Destructive effects on endothelial cells of grafts in cytomegalovirus DNA-positive patients after keratoplasty
2023-02-11YunXiaoZangRongMeiPengHanZhiBenJingHaoQuGeGeXiaoLiXueShuaiPeiZhangLiNaFengJingHong
Yun-Xiao Zang, Rong-Mei Peng, Han-Zhi Ben, Jing-Hao Qu, Ge-Ge Xiao, Li-Xue Shuai,Pei Zhang, Li-Na Feng, Jing Hong
1Department of Ophthalmology, Peking University Third Hospital, Beijing 100191, China
2Key Laboratory of Vision Loss and Restoration, Ministry of Education, Beijing 100191, China
Abstract
● KEYWORDS: keratoplasty; cytomegalovirus; ocular comorbidities; endothelial cell density; central corneal thickness
INTRODUCTION
Corneal transplantation has been performed for over a hundred years. However, the graft survival rate remains a major concern. Endothelial cell density (ECD)plays an important role in corneal graft survival[1]. ECD loss can occur during and after keratoplasty[2]. One study reported no significant change in ECD loss 1‐3y after penetrating keratoplasty (PKP) and Descemet’s stripping automated endothelial keratoplasty (DSAEK)[3]. Ocular comorbidities,such as drainage valve devices before DSAEK[4], vitrectomy before keratoplasty[5], laser iridotomy[6], could also increase graft endothelial cells (ECs) loss. In recent years, the influence of herpesvirus on graft ECs has attracted attention.We screened for herpesvirus in the edges of donor corneal grafts and had confirmed that ECD loss was much higher in the herpesvirus positive group than in the virus negative group[7‐8]. Cytomegalovirus (CMV), a widespread member of theHerpesviridaefamily, could cause CMV endotheliitis and contribute to endothelial decompensation in a short period of time[9]. Studies have confirmed CMV activation in the anterior segment after keratoplasty. Jeng[10]examined ocular samples after PKP and found high positivity rates for herpes simplex virus (HSV) and CMV, which may be correlated with a high risk of graft failure. Currently, there has been only one case series describing the effects of CMV on the ECs of corneal grafts. Koizumi[11]reported 4 cases of CMV endotheliitis following DSAEK, in which a sudden decrease in ECD was observed with an average loss of more than 50% of ECs at 6mo postoperatively. This study suggested that graft failure may be correlated with CMV activation after keratoplasty;however, considering the small sample size, further large, in‐depth studies are needed. Herein, in the largest sample thus far,we compared graft survival rates and postoperative graft ECD between CMV DNA positive (CMV+) patients and viral DNA negative (virus‐) patients. To eliminate confounding effects introduced by ocular comorbidities on EC loss, patients in the virus‐ group were selected to matched to those in the CMV+group according to ocular comorbidities. Additionally, patients with a better prognosis after keratoplasty were included as normal controls for comparison with the CMV+ and virus‐groups.
SUBJECTS AND METHODS
Study SubjectsWe retrospectively analyzed the clinical data of patients who underwent keratoplasty from March 2015 to December 2018 at the Peking University Third Hospital,Beijing, China. Aqueous humor/corneal tissue was examined for viral DNA in patients who were hospitalized for corneal keratoplasties to analyze the prevalence of virus infection in eyes. The inclusion criteria were as follows: 1) patients who had undergone PKP or DSAEK in our ophthalmic ward and performed virus DNA detection of aqueous humor/cornea during keratoplasties; 2) patients with complete follow‐up data.The cases in CMV+ group were only CMV DNA polymerase chain reaction (PCR) positive; tests for HSV‐1, HSV‐2,varicella zoster virus (VZV) and Epstein‐Barr virus (EBV)were all negative. In the two other groups, none of the members tested positive for viral DNA. According to the presence of viral DNA and ocular comorbidities, the patients were divided into three groups with an average of 29 cases in each group. 1) The CMV+ group included 13 cornea tissue and 16 aqueous humor CMV DNA positive cases. These cases were highly suspected of corneal CMV infection before surgery,with or without other ocular comorbidities. 2) The virus‐group included 15 cornea tissue and 14 aqueous humor virus DNA negative cases; these patients had no clinical evidence of viral infection were pairwise matched with the CMV+group according to ocular comorbidities. 3) The control group included 11 cornea tissue and 18 aqueous humor virus DNA negative cases; patients in this group were diagnosed with either keratoconus or bullous keratopathy with no other ocular morbidities or surgical histories except intraocular lens (IOL)implantation. Adjustment variables included age, sex, primary graft ECD and keratoplasty patterns among three groups.
