Unique Features of Zeolites Crystallized in Strongly Acidic Fluoride Medium
2023-01-31ZHAOHaonuanDlBEddySHlDiandianFUGuangyingDlNGRuiqinWANGSongxiaYANGYangLANGQiaolinYANGXiaoboFANGQianrongVALTCHEVValentin
ZHAO Haonuan ,DlB Eddy ,SHl Diandian ,FU Guangying ,DlNG Ruiqin ,WANG Songxia ,YANG Yang,LANG Qiaolin,YANG Xiaobo,FANG Qianrong,VALTCHEV Valentin
(1.The Zeo Mat Group,Qingdao Institute of Bioenergy and Bioprocess Technology,CAS,Qingdao Shandong 266101,China;2.Laboratoire Catalyse et Spectrochimie,CNRS,Caen 14050,France;3.State Key Laboratory of Inorganic Synthesis and Preparative Chemistry,Jilin University,Changchun Jilin 130012,China)
Abstract:Extending the chemistry filed of zeolite crystallization from the basic and near-neutral conditions to strongly acidic region generates a series of new features to the zeolite materials,such as substantially perfect lattices,hydrophobic surfaces,extremely high thermal stability,and predictable diffusivity.The present paper elaborates using both previously published and newly acquired data,that the acidic-medium crystallization method is generally applicable to a number of zeolites with various framework topologies,and that an unprecedentedly ordered lattice structure can be achieved for the products of such syntheses.
Keywords:zeolite crystallization in acidic medium;perfect crystalline lattice;highly ordered zeolite structure;solid-state NMR of zeolites
Zeolites are the most important adsorbents and catalysts in the oil refinery and petrochemical industry[1-2],and are increasingly applied in the processing of renewable feedstocks[3-5],in remediation of air and water pollutions[6-7],in advanced functional materials[8],as well as in medicine deliveries[9].The applications of zeolites are based on the fundament,that the uniform micropores delineated by the crystalline lattices possess unique surface and diffusion properties that discriminate molecules according to their sizes and polarities,i.e.,molecular sieving.The new applications often require that zeolites work under hitherto unexperienced conditions,such as extreme temperatures and new chemical environments,that expect the materials to possess reliable stabilities,surface characteristics and diffusion properties.Therefore,reviewing and improving the synthetic methods,in order to fulfill the ever-renewed property requirements,remain a long-standing research task[10].
Traditionally zeolites are synthesized through hydrothermal crystallizations in alkaline media of pH=9-14,where Al-rich aluminosilicate zeolites with hydrophilic surfaces and big amounts of acid sites are produced[11-12].The introduction of organic structure-directing agents(SDA),such as alkylammonium ions,into the synthetic system has been successful to create high-silica and pure-silica zeolites with hydrophobic surfaces and less but stronger acid sites[13-14].In such synthetic systems,hydroxide anion plays the role of a “mineralizer”that acts in the scenario of dissolving aluminate and silicate species from amorphous gel particles,transporting them to the growing crystal surfaces,and depositing them for the further surface growth.Due to the fast crystallization at the present of hydroxide ions the obtained crystals possess various kinds of structural defects,such as point defects of framework T atoms and associated silanol nests,broken T-O-T bridges[15],linear displacements[16],misoriented microdomains and associated domain-boundaries[17],intergrown of polymorphs[18],etc.These different types of defects may lead to uncontrollable surface hydrophilicity or random blockage of the micropores.Therefore,the development of zeolitic sorbents and catalysts relies often on trial-anderror processes,rather than on theoretical deductions from the structure.
Significant improvements of the crystal quality have been achieved,when fluoride was discovered being able to work as a mineralizer in the zeolite crystallization procedure instead of the hydroxide ions.In the “fluoride route”synthesis the zeolite crystallization occurs from the hydrogels containing the usual alumina and silica sources,the SDA,optionally an alkaline cation,and the fluoride anion at pH=5-8[19-21].The fluoride ion presents several advantages as the mineralizer,such as the generation of high-silica zeolites,large crystal sizes,hydrophobic surfaces,etc.And,most noticeably,the products of the fluoride route possess ordered lattice structures that are substantially defect-free[22-23].
