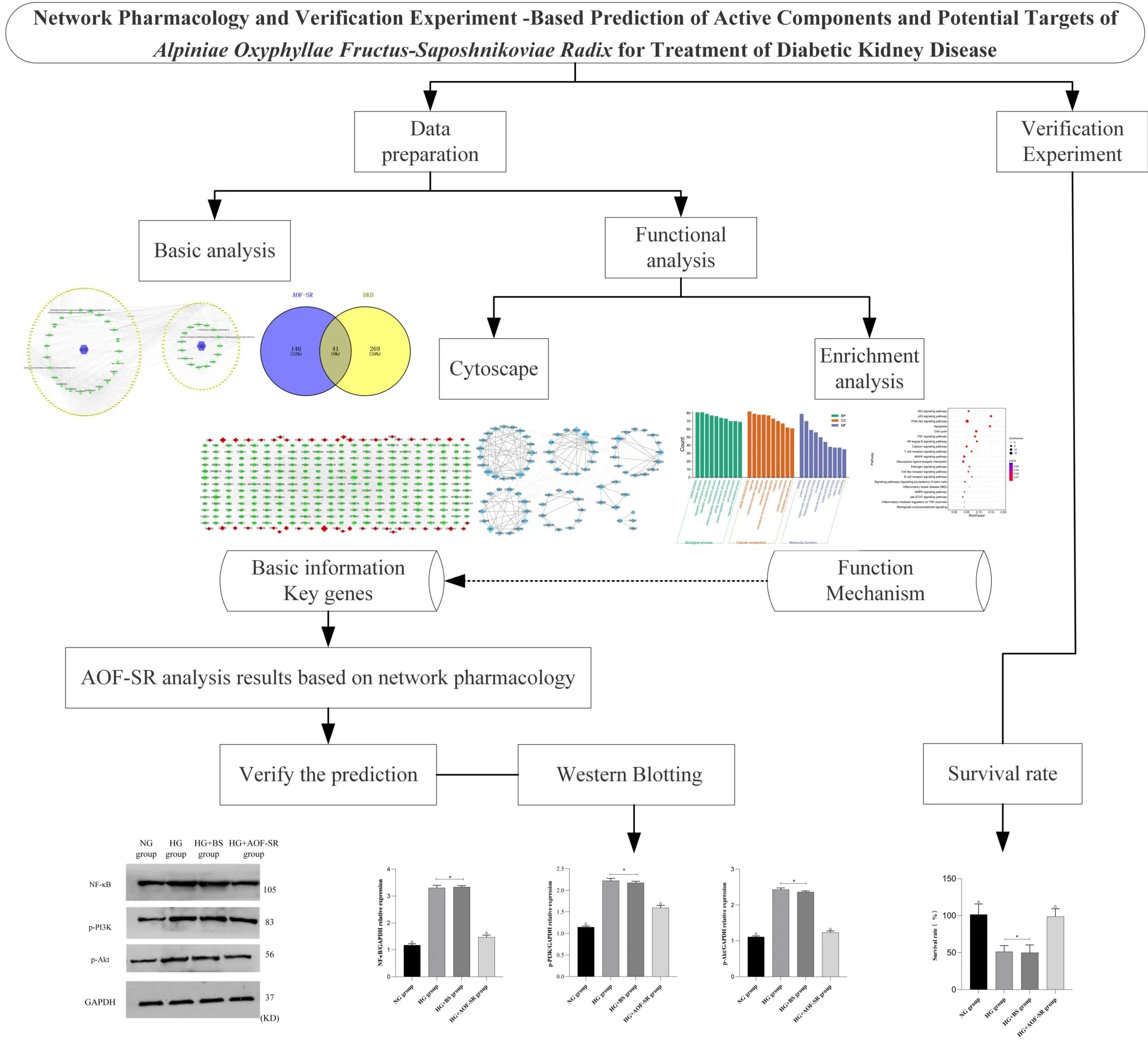
Figure 1 The brief flow chart of this study. DKD, diabetic kidney disease; AOF‑SR, Alpiniae Oxyphyllae Fructus and Saposhnikoviae Radix; GO,Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; BS,blank serum; HG, high glucose; NG, control group; CC,cellular component;MF, molecular function; BP, biological process.