Surgical Technique and Viral DNA DetectionIn this study, all keratoplasty surgeries, including PKP and DSAEK,were performed by the same surgeon (Hong J) at the Peking University Third Hospital. The surgical procedures were performed as previously described[12]. DNA was extracted from recipient aqueous humor/corneal tissue using a QIAamp DNA Mini Kit (catalog no. 51 304; Qiagen, Hilden, Germany)according to the manufacturer’s instructions. In brief, detection samples were placed in a 1.5‐mL microcentrifuge tube and digested with Buffer ATL and proteinase K. The extracted DNA was diluted in water; a total of 50 ng was tested by PCR.HSV‐1, HSV‐2, VZV, CMV, and EBV were detected using qualitative commercial, TaqMan based methods (HSV‐1/HSV‐2 Typing Real‐Time PCR Kit, Z‐SD‐0136‐02; VZV Real‐Time PCR Kit, OD‐0024‐02; CMV Real‐Time PCR Kit, Z‐OD‐002‐02; EBV Real‐Time PCR Kit, Z‐OD‐0023‐02; Liferiver Bio‐Tech Corp, China) in accordance with the manufacturer’s instructions. Real‐time PCR was performed using reagents from PE Biosystems (PE Applied Biosystems, Foster City, California).The limit of detection for all viral DNA was 10 copies/μg.Each sample was processed with an internal control to assess isolation and amplification efficacy.
Postoperative TreatmentThe standard postoperative treatment for PKP and DSAEK consisted of topical 0.5%levofloxacin and artificial tears (4 times per day) for 1mo;topical 0.1% dexamethasone eye cream (once every night) for 1wk; and topical 1.0% prednisolone acetate (4 times per day),tapered over 3‐6mo. Topical 1% cyclosporin (4 times per day)was added 1wk after surgery and was tapered depending on the status of the graft. Patients with CMV DNA positivity in aqueous humor/corneal tissue were treated withi.v. ganciclovir(5 mg/kg) for 7‐14d andp.o. ganciclovir (1 g) 3 times per day combined with topical 0.15% ganciclovir eye cream (4 times per day) for a long time.
Postoperative Follow UpThe follow‐up indicators collected were graft survival, ECD, ECD loss, and central corneal thickness (CCT). In this study, ECD and ECD loss in corneal grafts were examined at 1, 3, 6, 12, and 24mo after keratoplasty along with the CCT was evaluated at 3 and 12mo after keratoplasty in the CMV+, virus‐, and control groups.We defined the first 6mo after keratoplasty as the early stage,6‐12mo as the middle stage, and 12‐24mo as the late stage[3].Cornea graft failure was defined as irreversible corneal graft opacity without incident keratopathy. Corneal endothelial decompensation primarily manifested as a decreased ECD(less than 400 cells/mm2in our study)[13], persistent corneal edema and an increased CCT. We excluded cases in which the ECD was unable to be measured for the ECD loss calculation.The average ECD in the central area was measured byin vivoconfocal microscopy (HRT III, Heidelberg Engineering,Heidelberg, Germany). Graft attachment and the CCT were assessed with anterior‐segment optical coherence tomography(Carl Zeiss Meditec, Dublin, California, USA). The same certified ophthalmic technician performed all postoperative patient examinations using the same microscope.
