3‑((4,6‑二甲基‑2‑嘧啶基)硫代)-丙酸和菲咯啉三元稀土配合物的晶体结构和发光性能
2023-02-03申偲伯贾丹丹王东军
兰 帅 张 雨 任 杰 申偲伯 曹 磊 贾丹丹 王东军
(1河北科技师范学院化学工程学院,秦皇岛 066000)
(2河北科技师范学院分析测试中心,秦皇岛 066000)
Due to the forbidden nature of the 4ftransitions and the lower molar absorption coefficients,lanthanide ions show very weak luminescence.Thus,the“anten‑na”ligand is mandatory.The triplet energy level of the antenna should meet the energy level of the lanthanide ion[1‑2].Among the wide range of antenna ligands,car‑boxylate and 1,10‑phenanthroline(Phen)are proven to be the widely accepted antenna.Some ternary rare earth complexes based on carboxylate and Phen have been established.Here gives some carboxylate ligands:N‑((benzoylamino)thioxomethyl)glycine,N‑(p‑acetami‑dobenzenesulfonyl)glycine,malic acid,mandelic acid,N‑((4‑methylphenyl)sulfonyl)glycine,L‑proline,2,4‑dinitro ‑benzoic acid,andα‑naphthyl acetic acid[3‑14].Furthermore,the biological activity of some rare earth complexes based on 2‑((4,6‑dimethyl‑2‑pyrimidinyl)thio)‑acetic acid has been reported[15‑16].
In this work,considering the potential antenna property of carboxylate and Phen,the 3‑((4,6‑dimethyl‑2 ‑pyrimidinyl)thio)‑propanoic acid(HL),as well as Phen,were introduced to react with lanthanide(Eu,Tb)ions giving the corresponding ternary lanthanide complexes.Meanwhile,we proved the prepared prod‑ucts are two isostructural lanthanide complexes.Impor‑tantly,some unique solid‑state photoluminescent prop‑erties were found in this system.
1 Experimental
1.1 Material and methods
HL was prepared following the published litera‑ture[16].All other chemicals were analytically pure from commercial sources and used without further purifica‑tion.Elemental analyses on C,H,S,and N were per‑formed on a German Elementary Vario EL Ⅲ instru‑ment.The IR spectra were recorded on KBr disks using a Nicolet‑Avatar 370 spectrometer between 400 and 4 000 cm-1.Thermogravimetric(TG)analyses were carried out on a NETZSCH STA 449 C unit at a heat‑ing rate of 10 ℃·min-1.Photoluminescence studies were performed on an Edinburgh FLS920 fluorescent spectrometer in a solid state.
1.2 Syntheses of complexes 1 and 2
A mixture of HL(0.641 g,3.0 mmol)and Phen(0.198 g,1.0 mmol)was dissolved in 50 mL ethanol.Then,the lanthanide nitrate hexahydrate(1.0 mmol)was added to the mixture.NH3·H2O was added drop‑wise until the pH value was 6.5.The mixture was kept stirring overnight at room temperature.A large amount of white precipitate was formed.After filtration and washing with water and ethanol,the white solid was obtained.Single crystals suitable for single‑crystal X‑ray diffraction were obtained from the filtrate afterca.8 d.
Complex[Eu(L)3(Phen)]2·2H2O(1):white solid,Yield:0.104 g,54% .IR(ATR,cm-1):3 442(m),2 924(w),1 608(vs),1 582(vs),1 557(s),1 478(w),1 423(s),1 393(s),1 340(w),1 269(s),1 225(m),1 176(w),1 013(w),883(w),764(m),710(w),545(w).Anal.Calcd.for C39H43EuN8O7S3(% ):C,47.61;H,4.41;N,11.39;S,9.77.Found(% ):C,48.09;H,4.48;N,11.98;S,10.39.
Complex[Tb(L)3(Phen)]2·2H2O(2):white solid,0.114 g,59% .IR(ATR,cm-1):3 422(w),3 050(w),2 923(w),1 604(vs),1 580(vs),1 551(s),1 425(vs),1 339(m),1 304(m),1 264(vs),1 208(m),1 139(m),1 102(m),1 004(w),948(w),845(m),730(m),690(m),545(w).Anal.Calcd.for C39H43N8O7S3Tb(% ):C,47.27;H,4.37;N,11.31;S,9.71.Found(% ):C,47.39;H,4.61;N,11.96;S,10.00.