Materials and Methods
Collection of compounds targets
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com/tcmsp.php) is a commonly used traditional Chinese herb research platform, which contains the commonly used traditional Chinese herb active ingredients and potential targets reported in studies, which is used to collect AOF‑SR active components [23]. In TCMSP, pharmacokinetic information retrieval filters for absorption, distribution, metabolism,and excretion screening are based on oral bioavailability ≥20% and drug‑likeness ≥0.1. To make the TCMSP data results rigorous to guide the verification experiment, oral bioavailability and drug‑likeness were selected according to the “suggested drug screening criteria” (https://old.tcmsp‑e.com/load_intro.php?id=29)in TCMSP [24]. The Encyclopedia of Traditional Chinese Medicine includes comprehensive and standardized information on the commonly used herbs and formulas of TCM, as well as their components. Drug‑likeness weight is an important indicator for screening the active components of Encyclopedia of Traditional Chinese Medicine.Based on the research published by Liang et al.,the drug‑likeness weight was set to > 0.49 [25]. The potential targets of active compounds collected were supplemented through the PubChem database(https://pubchem.ncbi.nlm.nih.gov/).
Collection of disease targets
Disease targets were collected from OMIM (http://www.omim.org/),Drugbank (http://www.drugbank.ca/), and DisGeNET(https://www.disgenet.org/search/) databases with “diabetic kidney disease” and “diabetic nephropathy” as keywords. All gene names ofAlpiniae Oxyphyllae Fructuscompounds‑targets,Saposhnikoviae Radixcompounds‑targets,and DKD disease‑targets were converted to HUGO Gene Symbol in the String (https://www.string‑db.org/).Furthermore, the online tool Venny 2.1(https://bioinfogp.cnb.csic.es/tools/venny/) was used to analyze the intersection of compound targets and disease targets, and the intersection results were used as possible targets for AOF‑SR treatment of DKD.
Construction of Protein–Protein Interaction (PPI) networks
In order to further analyze the possible mechanism of AOF‑SR in the treatment of DKD, the herb‑compound‑target‑disease network was established using Cytoscape 3.6.0. Cytoscape provides powerful mapping capabilities by which disease and drug targets are mapped separately. Based on these results, a PPI network was successfully constructed. In the PPI network, nodes with different colors, shapes and sizes directly represent the role of the target in the PPI network,among which the lines are considered to be the role of PPI. Degree,betweenness centrality, closeness centrality and average shortest path length are regarded as the main network topological parameters.These values are used as indicators for screening important nodes in a network [26].
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes(KEGG) enrichment analysis
The correlative targets of AOF‑SR treatment in DKD and possible signaling pathways were analyzed for further functional investigation.To this end, GO and KEGG pathway enrichment analyses were carried out using the DAVID database (https://david.ncifcrf.gov/).
Drugs and reagents
AOF‑SR granules were purchased from Guangdong EFONG Pharmaceutical Co., Ltd. (Guangdong, China). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Wuhan Punoxai Life Science and Technology Co., Ltd. (Wuhan, China). Fetal bovine serum was purchased from Bioind(Shanghai,China).Cell culture bottles and plates were procured from Corning (Shanghai, China). Cell counting kit‑8 (CCK‑8) kit was obtained from Bimake (Shanghai, China). BCA protein quantitative kit was purchased from Beijing Kangwei Century Biotechnology Co., Ltd. (Beijing, China, license No. CW10045). Skim milk powder was purchased from BioRuler(Danbury,CT,USA,license No.151‑21‑3).The protein color predyeing marker was purchased from Thermo Fisher Scientific (Shanghai, China, license No. 26616). PVDF membranes (0.45 μm) were procured from Millipore/Merck (Bedford,MA,USA).The antibodies of NF‑κB,p‑PI3K,and p‑Akt were purchased from Bioss Biotechnology Co., Ltd. (Beijing, China, license No.bsm‑52131R).
Resuscitation and passage of cell lines
HK‑2 cells (Wuhan Procell Co., Ltd., No. CL‑0109), frozen in a liquid nitrogen tank, were resuscitated. The HK‑2 cell suspension was absorbed and centrifuged in a sterile centrifuge tube at 1,000 r/min for 5 min, and the supernatant was discarded. 10 mL DMEM was then added to the centrifuge tube for further centrifugation. DMEM medium (10% fetal bovine serum and 100 U/mL penicillin) was inoculated into the culture flasks and then placed in an incubator for culture (saturated humidity, 37 °C, 5% CO2). After the HK‑2 cells adhered to the wall and grew to 80%confluence,trypsin digestion and centrifugation were performed. Morphology of HK‑2cells was observed continuously. Cells in the good and exponential growth stages were selected for follow‑up experiments.