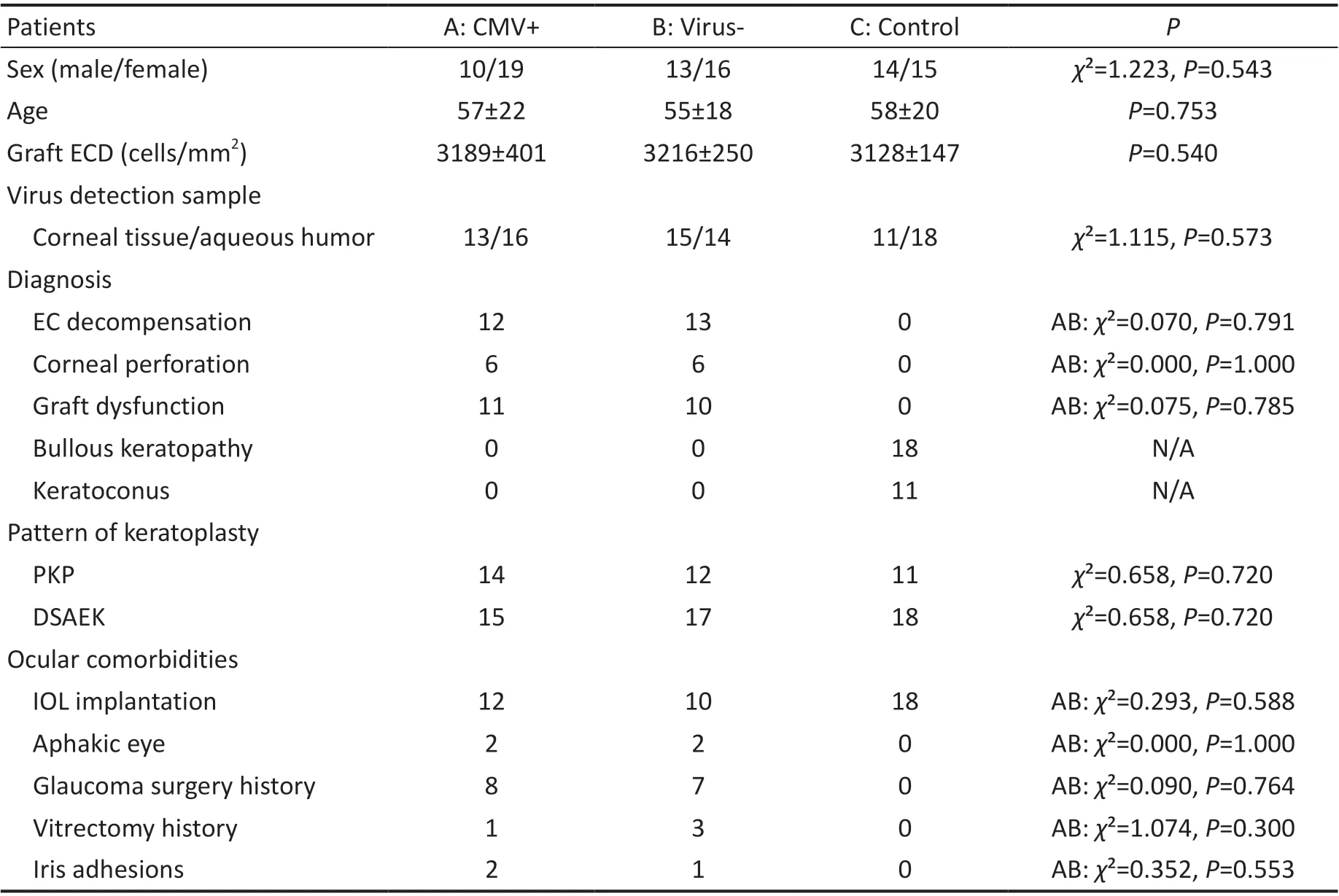
Table 1 Clinical information of patients in the CMV+, virus-, and control groups
Statistical AnalysisStatistical analysis was performed with SPSS 23.0 (SPSS, Chicago, Illinois, USA). Chi‐square and one‐way ANOVA tests were used for comparisons among three groups. Kaplan‐Meier survival analysis was used to generate corneal graft survival curves, and the log‐rank test was used to compare the graft survival rate in each group. Tukey’s HSD test was used for comparisons of ECD, ECD loss, and the CCT between two groups among the CMV+, virus‐, and control groups. The correlation between ECD and CCT was examined by Pearson’s test. One‐way ANOVA was employed to examine within‐group differences. All tests were 2‐tailed,P<0.05 was considered statistically significant, andP<0.01 was considered very statistically significant.