These advantages become even more pronounced when the pH of the synthetic medium expands further to t he strongly acidic region[24].We have recently demonstrated a method of synthesis and morphology control for silicalite-1,high-silica ZSM-5,and some other microporous silicate materials in the pH range of 2-5[24-27].The method is based on the fact that the isoelectronic point of the silicate species in such systems bares a value of ca.pH=2.3,and above this point the paradigm applies that the positively charged SDA and negatively charged silicates interact and facilitate the crystallization of the target zeolites.The high solubility of monomeric hexafluoro-silicates in acidic solutions renders a oneby-one deposition of silicate units to the growing surfaces.Thus,the product has a perfect lattice structure without point-defects[24-25].The crystals obtained in such system has usually very big sizes at hundreds of micrometers,but can be tuned down to a few hundred nanometers through seeding by colloidal nano-crystals without ruining the highly ordered framework structures[25].
The present paper summarizes,that the acidic-medium synthesis method is generally applicable for the syntheses of high-silica zeolites with various framework topologies;that highly ordered structures and thus high hydrophobicity,high thermal stability are the common features of the acidic-medium synthesis products.Using silicalite-1 crystals of various sizes as examples,the unprecedentedly ordered structure has been analyzed in details.
1 Experimental
1.1 Synthesis of zeolites in the acidic medium
The synthesis of zeolites in the acidic medium was carried out following a recipe with the general composition 1 SiO2∶(0.3-0.5)SDA∶(0.1-0.5)HF/NH4F∶(0-0.3)HBr∶(10-50)H2O in the pH range of 2-6,where SDA stands for organic amines or ammonium ions as the structure-directing agents.The hydrothermal crystallization was performed in the temperature range of 150-200℃.Tab.1 lists the SDA,the detailed recipe composition,the hydrothermal conditions,and the corresponding crystalline zeolite obtained.For the individual synthesis,the SDA was dissolved in the appropriate solution of HF,NH4F,HBr,and H2O.Colloidal silica sol(Ludox AS40)was added dropwise under stirring.The resulting gel was stirred further at room temperature for longer than 4 hours.Then the gel was sealed in a Teflon-lined autoclave for the crystallization at the given temperature and duration.The solid product was recovered,washed with plenty w ater,and dried at 80℃in air.Calcination was performed at 550℃in air for 6 hours.Beside Ludox AS40,other silica sources were also tested,such as tetraethylorthosilicate(TEOS),fumed silica and other types of silica sols.
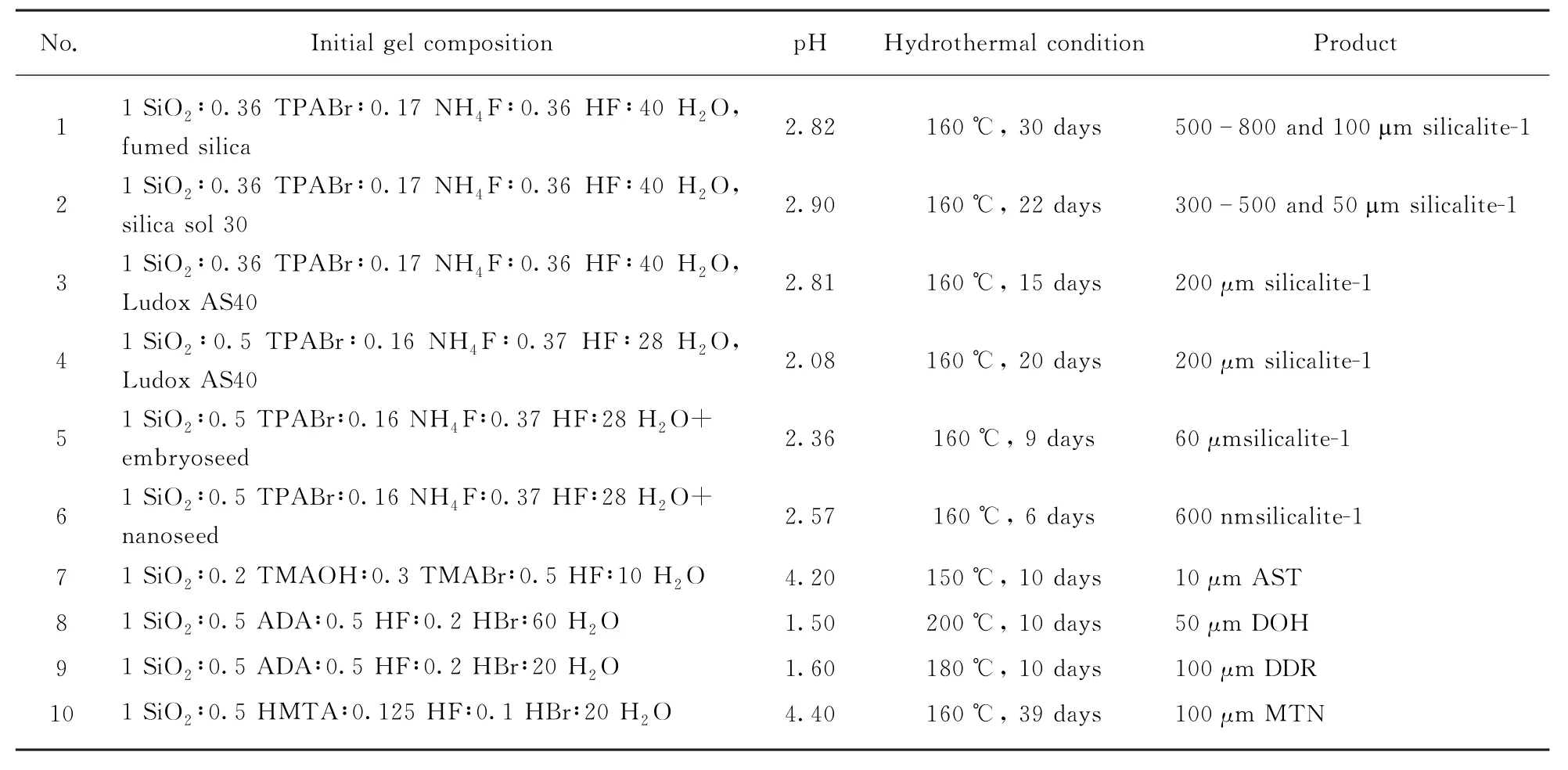
Tab.1 The synthetic recipes and corresponding products
1.2 Characterization
Crystalline products were identified by powder X-ray diffraction at room temperature in ambient air on a RIGAKU Smart Lab Diffractometer.The diffractometer uses Cu Kαradiation(λ=1.541 8Å,40 kV,150 mA)with a scanning speed of 20.00°/min at 0.01°steps in the theta-theta geometry.Samples were pressed on a glass plate.Scanning electron microscopy(SEM)of the samples were recorded on JEOL JSM-6700F microscope,which was equipped with a cold field emission gun operated at 1-2 kV.29Si{1H}and29Si{19F}cross-polarization(CP)magic-angle spinning(MAS)NMR spectra were acquired at 99.3 MHz on a Bruker Avance III-HD 500(11.7 T),using 4 .0-mm outer diameter probe spun at 12 kHz.A recycle delay of 2 s and a contact time of 7.5 ms were used.19F MAS NMR spectra were acquired using Hahn echo at 470.6 MHz on a Bruker Avance III-HD 500(11.7 T),using 4.0-mm outer diameter probe spun at 12 kHz.A recycle delay of 5 s and a nutation radiofrequency power of 67.6 kHz were used.The fitting of the NMR spectra is done using Dmfit software[28].
2 Results and discussions
2.1 Acidic medium synthesis is a generally applicable method
Silicalite-1 is the first zeolite that has been successfully crystalized in the acidic medium down to pH=- 2.3[24].Slow crystallization kinetics were observed,depending on the silica source,e.g.,the zeolite forms slightly faster out of fumed silica and a few different colloidal silica(13-30 days at 160℃)than TEOS(longer than 30 days).Due to the high solubility of hexafluoro-silicate,thus the difficulty in establishing a supersaturation state,the nucleation of the zeolite is retarded.The heterogeneity of the initial hydrogels helps the nucleation,but extreme heterogeneity of the initial gel leads also to non-uniform sizedistribution in the product.Fig.1 on page 760 shows the SEM pictures of silicalite-1 crystals obtained from different silica sources.The 15-day crystals out of Ludox AS40(a silica sol with Si/Na~200)are prismatic with obelisk-like tips on both ends.They have average dimensions of 30×30×200μm with a narrow size distribution.Two populations of crystals were observed in the product issued from Silica sol 30(a silica sol with Si/Na~100).The great majority of the crystals were 300-500μm large.A population of smaller(50-100μm)crystals was detected as well.Many of the smaller crystals are intergrown.The crystals obtained within 30 days using fumed silica as the silica source also show bimodal size distribution.The bigger ones exhibit a length of 500-800μm,and the smaller intergrown ones are 100μm long[25].