Animals and preparation of medicated serum
Eight specific pathogen free Sprague‑Dawley male rats were selected(5‑week‑old; 250 ± 20 g) (Hunan SJA Laboratory Animal Co., Ltd.,quality certificate No. 430727201101514646). They were raised in the animal room of Hainan Medical University with free access to food, water, and natural light (20–25 °C and 50% humidity). The Ethics Committee of Hainan Medical University reviewed and approved all experimental procedures (grant No. HYLL‑2022‑216).Eight Sprague‑Dawley rats were divided into blank serum (BS) and AOF‑SR groups. According to the conversion method of human and animal surface areas, the AOF‑SR group was administered 5.2 g/kg·d of active drug, and the ratio ofAlpiniae Oxyphyllae FructustoSaposhnikoviae Radixwas 1:1. Under the same conditions, the BS group was only given 10 mL/kg 0.9% normal saline. Gavage was administered twice daily (8:00 and 16:00) for five days.Sprague‑Dawley rats were anesthetized by 3% isoflurane inhalation after fasting for 12 h. Blood was collected from the rat abdominal aorta under sterile conditions and left at room temperature for 1 h.The blood was centrifuged at 3,000 r/min for 15 min, and the supernatant was collected. Finally, the serum was inactivated at 56 °C for 30 min. After filtration through a 0.22 μm sterile filter membrane, the serum was stored in a sterile Eppendorf tube at−80°C.
Modeling, grouping,and intervention of HK-2 cells
The modeling method of HK‑2 cells induced by high glucose(HG)was carried out in accordance with the pre‑experimental method of our group and blood glucose concentration (30 mmol/L) reported in reference [27]. HK‑2 cells at the exponential growth stage were harvested and inoculated into 6‑well culture plates after pancreatin digestion(16 h,60%confluent).After 24 h of paving,the groups were intervened as follows: control group (NG group, 5.6 mmol/L glucose),HG group (30 mmol/L glucose), BS group (HG + BS group, 30 mmol/L glucose+10% normal rat serum),and AOF‑SR group(HG+AOF‑SR group, 30 mmol/L glucose + 10% AOF‑SR‑containing serum).After 24 h of treatment,HK‑2 cells were digested with trypsin,and the density was adjusted to 5× 104/mL.Finally, HK‑2 cells were inoculated into a 6‑well culture plate, 100 μL of cell suspension was added to each well, and six multiple wells were set up.
CCK-8 experiment
To detect cell proliferation, exponential growth phase cells in good growth conditions were chosen and digested with routine trypsin in 6‑well culture plates, and the corresponding growth environment was provided. After 24 h of digesting with trypsin, the cell density was adjusted to 5×104 cells/mL,and the cells were inoculated in 96‑well culture plates with 100 μL of cell suspension added to each well. Each group contained six wells. After 24 h of intervention, 10 μL of CCK‑8 solution was added to each well and incubated for 1 h in a cell culture incubator. The optical density was measured at 450 nm using a microplate reader. The experiment was repeated three times.
Western blotting
Western blotting was used to detect the expression levels of NF‑κB,p‑PI3K, and p‑Akt. The proteins were extracted after 24 h of intervention, and the protein concentrations were measured using a BCA kit. After separating the protein components using sodium dodecyl sulfate‑polyacrylamide gel electrophoresis, the proteins were transferred onto a PVDF membrane.After blocking with 5%skim milk powder for 1 h, different primary antibodies were added and incubated overnight at 4 °C, followed by incubation with horseradish peroxidase labeled secondary antibodies at room temperature for 1 h.Finally, the film was scanned using a gel imaging system. The target strip was analyzed using ImageJ software, and each experiment was repeated three times.
Mapping and statistical analysis
The PPI network was derived from Cytoscape version 3.6.0, and the results are presented as network charts. The bubble charts was used for enrichment analysis, which was derived from Omicshare(https://www.omicshare.com/). GraphPad Prism 9.0 was used to statistical and analyze experimental results. Count data are presented as± s. One‑way analysis of variance was used to compare data between groups.P< 0.05 was considered statistically significant.
Results
Collection of targets
A total of 38 active compounds were screened for AOF‑SR, including 23 ofAlpiniae Oxyphyllae Fructusand 15 ofSaposhnikoviae Radix,were collected. A total of 146 and 71 proteins targeted by AOF‑SR components, respectively, were obtained. Finally, 187 AOF‑SR proteins were obtained by removing repeated proteins (Figure 2).Detailed information about these active ingredients (whole name,basic structure, molecular formula) is provided in Supplementary Table S1. A total of 310 potential DKD targets were selected, and 41 overlapping targets were initially selected by Venn diagram(Figure 3).
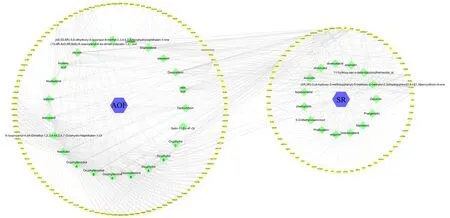
Figure 2 AOF-SR components-targets network.Square represents the drugs,diamond represents the active components,and circle represents the drug targets. AOF, Alpiniae Oxyphyllae Fructus; SR, Saposhnikoviae Radix.