RESULTS
DemographicsThe mean ages of the patients in the CMV+, virus‐, and control groups were 57±22, 55±18, and 58±20y respectively. There were no significant differences in age (P=0.753), sex (χ²=1.223,P=0.543), graft ECD(P=0.540) or keratoplasty patterns (PKP,χ²=0.658,P=0.720;DSAEK,χ²=0.658,P=0.72). Pairwise matching for ocular comorbidities, including IOL implantation (χ²=0.293,P=0.588), aphakia (χ²=0.000,P=1.000), glaucoma surgery history (χ²=0.090,P=0.764), vitrectomy history (χ²=1.074,P=0.300) and iris adhesion (χ²=0.352,P=0.553), was performed between the CMV+ and virus‐ groups, with no significant differences between these two groups. The primary diagnoses in the CMV+ and virus‐ groups were corneal endothelial decompensation (χ²=0.070,P=0.795), corneal perforation (χ²=0.000,P=1.000) and corneal graft failure(χ²=0.075,P=0.785), with no significant differences between these two groups. The clinical data are listed in Table 1.
Kaplan-Meier Curves of Graft SurvivalIn the CMV+group, 3 graft failures (10.34%) occurred within 6mo after keratoplasty, 10 graft failures (34.48%) occurred within 12mo, and 18 graft failures (62.07%) occurred within 24mo after keratoplasty. There was 1 graft failure (3.45%) in thevirus‐ group at 12mo and 3 graft failures (10.34%) at 24mo after keratoplasty. There were significant differences in graft survival among three groups (χ2=38.07,P=0.000; Figure 1).Among the 29 patients in the CMV+ group, 62.07% of corneal grafts failed after keratoplasty, which was higher than 10.34%among the 29 patients in the virus‐ group. The relative risk of graft failure post‐keratoplasty in the CMV+ group compared with the virus‐ group was 6.000 (95% confidence interval:1.980‐18.181) and was significantly different (P=0.000).
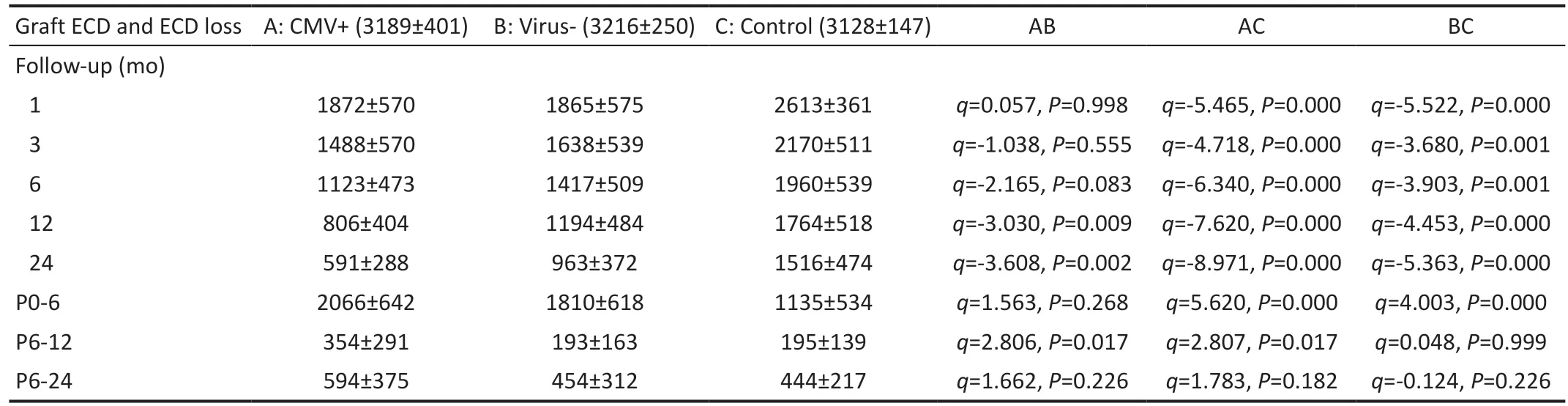
Table 2 Average ECDs and ECD losses at 1, 3, 6, 12, and 24mo after keratoplasty
Graft Endothelial Cell Density and Endothelial Cell Density LossGraft ECD and ECD loss values after keratoplasty in the three groups are shown in Table 2. The graft ECDs at each timepoint were significantly lower in the CMV+and virus‐ groups than in the control group (P<0.01). There were no significant differences in the graft ECDs between the CMV+ and virus‐ groups at 1 (q=0.057,P=0.998), 3(q=‐1.038,P=0.555) and 6mo (q=‐2.165,P=0.083) after keratoplasty, while there were significant differences at 12 (q=‐3.030,P=0.009) and 24mo (q=‐3.608,P=0.002).