Fig.1 SEM images of silicalite-1 crystallized in acidic medium using
The very slow crystallization can be accelerated,and the giant crystal sizes that are undesired in adsorption and catalysis due to diffusion limitations can be reduced,when seeds are added in the initial gels.The SEM images in Fig.2 show that the initial 200μm long crystals can be made smaller to 60μm when zeolite embryos are used as seeds;to ca.600 nm when seeded with the colloidal nano-zeolite.The smaller crystals are sword-blade shaped plates with various thicknesses casional intergrowths are observable[25].

Fig.2 SEM images of silicalite-1 crystallized in acidic medium using Ludox AS40 as the silicon source
The acidic medium synthesis method is not only applicable to silicalite-1 but has also been successfully applied to the crystallization of a few other zeolitic materials with different framework topologies,when respective SDAs are employed.Fig.3 shows the XRD patterns of the clathrasil materialASTcrystallized in 10 days at the presence of TMA+at pH=4.2 andT=150 ℃;DOHat the presence of 1-aminoadamantane at pH=1.5 andT=200 ℃;DDRat the presence of 1-aminoadamantane at pH=1.6 andT=180℃.In the SEM pictures in Fig.4,ASTare octahedral crystals with edge lengths around 10μm;DOHare hexahedral prisms of ca.50μm;DDRare 100μm large octahedra.