Figure 3 The intersection of AOF-SR-targets and DKD-targets. Blue and yellow represent AOF‑SR and DKD, respectively. The common area represents overlapping protein targets. DKD, diabetic kidney disease; AOF‑SR, Alpiniae Oxyphyllae Fructus and Saposhnikoviae Radix.
Network construction and analysis
The “compounds‑diseases‑targets” network was constructed using Cytoscape. PPI were identified using topological analysis and visualized using Cytoscape. A network of 1,644 points and 11,100 edges was constructed, and 110 potential targets of AOF‑SR were identified in this network.The network was more clearly presented by setting the“Degree”,“Betweenness Centrality”,“So‑called Centrality”,and “Average Shortest Path Length” (Figure 4). These specific values are included in Supplementary Table S2. To further clarify the mechanisms of action of AOF‑SR, the targets of AOF‑SR in the compounds‑diseases‑targets network were analyzed using ClusterMaker analysis in Cytoscape. As shown in Figure 5, the network had 82 targets and seven modules, which were presumed to be the main links between AOF‑SR and DKD.
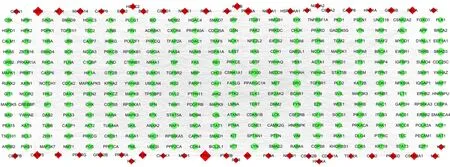
Figure 4 The compounds-diseases-targets network (334 nodes and 3567 edges).The diamond (bottom) represents the targets of AOF‑SR, and the circle (top) represents the targets of DKD. The size of the targets positively correlates with degree. DKD, diabetic kidney disease; AOF‑SR,Alpiniae Oxyphyllae Fructus and Saposhnikoviae Radix.