ECD loss in the middle stage (6‐12mo) after keratoplasty was significantly higher in the CMV+ group than in the virus‐group (q=2.806,P=0.017), while there was no significant difference between these groups in the early stage (0‐6mo)post‐keratoplasty (q=1.563,P=0.268). Graft ECD loss was significantly higher in the early stage (q=4.003,P=0.000) but not in the middle stage (q=0.048,P=0.999) post‐keratoplasty in the virus‐ group than in the control group. Graft ECD loss was significantly higher in the CMV+ group than in the control group in the early stage (q=5.620,P=0.000) and middle stage(q=2.807,P=0.017) post‐keratoplasty. The above findings suggest that CMV may play an important role in ECD loss in the middle stage after keratoplasty.
当前,我国化肥行业整体形势严峻,一方面,产能结构性过剩、产品同质化严重、低水平竞争等严峻现状倒逼肥企转型升级;另一方面,国家扶持政策取消、农业供给侧改革深化、安全环保从严、肥料分级等政策迫使行业深化改革。在整体市场环境较差、产能迅速退出的大趋势下,倍丰盐湖农业科技却横空出世,强势入驻中国复合肥之乡。这不仅仅彰显了倍丰盐湖科技激流勇进、逆势而行的勇气,也彰显了它运筹帷幄、志在必得的底气。
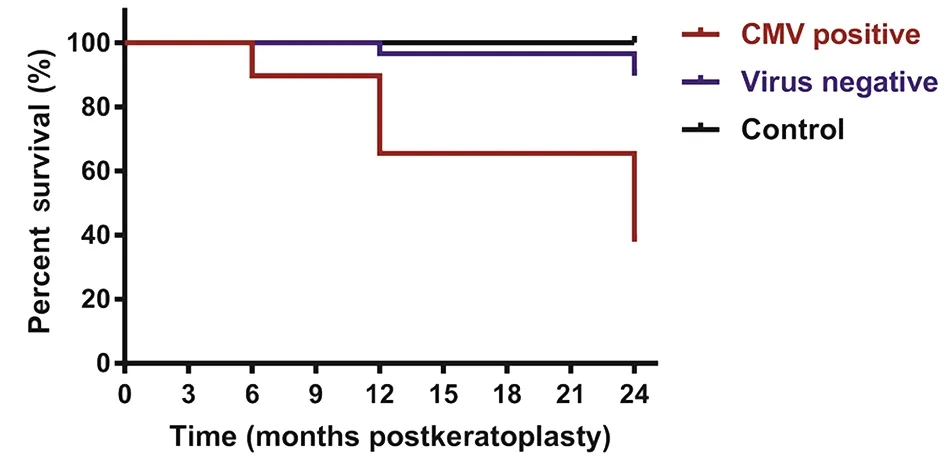
Figure 1 Graft survival in the three groups The difference in graft survival among the three groups was analyzed by the log-rank test.CMV: Cytomegalovirus.
ECD in PKP and DSAEKIn the CMV+, virus‐, and control groups, the ratio of PKP to DSAEK cases were 14:15,12:17 and 11:18, respectively, with no significant difference among three groups. Differences in ECD between PKP and DSAEK patients in the same group at 1, 3, 6, 12, and 24mo after keratoplasty were compared (Figure 2). There was no statistical difference in the recipient ECD between the DSAEK and PKP groups (P>0.05). In the CMV+ and virus‐ groups,there was no difference in ECD between the DSAEK and PKP subgroups (P>0.05). In the control group, the ECD was significantly lower in the DSAEK subgroup than in PKP subgroup (P<0.01). The above results suggested that CMV and ocular comorbidities had more serious negative effects on ECD in the PKP subgroup than in the DSAEK subgroup.