Fig.3 XRD patterns of the zeotype materials crystallized in the acidic medium using various

Fig.4 SEM images of the zeotype materials crystallized in the acidic medium
The common phenomena in these syntheses are the slow formation of big crystals.In the slow crystallization procedures,the thermodynamic stability of the SDA@framework compounds plays an overwhelming role in the phase selection.Simulated annealing indicates that the heat of formation of the TMA+@AST compound out of the host framework and the SDA guest is 10.5 kcal/mol;ADA@DOH 26.4 kcal/mol;ADA@DDR 27.6 kcal/mol;and TPA+@MFI 41.8 kcal/mol.TPA+@MFI has the highest thermodynamic stability,therefore has become the easiest one to crystalize[26].
It is expected that in the extended chemistry field of zeolite crystallization new SDAs and new chemical building blocks can be discovered that has not worked previously in the basic and neutral systems but only become feasible in the acidic medium.The crystallization ofMTNtype clathrasil using urotropine as an SDA in the acidic medium provides a first successful example.
Urotropine,or hexamethylene-tetramine(HMTA),with its C/N ratio of 1.5 is a very hydrophilic molecule which does not possess enough power to interact with silicate precursors and facilitate zeolite crystallization[29-30].However,in the acidic medium,specifically in hydrogels with pH lower than the isoelectric point of the molecule,which has been reported as 8.85,5.18 and 4.89 for various solutions[31-33],HMTA become mono-protonated to a cation HMTA+.The cation can interact properly with silicate species,and become a very robust SDA that promotes the crystallization ofMTNzeolite in a very broad range of gel compositions,i.e.,pH=4-7 and H2O/SiO2=3-80[27].Fig.5 shows the XRD of selected crystals obtained at various pH.The crystallizations occurred at 160℃in 21-39 days at very slow paces to big crystals.The lower the pH value,the longer the crystallization time.