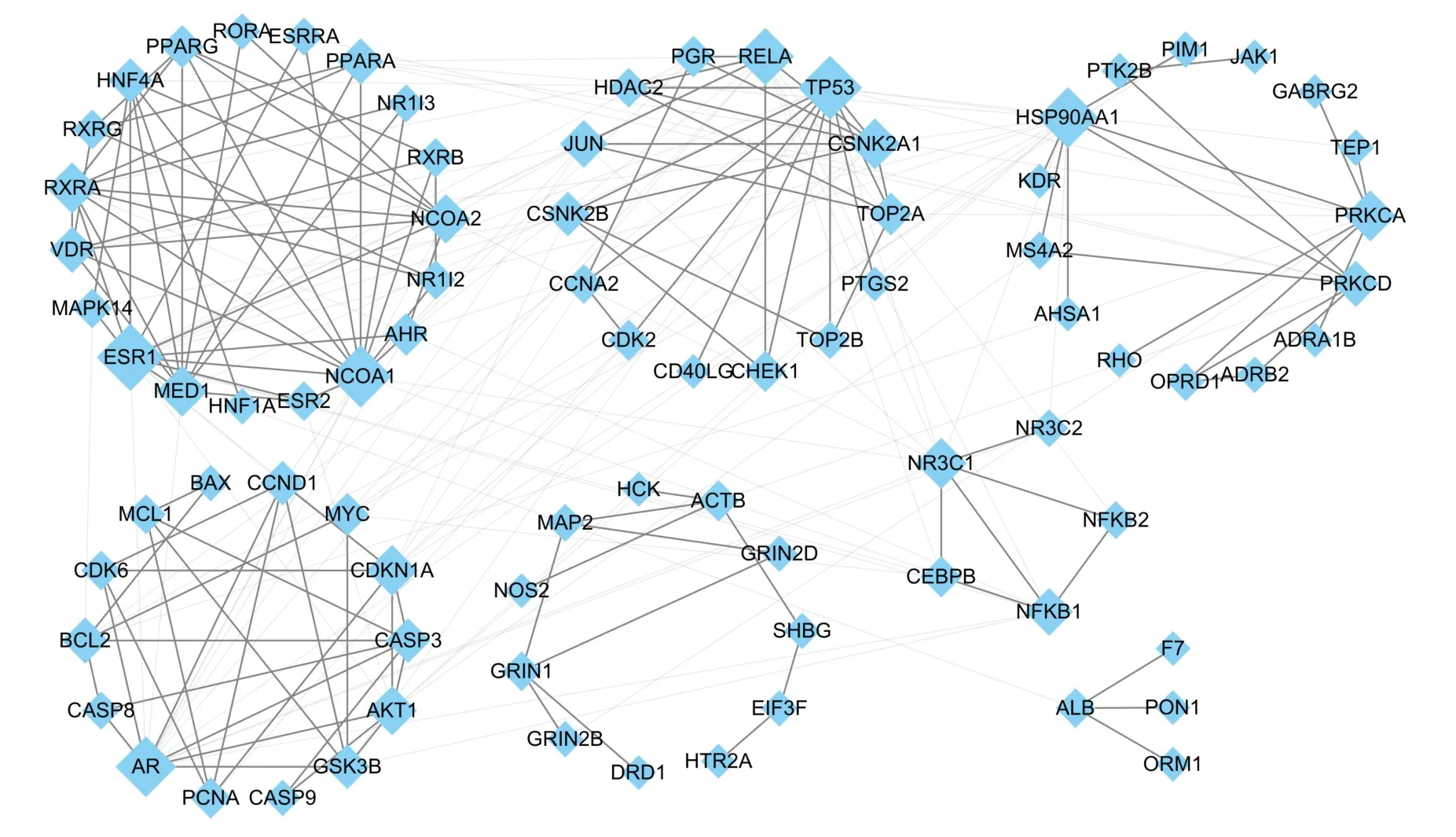
Figure 5 Clusters of screened core networks.This network hints the main targets and functional settlements of AOF‑SR in treating DKD.The size of the targets positively correlates with degree. DKD, diabetic kidney disease; AOF‑SR, Alpiniae Oxyphyllae Fructus and Saposhnikoviae Radix.
GO and pathway enrichment analysis
Cellular component (CC), molecular function (MF), and biological process(BP)are three important aspects of GO enrichment analysis.In our study,344 GO entries were enriched(P<0.05).The results of the GO analysis are detailed in Supplementary Table S3. According to the GO analysis results,the top 10 CC,MF and BP are shown in Figure 6A.The positive regulation of transcription from RNA polymerase II promoter, transcription initiation from RNA polymerase II promoter,and steroid hormone‑mediated signaling pathway were the top three GO terms in BP with a high count. Nucleoplasm, nucleus, and cytosol were the top three GO terms in CC with a high count.Steroid hormone receptor activity, RNA polymerase II transcription factor activity,ligand‑activated sequence‑specific DNA binding, and transcription factor binding were the top three GO terms in MF with a high count.In KEGG analysis, 88 most enriched signaling pathways were