There was no difference in recipient ECD among the three groups in DSAEK patients before keratoplasty (P=0.59).Significant differences in ECDs were observed between the CMV+ and control groups at 1, 3, 6, 12, and 24mo after DSAEK (P<0.01). Significant differences in the ECD were observed between the virus‐ and control groups at 1, 3, 6, 12,and 24mo after DSAEK (P<0.01). The ECD was lower in the CMV+ group than in the virus‐ group at 24mo (P=0.033,Tukey’s test) after DSAEK, which suggested that CMV had the strongest negative effect on ECD at 24mo post‐keratoplasty in those who underwent DSAEK.

Figure 2 Differences in the ECD between the DSAEK and PKP patients in the CMV+, virus-, and control groups Differences of the ECD between PKP and DSAEK patients were compared by one-way ANOVA. A: ECD in CMV+ DSAEK and PKP patients; B: ECD in Virus- DSAEK and PKP patients;C: ECD in Control DSAEK and PKP patients. The ECD was significantly lower in the DSAEK patients than in the PKP patients (P<0.01). CMV:Cytomegalovirus; ECD: Endothelial cell density; PKP: Penetrating keratoplasty; DSAEK: Descemet's stripping automated endothelial keratoplasty.bP<0.01.
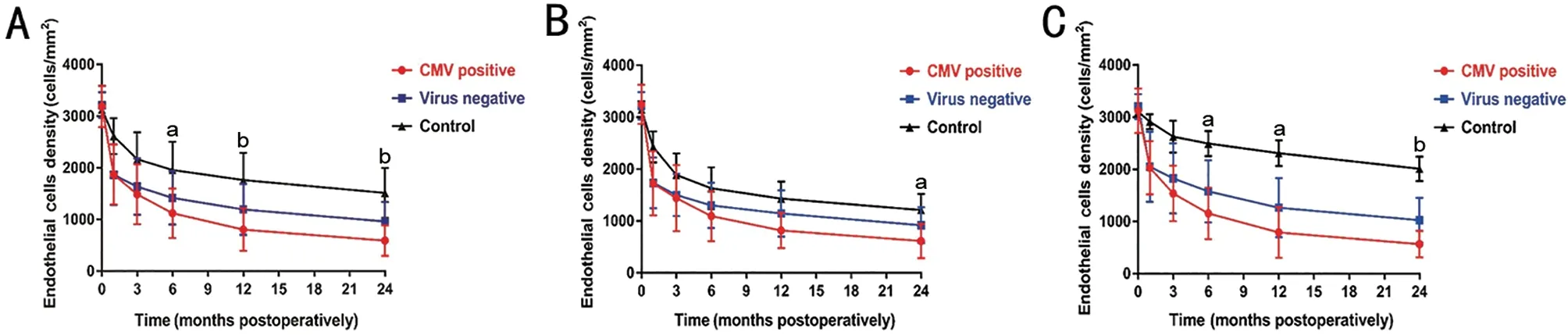
Figure 3 ECD in the DSAEK and PKP subgroups in the CMV+, virus- and control groups Differences of the ECD in the DSAEK and PKP subgroups among CMV+, virus- and control group were compared by Tukey’s HSD test. A: ECD in DSAEK and PKP patients; B: ECD in DSAEK patients; The ECD was lower in the CMV+ group than in the virus- group at 24mo (P=0.033) after DSAEK. C: ECD in PKP patients. The ECDs were lower in the CMV+ group than in the virus- group at 6 (P=0.020), 12 (P=0.039), and 24mo (P=0.002) after PKP. CMV: Cytomegalovirus; ECD: Endothelial cell density; PKP: Penetrating keratoplasty; DSAEK: Descemet's stripping automated endothelial keratoplasty. aP<0.05, bP<0.01.
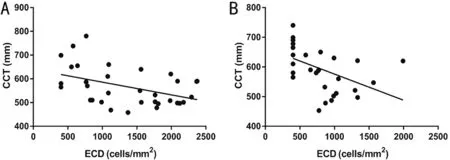
Figure 4 Correlation between the ECD and CCT at 3 and 12mo after keratoplasty in the CMV+ group Correlations were analyzed by Pearson’s test. A: Correlation between ECD and CCT at 3mo after keratoplasty; B: Correlation between ECD and CCT at 12mo after keratoplasty. In the CMV+ group, there were negative correlations between the ECD and CCT at 3 (r2=0.223, P=0.013) and 12mo (r2=0.218, P=0.011) after keratoplasty. CMV: Cytomegalovirus; ECD: Endothelial cell density; CCT: Central corneal thickness.