Fig.5 XRD patterns of MTN-type zeolites crystallized at various pH
Fig.6 shows the corresponding SEM images.At the lower pH the crystals grow into big agglomerates of giant octahedra with edges closing 100μm.

Fig.6 SEM images of MTN-type zeolites crystallized at various pH(Reproduced from Ref[27]with permisson from the Royal Society of Chemistry)
2.2 The acidic-medium synthesized crystals have highly ordered structures
It is generally recognized,that high-silica zeolites synthesized via the “fluoride route”in neutral systems have fewer point defects and better integral frameworks than the materials synthesized in traditional basic media[22-23,34].This fact has been proven,e.g.,by29Si MAS NMR spectroscopy,which shows higher resolutions of the Q4(Si-(O-Si)4)signals[35]for the materials obtained via the fluoride route[36-37].In strongly acidic medium the produced zeolite crystals exhibit even higher29Si MAS NMR resolutions than the products in the neutral medium.
Fig.7 shows the29Si{1H}CP MAS spectra of the silicalite-1 samples of various sizes crystallized in the acidic medium,i.e.,the crystals made with Ludox and have lengths of 200μm,60μm,and 600 nm.For comparison,the spectrum of a 50μm sample made in the neutral medium are given in the sa me figure(synthesized according to Ref.[22]).For all four samples,the Q4sites are well resolved,and few or no Q3sites are observed.Because of the presence of F-anions,the siloxyl/silanols defects,which are often observed in the materials synthesized in the traditional basic medium using hydroxide anions as a mineralizer to balance the positive charge of the TPA+cation[14],are no longer necessary.The spectral resolution of the samples synthesized in the acidic media is clearly higher than the one observed for the sample synthesized in neutral pH.Furthermore,looking closer to the three acidicmedium synthesized samples,there is a decreasing local order with the smaller crystal sizes.However,even the smallest acidic medium synthesized crystals(ca.600 nm)have a significantly higher local structure order than the neutral counterpart of a much bigger size.
19F MAS NMR in Fig.7 shows the same order of peak-broadening with the decreasing sizes of the acidic-medium crystals.And th e peak for the neutral crystallized sample is broader than all acidicmedium materials.Furthermore,in all samples,the fluoride anions occupy the [415262]cage[38].Correspondingly,there is a broad resonance at-125 ppm observable in the29Si NMR spectra,which is the 5-coordinated Si including the weak bond to F-[36].

Fig.7 29 Si and 19 F MAS NMR spectra of silicalite-1 crystals synthesized in acidic and neutral media
Fig.8 displays29Si MAS NMR spectra of the other zeotype materials crystallized in the acidic medium,showing well resolved peaks,thus high local structural orders,too.The29Si MAS NMR of theASTsample synthesized at pH=4.2 exhibits two well-resolved sharp Q4(Si-(O-Si)4)peaks with an area ratio of 4∶1 atδ=-106.15 and -107.47 ppm.In the I4/m(#87)unit cell,ASThas two unique framework T positions:Si1 on the general position of(0.232 8,0.045 3,0.112 74)and Si2on the special position(0,0.5,0.25).The multiplicities of these two sites are 16 and 4,respectively[39-40].The NMR curve matches exactly this ratio,i.e.,the sample has a perfect framework.