1.3 X‑ray crystallography
A suitable crystal was covered in mineral oil and mounted on a glass fiber and directly transferred to a Brucker D8 advance diffractometer equipped with a sealed Mo tube and a graphite monochromator using MoKαradiation(λ=0.071 073 nm).All structures were solved by the direct methods using SHELXS[17‑18]and refined onF2with SHELXL and Olex2[19].All non‑hydrogen atoms were refined anisotropically and hydro‑gen atoms were determined with theoretical calcula‑tions.Multi‑scan absorption correction was applied to the intensity data using the SADABS program[20].SQUEEZE routine in PLATON was employed to remove the corresponding Q peaks,which may be iden‑tified as two water molecules[16,21].
The crystal data and structure refinement parame‑ters for the complexes are summarized in Table 1.Images of the crystal structures were generated by Diamond,version 3.2 (software copyright,Crystal Impact GbR).
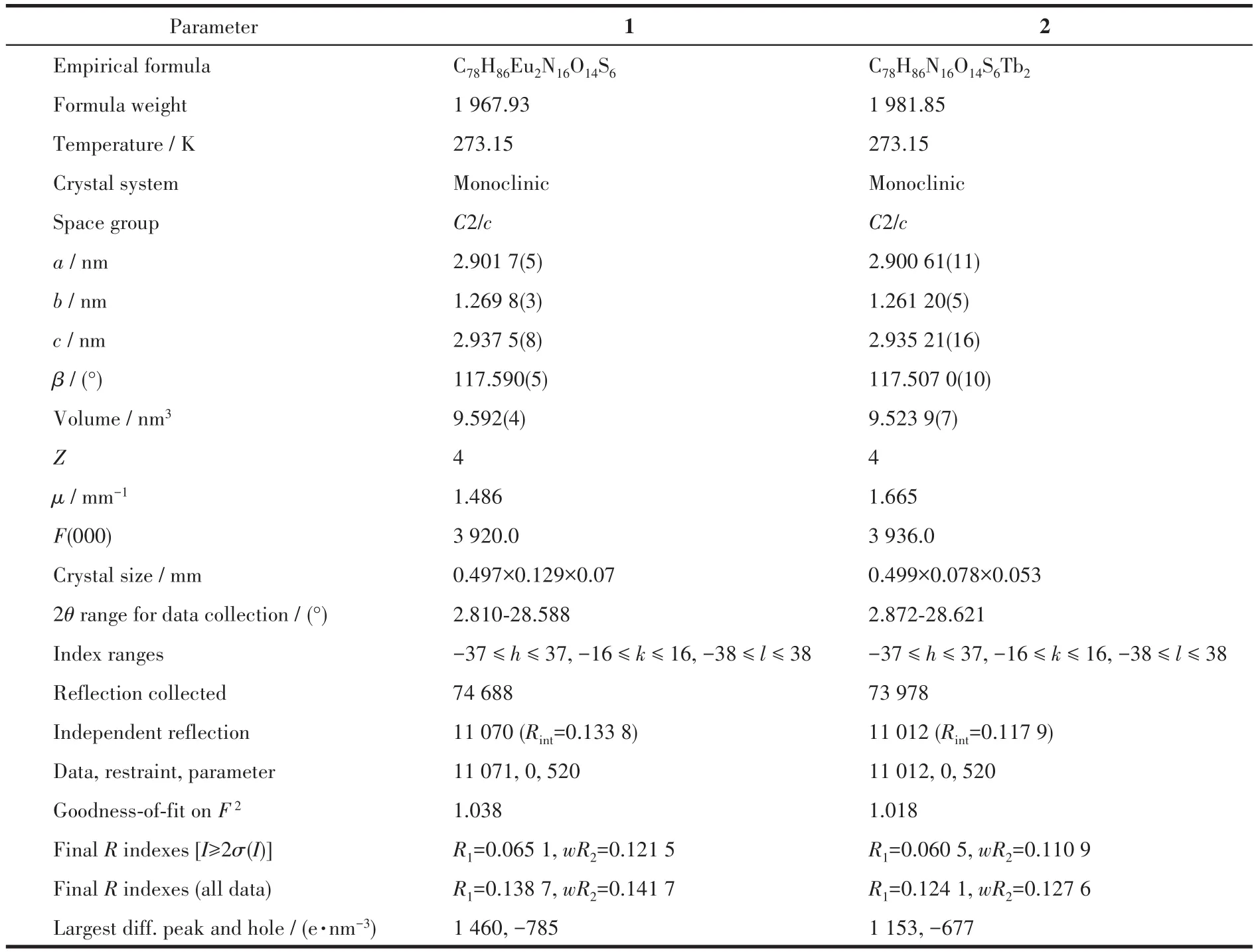
Table 1 Crystallographic data of complexes 1 and 2
CCDC:2129656,1;2129657,2.