identified(P<0.05).Detailed KEGG pathways results are included in Supplementary Table S4.We screened 20 signaling pathways that may be related to DKD, including Wnt, p53, PI3K/Akt, and NF‑κB, as shown in Figure 6B. Finally, the PI3K/Akt and NF‑κB signaling pathways were selected for verification experiments.
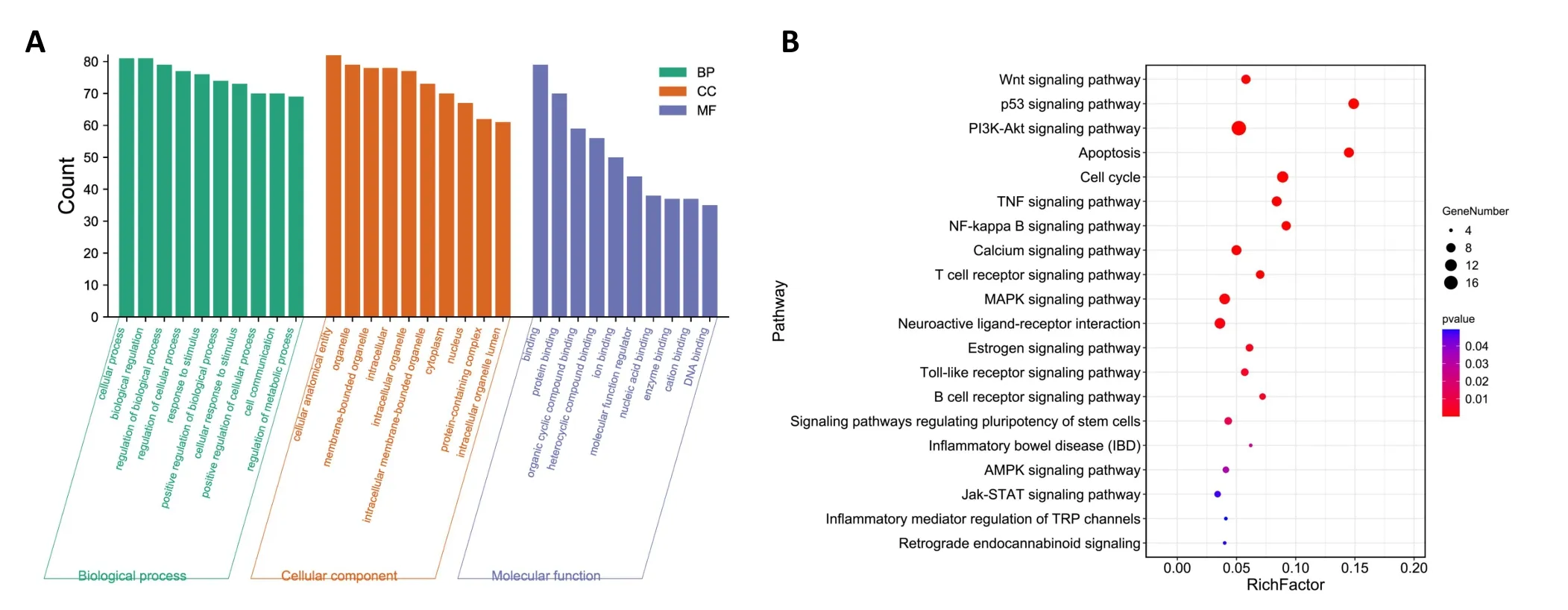
Figure 6 Possible mechanism of AOF-SR treatment of DKD.(A)A bar chart of GO analysis results.(B)Bubble diagram of KEGG analysis results.DKD, diabetic kidney disease; AOF‑SR, Alpiniae Oxyphyllae Fructus and Saposhnikoviae Radix; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; CC, cellular component; MF, molecular function; BP, biological process.
Effects of AOF-SR on survival rate of HK-2 Cells
The survival rate of HK‑2 cells after 24 h of HG modeling and drug intervention is shown in Figure 7. Compared with NG group, the survival rate of HK‑2 cells in HG and HG + BS groups was significantly decreased (P< 0.05). The survival rate of HK‑2 cells in HG + AOF‑SR group was significantly higher than that in HG group(P< 0.05).
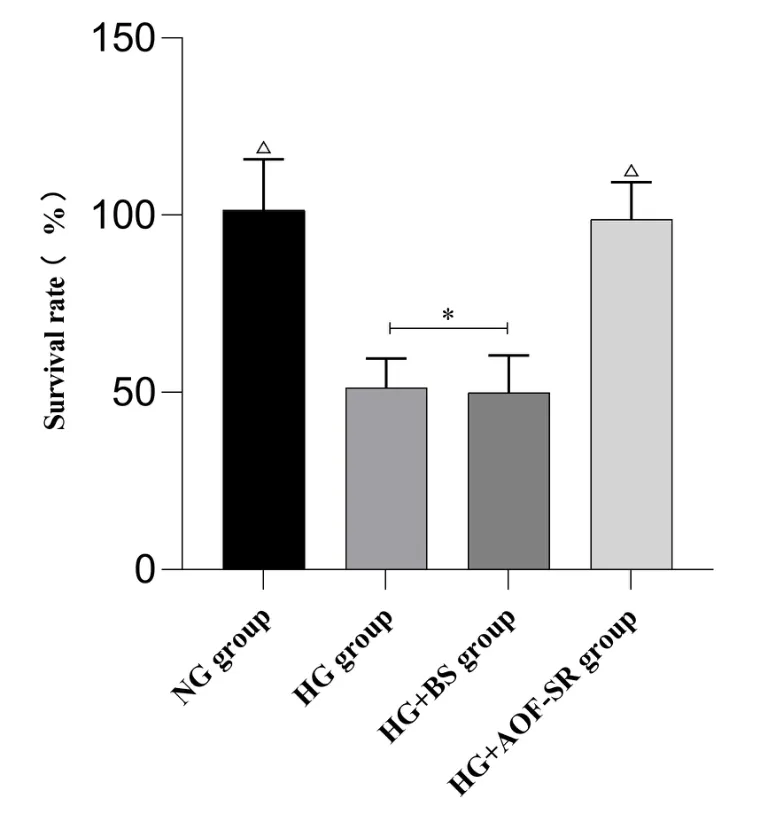
Figure 7 Comparison of cell survival rate in each group (n = 6).△ compared with HG group P < 0.05; * compared with HG +AOF‑SR group P < 0.05. AOF‑SR, Alpiniae Oxyphyllae Fructus and Saposhnikoviae Radix; BS, blank serum; HG, high glucose; NG, control group.