There were no differences in recipient ECD among the three groups in PKP patients before keratoplasty (P=0.71).Significant differences were observed in ECD between the CMV+ and control groups at 1, 3, 6, 12, and 24mo after PKP(P<0.01). Significant differences were observed in the ECDs between the virus‐ and control groups at 1, 3, 6, 12, and 24mo after PKP (P<0.01). ECDs were lower in the CMV+ group than in the virus‐ group at 6 (P=0.020, Tukey), 12 (P=0.039,Tukey), and 24mo (P=0.002, Tukey) after PKP (Figure 3), which suggested that CMV had a stronger negative effect on ECD in 6‐24mo post‐keratoplasty in those who underwent PKP.
Central Corneal Thickness in the Three GroupsThe mean CCTs at 3 and 12mo post‐keratoplasty were 561±81 and 582±91 μm in the CMV+ group, 529±79 and 536±70 μm in the virus‐ group, and 521±38 and 525±50 μm in the control group. There were no differences in the CCT among CMV+ ,virus‐, and control groups at 3mo after keratoplasty(P>0.05). The CCT was greater in the CMV+ group than in the virus‐ group and the control group at 12mo after keratoplasty(t=2.999,P=0.010;t=3.855,P=0.001, Tukey’s test).In the CMV+ group, there were negative correlations between ECD and CCT at 3 (r2=0.223,P=0.013) and 12mo(r2=0.218,P=0.011) after keratoplasty (Figure 4). There were no correlations between ECD and CCT at 3 or 12mo after keratoplasty in the virus‐ group (3mo:r2=0.000,P=0.971;12mo:r2=0.001,P=0.849) or the control group (3mo:r2=0.007,P=0.666; 12mo:r2=0.130,P=0.054). The above results suggested that the decrease in ECD caused by CMV infection was the main cause of corneal edema when ocular comorbidities were excluded as confounding factors.
DISCUSSION
There were significant differences in the Kaplan‐Meier curves of graft survival among the three groups, which suggesting that CMV could reduce the graft survival after keratoplasties.This finding was consistent with that of previous studies[14].López and Chan[15]examined the therapeutic prognosis of patients with CMV endotheliitis after DSAEK and found a postoperative rejection rate of 70%. Additionally, Dockeryet al[16]reported that CMV infection occurred after corneal transplantation and was strongly associated with rejection.
Based on the results of this study, the ECDs in the CMV+and virus‐ groups were significantly less than those in the control group at 1, 3, 6, 12, and 24mo after keratoplasty,suggesting that both CMV and ocular comorbidities before surgery could both affected ECD post‐keratoplasty. The ECD in CMV+ group was less than virus‐ group at 12 and 24mo after keratoplasty while showing no statistical difference at 1, 3, and 6mo, which meant CMV could cause persistent ECs destruction after keratoplasty. Normally, graft ECD loss mainly happened in the early stage then the rate of ECD loss was decreased gradually in the middle and late stage after keratoplasties[3]. According to our results, ocular comorbidities mainly cause ECD loss in the early stage and there was significant difference between virus‐ and control groups. During the middle stage, CMV infection played a more important role in ECD loss than ocular comorbidities,as CMV+ group showed significant difference from the virus‐group. The aforementioned result revealed that CMV exerted persistent detrimental changes on ECs in grafts, likely due to the effects of viral replication in the early post‐keratoplasty period, a decrease in the ECD in the middle stage, and finally endothelial decompensation in the late stage. Although removing infected lesions during keratoplasty allowed CMV infection of the anterior chamber to be controlled, CMV might continue to replicate in the event of failed immune surveillance in the anterior chamber, thereby exerting a devastating effect on grafted ECs[17]. Therefore, to reduce ECD loss after keratoplasty, ocular comorbidities should be addressed well during the procedure[18]. On the other hand, ECD loss in CMV positive patients should be considered in the early and mid‐stages after keratoplasty, especially during the middle stage (6‐12mo postoperatively). If graft ECD loss occurs within a short period, CMV‐related damage should be considered in addition to graft rejection.