2 Results and discussion
2.1 Crystal structure
Complexes 1 and 2 are isostructural with mono‑clinic space groupC2/c(Table 1).Selected bond lengths and bond angles are listed in Table 2 and Table 3,respectively.Both complexes feature the dimeric arrangement(Fig.1).Two metal centers are bridged by four carboxylate ligands with Eu…Eu sepa‑ration of 0.391 57(8)nm and Tb…Tb separation of 0.387 68(4)nm.The metal center is nine‑coordinated with one Phen molecule(chelatingη2mode)and three L-ions,in which three coordination modes can be confirmed:μ3∶η2‑η1,μ2∶η1‑η1,andη2.Thus,the coordi‑nation polyhedron of Ln is a distorted mono‑capped square antiprism,which can be widely observed in the published lanthanide complexes.The Eu—O bond lengths ranging from 0.236 0(3)to 0.254 5(3)nm and Tb—O bond lengths ranging from 0.232 4(3)to 0.251 9(3)nm agree with the reported single bond values in the carboxylate lanthanide complexes[22‑24].As is apparent,the O—C—O bond angle ofμ2∶η1‑η1mode(126.3(5)°)is larger than those ofμ3∶η2‑η1mode(120.6(5)°)andη2mode(121.8(5)°).It should be noted that the molecular structures of complexes 1 and 2 are highly consistent with the published complexes[RE(L)3(Phen)]2·nH2O(RE=Nd,Sm,and Y;for Sm and Nd:n=2;for Y:n=0)[16].
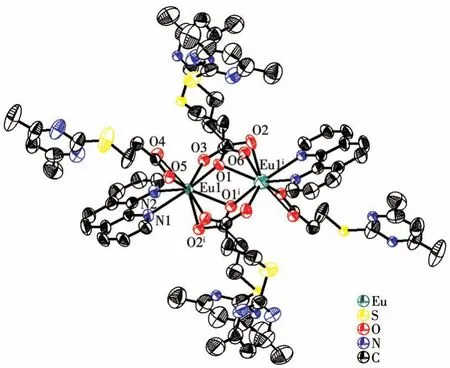
Fig.1 Drawing of the molecular structure of dinuclear Eu complex 1 with an ellipsoid probability of 50%
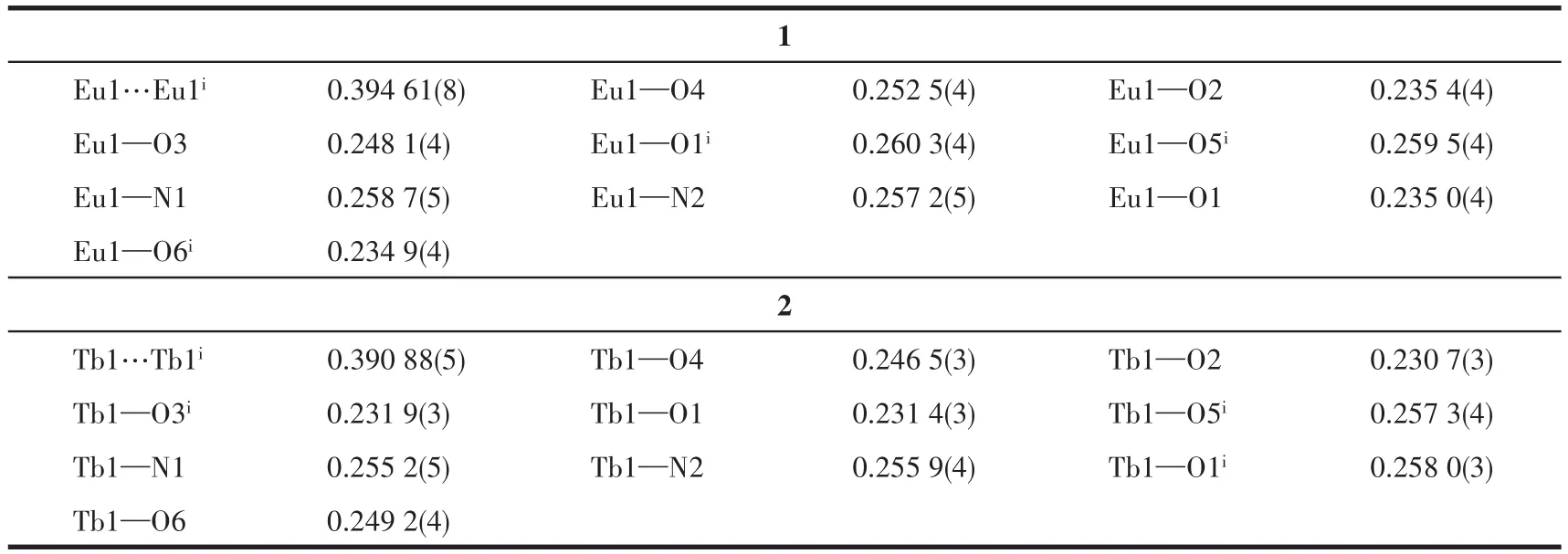
Table 2 Selected bond lengths of complexes 1 and 2
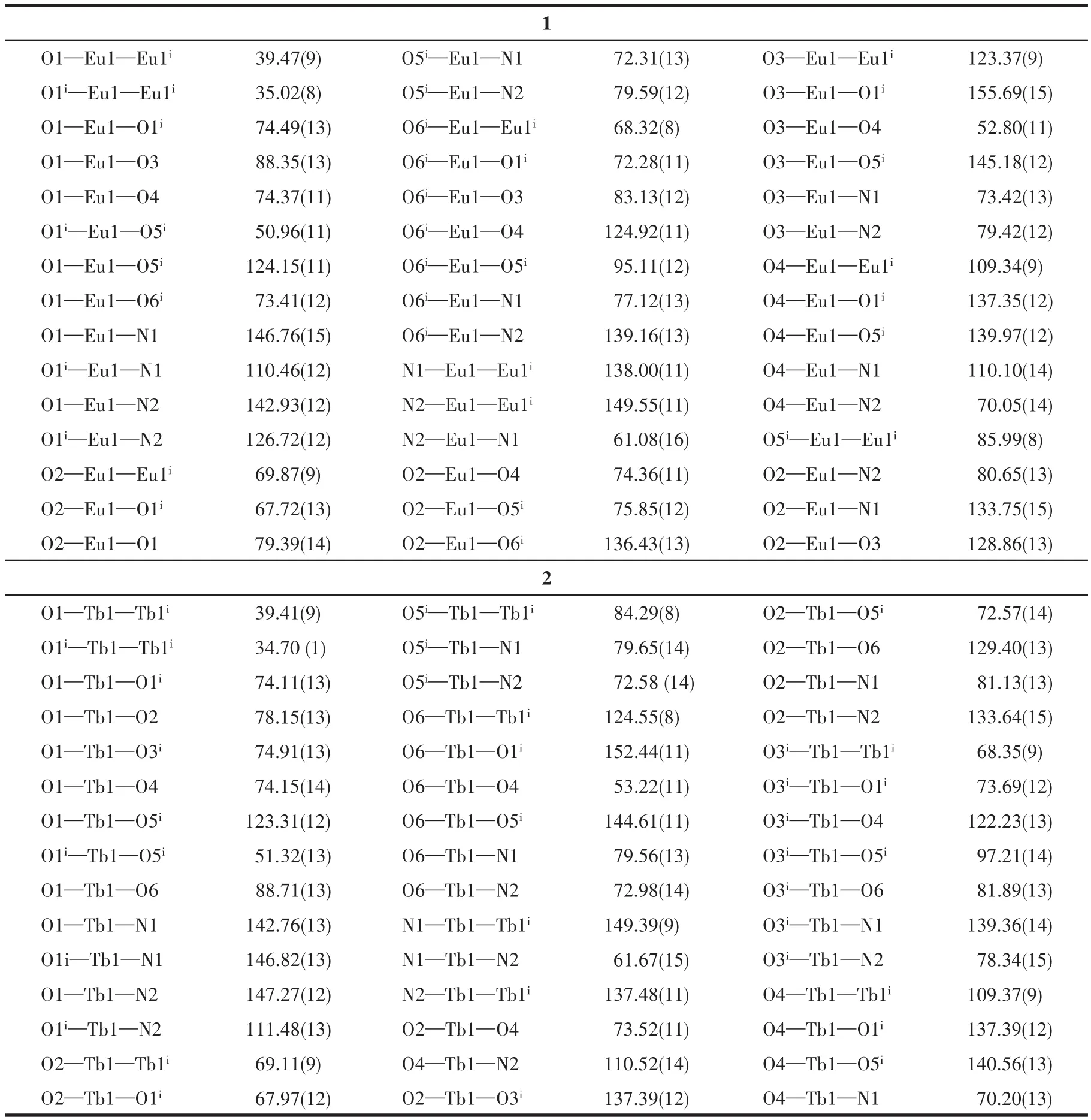
Table 3 Selected bond angles of complexes 1 and 2
2.2 TG analysis
The TG properties of complexes 1 and 2 were measured from 25 to 1 000℃(Fig.2).They showcase almost identical TG curves.Thus,only complex 1 will be discussed in detail here.A slight weight loss can be observed before 225℃,which may indicate the remov‑al of two water molecules(Obsd.1.3% ;Calcd.1.8% )in the sample.These results agree with the single crystal X‑ray diffraction analyses and the published isostruc‑tural complexes[RE(L)3(Phen)]2·2H2O(RE=Nd,Sm)[16].Further weight loss can be observed at around 1 000℃.