Effects of AOF-SR on NF-κB,p-PI3K,p-Akt protein expression
The NF‑κB and PI3K/Akt signaling pathways were used for validation based on network pharmacology analysis. The protein expression levels of NF‑κB, p‑PI3K, and p‑Akt are shown in Figure 8. Compared with NG group, the protein expression levels of NF‑κB, p‑PI3K, and p‑Akt in HG and HG + BS groups were significantly increased(P< 0.05). The increase of NF‑κB,p‑PI3K, and p‑Akt was inhibited in HG + AOF‑SR group compared with HG group (P< 0.05).

Figure 8 Effects of AOF-SR on NF-κB,p-IP3K,and p-Akt protein expression(n=3).(A)Western blot representing the protein levels;(B)NF‑κB protein expression level; (C) p‑PI3K protein expression level; (D) p‑Akt protein expression level. △ compared with HG group P < 0.05; *compared with HG + AOF‑SR group P < 0.05. AOF‑SR, Alpiniae Oxyphyllae Fructus and Saposhnikoviae Radix; BS, blank serum; HG, high glucose;NG, control group.
Discussion
With the supplement of clinical data in recent years, the advantages of TCM in DKD complementary therapy have been gradually confirmed[28, 29]. Network pharmacology is an important method to study the effect of drugs on disease treatment [30]. In this study, through the method of network pharmacology, we preliminarily elucidated the compounds‑ targets basis and possible mechanism of AOF‑SR treatment of DKD. A total of 38 active components were screened, 82 of which were considered important targets for AOF‑SR treatment of DKD. After KEGG enrichment analysis of these targets, 88 signaling pathways possibly involved in the effect of AOF‑SR in DKD treatment were screened. However, this analysis is based on a database and has not been verified experimentally. Therefore, we verified the authenticity and feasibility of some KEGG signaling pathway results through experimental verification. In previous studies, we confirmed that theAlpiniae Oxyphyllae Fructusreduction of inflammatory inflammasome expression in DKD mice is related to the reduction of p47phox, thereby reducing the renal inflammatory state [31]. At the epigenetic level, in the type 2 diabetic animal model (C57BIKsj db‑/db‑) study of differential miRNAs, differential regulation of 17 and 13 miRNAs was found in the model andAlpiniae Oxyphyllae Fructustreatment groups, respectively [32]. In addition, at the metabolic level,Alpiniae Oxyphyllae Fructushas a significant regulatory effect on intestinal flora [33]. Clinical trials have shown thatSaposhnikoviae Radixcan enhance immune function and bactericidal effects in elderly patients [34].Saposhnikoviae Radixreduced uric acid levels and renal failure in rats in animal studies[18]. The use of AOF‑SR in the treatment of DKD comes from the clinical experience of Ziguang Guo, a master and professor of TCM.AOF‑SR are often used by Chinese doctors in the clinical treatment of DKD. Although in existing reports, there have been studies on the use ofAlpiniae Oxyphyllae FructusandSaposhnikoviae Radixalone in the treatment of DKD, the role of AOF‑SR in DKD remains to be further clarified. We predicted the KEGG signaling pathway mechanism of AOF‑SR treatment for DKD using a network pharmacology method and verified the prediction results in HK‑2 cells.AOF‑SR enhanced the survival rate of HK‑2 cells under high‑glucose conditions and reduced the protein expression levels of NF‑κB, p‑PI3K, and p‑Akt. This result suggests that AOF‑SR possibly treats DKD via the above approaches.The directions of these validation experiments were all extracted from the results of this network pharmacology study, confirming that the prediction results can guide future research directions.
Traditional Chinese herb is characterized by multiple targets and pathways involved in the treatment of diseases. Network pharmacology provides avenues for revealing active compounds of traditional Chinese herb and predicting its possible mechanisms for treating disease [35, 36]. ClusterMaker is a plug‑in in Cytoscape used to analyze and visualize biological datasets and confirm or generate assumptions about biological functions [37]. Xu et al. used this approach in the network pharmacology of baicalin[38].In addition to the experimentally validated PI3K‑Akt and NF‑κB signaling pathways,88 pathways were derived from the seven modules analyzed by ClusterMaker.In a randomized controlled clinical study of 72 patients,Berthier et al. found that JAK‑STAT pathways were highly and significantly regulated in human glomerular and tubulointerstitial samples [39]. Zitman‑Gal et al. found that p‑STAT3 was mainly expressed in the glomeruli, while p‑JAK2 was expressed in the renal tubules in DKD mouse kidneys [40]. They also confirmed that the JAK/STAT signaling pathway is involved in intracellular processes in an experimental diabetes model. Ma et al. knocked out the IL‑17 gene in DKD mice and confirmed that IL‑17 has pro‑inflammatory and pro‑fibrotic effects in DKD [41]. Wu et al. proposed that the inflammatory process in DKD is not only caused by the innate immune response mediated by macrophages but also by the adaptive immune response mediated by lymphocytes [42]. Zeng et al. found that the complications of diabetes were related to the massive activation of immune cells induced by T cells and Th‑17 through metabolomic study [43]. In addition, the mechanisms of the P53 signaling pathway in renal tubular injury have been confirmed in recent years; however,as with other signaling pathways, there are not enough clinical trials to support them [44, 45]. An in‑depth exploration of these signaling pathways can provide insights into the mechanisms of action of AOF‑SR in treating DKD.