Normally, PKP allow better preservation of ECD postoperatively than DSAEK and is associated with less ECs damage during surgery[3]. Among the CMV+ patients, there was no difference in postoperative ECD between those undergoing PKP and DSAEK. This suggested that, to some extent, CMV had a more negative influence on ECs in eyes that underwent PKP[19].Moreover, a significant difference in graft ECD between the CMV+ and virus‐ groups appeared earlier in the PKP subgroup than in the DSAEK subgroup (6mo after PKPvs12mo after DSAEK), suggesting that CMV replication had a shorter cycle in eyes that underwent PKP, which may be related to a stronger immunosuppressive effect of PKP on the anterior segment[20].Previous studies found that anterior‐segment CMV infections could occur after PKP[10,21], DSAEK[22‐23], and Descemet’s membrane endothelial keratoplasty (DMEK)[18], but were most common following the PKP procedure. Yoshimuraet al[21]first reported a case of CMV positivity after multiple PKP procedures. Cheeet al[24]reviewed patients who underwent PKP and developed postoperative CMV positivity that manifested as pigmented keratoplasty, corneal neovascularization, and Descemet’s folds.The aforementioned cases were cases of new CMV infection after PKP. There have also been reports of CMV recurrence after PKP. Marcus reviewed 32 cases of endotheliitis with preoperative CMV positivity treated with topical and systemic antiviral agents. PKP was performed after 6mo of keratitis quiescence, and patients who were CMV positive were more likely to relapse, with a mean time to recurrence of 10mo post‐keratoplasty[25], which was consistent with the ECD loss timepoint in our study.
It is commonly asserted that a decreased ECD, irreversible edema and an increased CCT in a corneal graft are prominent indicators of endothelial decompensation[17]. However, in the current study, in the CMV+ group, for whom the ECD was considerable, the CCT was greater than those in the virus‐ and control groups, which implied that fluid pumping function of ECs in the CMV+ group was weaker than those in the other groups. Moreover, a negative correlation between ECD and CCT after keratoplasty was observed in only the CMV+ group,which indicated that CMV+ was correlated with CCT and endothelial decompensation.
In conclusion, CMV can affect graft survival and exert persistent detrimental effects on the ECD after keratoplasty.We further demonstrate that graft ECD loss exerted by CMV mainly occurred in the middle stage (6‐12mo postoperatively),while ocular comorbidities mainly affected the ECD in the early stage (1‐6mo postoperatively). These findings suggested that the effects of CMV should be considered when addressing rapid ECD loss, especially in the middle stage post‐keratoplasty.
ACKNOWLEDGEMENTS
Authors’ contributions:Zang YX carried out the study and drafted the manuscript. Peng RM, Ben HZ and Qu JH participated in the design of the study and performed the statistical analyses. Xiao GG and Shuai LX conceived the study, and participated in its design and coordination and helped to draft the manuscript. Zhang P and Feng LN performed aqueous humor/cornea tissue virus DNA PCR detection. The corresponding author Hong J is responsible for ensuring that the descriptions are accurate and agreed upon by all authors. All authors read and approved the final manuscript.
Foundation:Supported by the National Natural Science Foundation of China (No.81970768).
Conflicts of Interest: Zang YX,None;Peng RM,None;Ben HZ,None;Qu JH,None;Xiao GG,None;Shuai LX,None;Zhang P,None;Feng LN,None;Hong J,None.
猜你喜欢
杂志排行
International Journal of Ophthalmology的其它文章
- Visual perception alterations in COVID-19: a preliminary study
- COVID-19 pandemic impact on ocular trauma in a tertiary hospital
- Apolipoprotein A1 suppresses the hypoxia-induced angiogenesis of human retinal endothelial cells by targeting PlGF
- Comparison of vegetable oils on the uptake of lutein and zeaxanthin by ARPE-19 cells
- Identifying a novel frameshift pathogenic variant in a Chinese family with neurofibromatosis type 1 and review of literature
- Recurrence risk factors of intravitreal ranibizumab monotherapy in retinopathy of prematurity: a retrospective study at one center