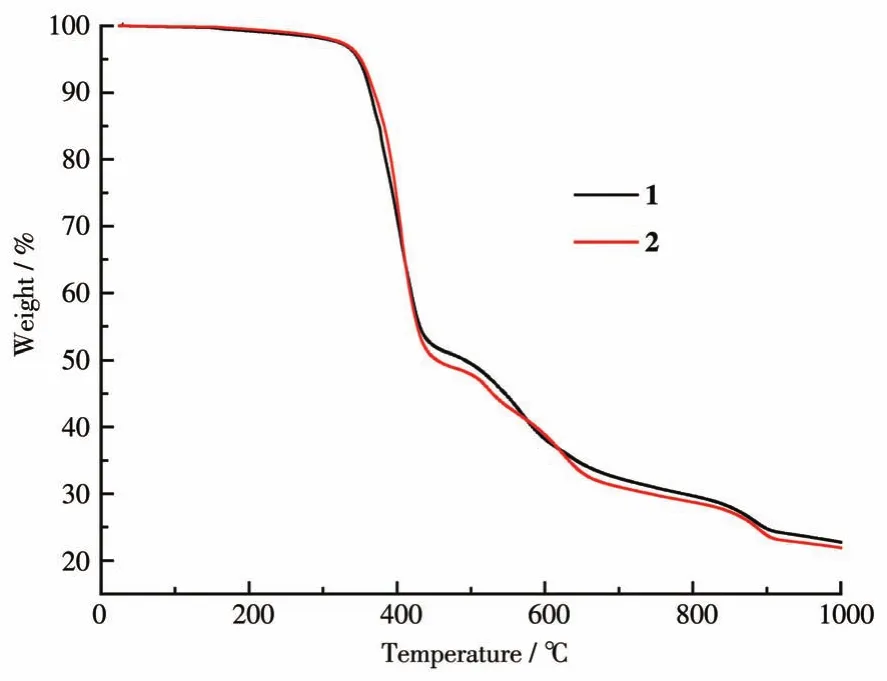
Fig.2 TG curves of complexes 1 and 2
2.3 Photoluminescent property
The solid‑state photoluminescent property of com‑plexes 1 and 2 was measured and the emission spectra are shown in Fig.3.After excited at 347 nm,three emis‑sion bands could be confirmed in the emission spectra of complex 1:593 and 595 nm(5D0→7F1transition);616,619,and 621 nm(5D0→7F2transition);689,694,and 699 nm(5D0→7F4transition).The most intense bands at 616,619,and 621 nm corresponds to the5D0→7F2transition.The intensity ratio between5D0→7F1and5D0→7F2transition indicates that the Eu is not located on the centrosymmetric site in the solid state,which agrees with the single crystal diffraction analy‑sis.The luminescent lifetime for complex 1 was 1.5 ms.The quantum yield was 87.3% .Due to the isostructural feature,the emission pattern of complex 2 was excited at 347 nm as well.Four characteristic emission bands were observed.Three weak bands at 490 nm(5D4→7F6transition),584 nm(5D4→7F4transition),and 622 nm(5D4→7F3transition)could be observed.The most intense band at 545 nm corresponds to the5D4→7F5transition.The luminescent lifetime of complex 2(1.3 ms)was almost identical to complex 1.However,the quantum yield(60.4% )was lower than complex 1.