Fig.8 29 Si MAS NMR spectra of the zeotype materials crystallized in the acidic medium
TheDOHsample shows a good resolution of Q4peaks,too.The P/mmm(#191)unit cell has four unique T sites.Three corresponding Q4peaks are resolved: δ=-115.12 ppm is the resonant of Si2/Si1;δ=-117.48 ppm is Si1/Si3/Si4;δ=-120.76 ppm is Si3[41]
The spectrum ofDDRis less resolved in the Q4region.Three Q4peaks can be recognized,while the rhombohedral R-3m(#166)unit cell has seven unique T sites.Expected Q4signals should populated in two groups,i.e.,T6,T3 and T4 around -119 ppm,and T2,T1,T5 and T7 around -113 ppm.[42]The broaden peaks are resolvable in 2 or 3 bundles.Unfortunately,there is an observable Q3(—Si—(O—Si)3)peak atδ-100 ppm,indicating the existence of point defects in this particular material.
The29Si and19F MAS NMR ofMTNare illustrated in Fig.9.There are three distinctive Si atoms in the cubic Fd3m(#227)unit cell ofMTN,corresponding to the three well-resolved Q4peaks in29Si MAS NMR at δ=-111.19,-115.52,and -118.87 ppm[43].In addition,there is a small Q3peak observable atδ=-101.65 ppm.The peak area of the Q3signal covers ca.2% of the total Si amount.And,19F MAS NMR shows that F atoms bond multiply to Si.Therefore,Q3Si arises from broken Si—O—Si bonds,which are terminated by the direct bonding of Fx—Si[27].

Fig.9 29 Si and 19 F MAS NMR spectra of MTN crystallized in the acidic medium(Reproduced from Ref[27]with permisson from the Royal Society of Chemistry)
2.3 The unprecedentedly perfect framework of silicalite-1
Taking the 600 nm silicalite-1 as an example,XRD shows that at the as-synthesized state it has an orthorhombic symmetry.In the space group Pnma(#62),it has 12 crystallographically unique T atoms with an equal occupancy.Fitting to the29Si{1H}CP MAS NMR spectrum,all 12 Si atoms are resolvable at equal peak areas(Fig.10)[44].This is a record resolution that has never been achieved before forMFItype zeolites.It means that the lattice structure of this material possesses an unprecedented perfection.

Fig.10 29 Si{1 H}(left)and 29 Si{19 F}(right)CP MAS NMR spectra of the 600 nm silicalite-1 crystallized in acidic medium,and fitted Si sites
In the29Si{1H}CP MAS NMR spectrum there is a broad and weak resonance around-125 ppm.It corresponds to the Si atoms of the [415262]cage,and weakly bond to the occluded F-ion in the cage with the“5th”coordinates[36].This configuration becomes pronounced in the29Si{19F}CP MASspectrum in Fig.10.An obvious broad peak can be 1℃ated at -127.40 ppm.
Perfect crystals are more stable than defective ones.Fig.11 shows the XRD of the 600 nm silicalite-1 crystallized in the acidic medium at temperatures from RT to 1 450 ℃in air,in comparison to the one crystallized in the traditional basic medium synthesized according to the reference[45].The acidic-medium product changes the symmetry from orthorhombic to monclinic upon the removal of SDA,and collapses between 1 350 to 1 400 ℃.The basic-medium product keeps monclinic only to 1 300-1350 ℃where the framework collapses.

Fig.11 XRD patterns acquired at temperatures from RT to 1 450℃of silicalite-1 crystallized in acidic(left)and basic(right)media
3 Conclusions
The crystallization of zeoliteMFIand several other topologies in acidic media at pH down to ca.2.0 was successfully conducted using different silica sources and SDAs.The method is generally applicable to the synthesis of high-silica and pure-silica zeotype materials.During the crystallization of zeolite in the acidic medium,the isoelectric point of gel particles was found to be the critical parameter determining the affinity between SDA and silicate species,which facilitates the crystallization.
The crystals synthesized in the acidic medium exhibit high local structure ordering.The silicalite-1 obtained in the acidic medium exhibits an unprecedentedly perfect lattice.It is important,that perfect crystals possess high stability,high surface hydrophobicity and predictable diffusion properties.
Usually,the crystals obtained from the slow crystallization are big in size.But a substantial reduction of the crystal sizes and the crystallization time can be achieved by seeding the initial system.For example,seeding with colloidal nano-zeolites leads to silicalite-1 of a few hundred nanometers.
The extension of the chemistry field of zeolite crystallization enables applying new SDA and new structural building blocks that has been unsuitable for the traditional basic and neutral systems in the zeolite crystallization,and offers opportunities to discover zeolites of new topologies and new compositions.