Based on the results of network pharmacology, some key cell signaling pathways in AOF‑SR therapy for DKD were identified. Cell signaling pathways mediate multiple cellular functions, including proliferation, autophagy, migration, metabolism, and apoptosis.Signaling pathways can explain the mechanisms of disease and drug therapy [46]. The PI3K/Akt signaling pathway plays a key regulatory role in the development of DKD. Under high‑glucose conditions, the PI3K/Akt signaling pathway in renal tubular cells is activated and regulates cell growth, epithelial‑mesenchymal transition, and lipid metabolism [47]. Xue et al. detected markers of the PI3K/Akt signaling pathway in the kidneys of DKD rats and found that the expression level of p‑Akt in the high‑glucose group was significantly higher than that in the normal group [48]. Similar results were obtained in HK‑2 cell experiments. Xu et al. used LY294002 to inhibit the expression of p‑Akt, effectively alleviating the epithelial‑mesenchymal transition process of HK‑2 cells caused by HG levels [49]. Recently, Wang et al. discovered that calcium dobesilate can attenuate glomerular enlargement and tubular fibrosis in DKD mice [50]. Western blotting revealed that calcium dobesilate decreased p‑PI3K and p‑Akt expression under high‑glucose conditions.Existing studies have confirmed that the expression of NF‑κB as a pro‑inflammatory factor is related to kidney inflammation [51].Experimental data showed that with increased NF‑κB expression in diabetic rats, mesangial membrane expansion and interstitial fibrosis occurred in the kidney, eventually leading to the thickening of the basement membrane, glomerular sclerosis, and tubular atrophy [52].Li et al. confirmed that in DKD mice, inhibiting the activation of the NF‑κB signaling pathway by knocking out cation transport regulator homolog 1 can reduce the inflammatory response of the kidney and delay DKD pathogenesis[53]. We conducted a pathway analysis of 82 potential targets screened by network pharmacology. A total of 88 pathways including PI3K‑Akt and NF‑κB were predicted. Western blotting confirmed that AOF‑SR could reduce the expression of NF‑κB,p‑PI3K, and p‑Akt in HK‑2 cells in a high‑glucose environment,providing insights into the possible mechanisms of AOF‑SR in DKD treatment.
We also confirmed that AOF‑SR can reduce the mortality of HK‑2 cells in a high‑glucose environment based on cell experiments.Combining the results of the enrichment and pathway analyses of 82 potential targets in seven modules of AOF‑SR suggested by network pharmacology,all of them may be considered potential mechanisms of AOF‑SR in treating DKD. However, there is still a lack of systematic and comprehensive animal and clinical trials to confirm the accuracy of these results, and subjectivity in the data‑collection process is unavoidable. In general, the targets and pathways obtained through network pharmacological analysis may provide directions for future studies on exploring the mechanism of AOF‑SR in DKD treatment.
Conclusion
In this study, active compounds of AOF‑SR and targets of AOF‑SR and DKD were screened. At the same time, network pharmacological analysis was used to screen the potential targets of AOF‑SR treatment of DKD and reveal the possible mechanism of AOF‑SR treatment of DKD.We found that the therapeutic effects of AOF‑SR on DKD may be related to 88 pathways,including the NF‑κB,JAK‑STAT, and PI3K‑Akt signaling pathways. Moreover, through verification experiments, it was confirmed that AOF‑SR could improve the survival rate of HK‑2 cells in a high‑glucose environment and downregulate NF‑κB, p‑PI3K,and p‑Akt expression. This suggests the feasibility of the results of this study, and provides research ideas for further research on AOF‑SR treatment of DKD.
杂志排行
Traditional Medicine Research的其它文章
- Theanine combined with cisplatin inhibits the proliferation and metastasis of TNBC cells through Akt signaling pathway
- Mechanism of Baihu Renshen decoction on T2DM rats based on mitochondrial autophagy mediated by PINK1/Parkin
- Comparison of polysaccharides from 10 species of genera Paris,Trillium,Aspidistra,and Polygonatum
- Research progress of artesunate in diabetes and its complications
- Exploring the compatibility theory of traditional Chinese medicine formulae: the disassembled prescriptions study