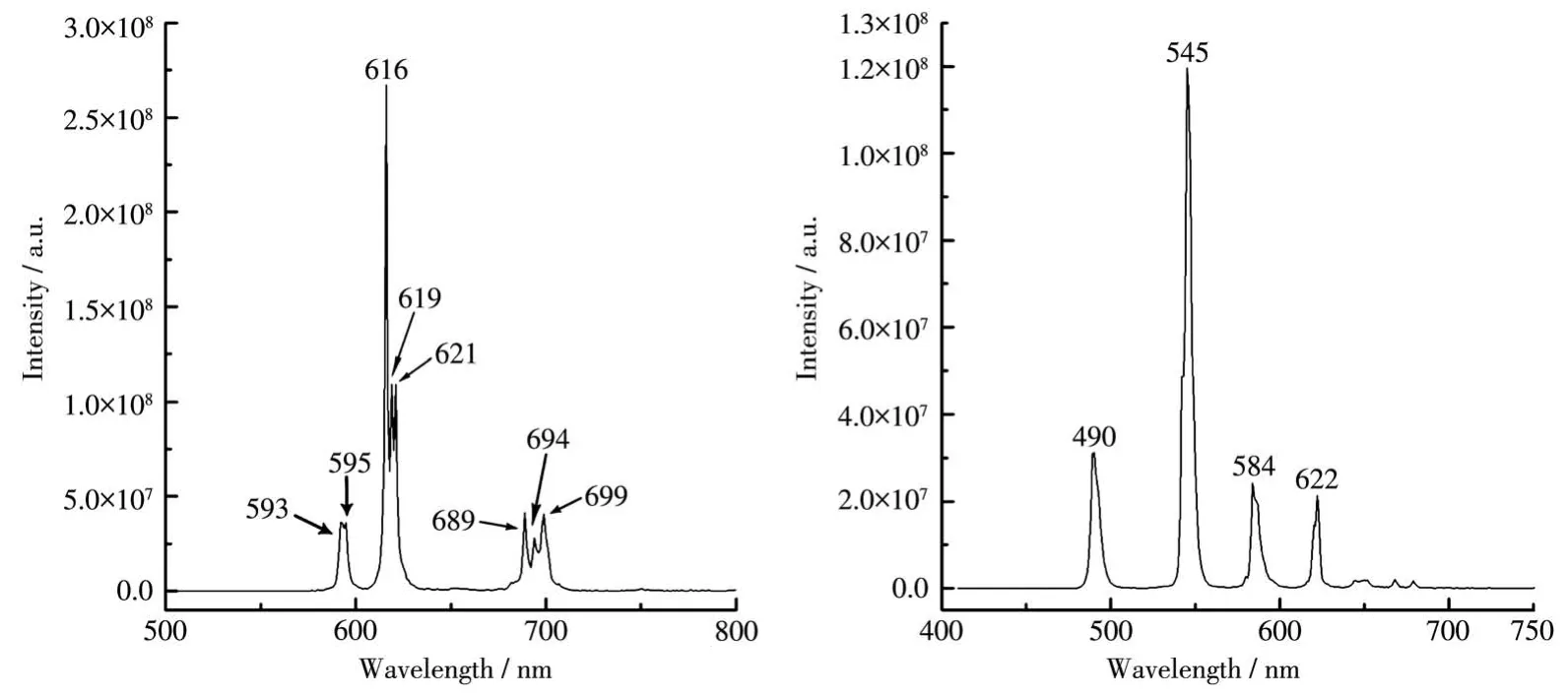
Fig.3 Solid‑state emission spectra of complexes 1(left)and 2(right)at room temperature
3 Conclusions
Two ternary lanthanide complexes of 3‑((4,6‑dimethyl‑2‑pyrimidinyl)thio)‑propanoic acid(HL)and Phen,[Ln(L)3(Phen)]2·2H2O(Ln=Eu(1),Tb(2)),were prepared and the molecular structures were established by single crystal X‑ray diffraction analysis.Both complexes feature a dimeric arrangement,in which two lanthanide metal ions are bridged by four carboxylate ligands.Solid‑state photoluminescent measurement reveals that complexes 1 and 2 manifest the character‑istic emission bands of the metal center.Complex 1 possessed a high quantum yield of 87.3% .
Conflicts of interest:There are no conflicts to declare.
