A Ru@Zeolite Catalyst for Highly Selective Synthesis of Primary Amines fromAlcohol Amination
2023-01-31HUANGLiangMElHuaqianLlSongbaiYANZhenSTRElFFStephaneSHENWeiXUHualong
HUANG Liang ,MEl Huaqian ,Ll Songbai ,YAN Zhen ,STRElFF Stephane ,SHEN Wei ,XU Hualong
(1.Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials,Department of Chemistry,Fudan University,Shanghai 200438,China;2.Laboratory of Advanced Materials,Fudan University,Shanghai 200438,China;3.Collaborative Innovation Center of Chemistry for Energy Materials,Shanghai 200438,China;4.Eco-Efficient Products and Processes Laboratory,UMI 3464 CNRS-Solvay,Shanghai 201108,China)
Abstract:The direct amination of aliphatic alcohols with ammonia is an environment-friendly route for the synthesis of primary amines.In this work,LTA zeolite-encapsulated Ru nanoparticles(Ru@LTA)were successfully synthesized and employed for the direct amination of 1-octanol with NH3 into octylamine at low NH3/alcohol ratios(2.1-2.4).A 76% selectivity to octylamine was obtained over a 5Ru@KA zeolite catalyst.The positive effect of Ru@LTA zeolite catalysts on primary amine selectivity stems from the steric hindrance.
Keywords:alcohols;amination;shape-selectivity;zeolite
Primary amines are extensively used in the production of agrochemicals,pharmaceuticals,organic dyes,and other fine chemicals[1].Up to now,many methods have been developed for the production of primary amines,including amination of alkyl halide compounds and ester compounds,direct reduction of nitriles,direct amination of aliphatic alcohols,and reductive amination of aldehydes or ketones[2].Among these processes,the direct amination of alcohols is an atom-efficient and environment-friendly route,and has brought widespread attention in recent years because a wide variety of biomass-based alcohols become available due to the development of green chemistry[3-5].The direct alcohol amination reactions operate via a “hydrogen borrowing”mechanism relying on metal catalysts,including Ni[6-9],Co[10-12],Ag[13],Ru[14],Pt[15],and Pd[16].The catalyst “borrows”H2from alcohols and“auto transfers”it to the intermediate imine to form primary amines.Because water is the only stoichiometric by-product,the route is more environment-friendly in comparison with the amination of alkyl or aryl halides[17].
Compared with non-noble metal catalysts,noble metal catalysts,especially Ru catalysts,exhibited higher activity[18].Recently,great efforts have been paid to prepare Ru-based catalysts for alcohol amination reactions,including homogeneous and heterogeneous Ru catalysts.Hamid et al[19]reported a facile method for the alkylation of primary amines with primary alcohols using [Ru(p-cymene)Cl2]2.Luo et al[20]successfully synthesized unsymmetrically substituted tertiary amines from primary alcohols and aliphatic tertiary amines by hydrated RuCl3.Supported heterogeneous Ru catalysts have the potential to realize industrial catalytic processes in comparison with homogeneous Ru-based organometallic catalysts and attract more attention.Yamaguchi et al[21-22]investigated heterogeneous Ru catalysts(Ru(OH)x/Al2O3and Ru(OH)x/TiO2)for the N-alkylation of amines.The results showed that Ru(OH)x/Al2O3catalyzed the formation of secondary amines while Ru(OH)x/TiO2catalyzed the formation of tertiary amines.Cano et al[23]reported that the N-alkylation of amines with benzylic alcohols could be achieved by Ru/Fe3O4.
For the synthesis of primary amines from the direct amination of alcohols with ammonia,the selectivity is always an issue due to the formation of secondary and tertiary amines,which are thermodynamically more favorable.Although relatively high selectivity can be obtained at high NH3/alcohol ratios(>4),it will result in high process pressures(>40 bar)which is not economic for the industry.Therefore,it is of interest to develop a mination catalysts which are selective at low NH3/alcohol ratios(<3).However,on conventional catalysts,the selectivity to primary amines drops sharply with the decrease in NH3/alcohol ratios,and poor selectivity to primary a mines(<30%)is often obtained at low NH3/alcohol ratios[24].Recently,a variety of efforts have been made to improve the selectivity.Liang et al[25]investigated the effect of the size of Ru nanoparticles on the octylamine selectivity in 1-octanol amination.The results showed that the self-coupling of octylamine was sensitive to the size of Ru nanoparticles,and a lower rate of self-coupling was obtained over smaller Ru nanoparticles.Ibáñez et al[12]prepared a series of Co-based bimetallic catalysts and found that Ru and Ag had positive effects on both the activity and selectivity.Niemeier et al[26]studied the influence of catalyst support and solvent.No significant differences between inorganic supports and activated carbon were observed,while water is a suitable solvent for the catalytic amination of alcohols.Ho et al[27]adopted hydroxyapatite(HAP)supported Ni catalysts for propanol amination to propylamine.The superior activity of Ni/HAP was attributed to the basic sites on hydroxyapatite,which are responsible for suppressing the self-coupling of propylamine.
Despite recent developments,the design of intrinsically selective catalysts for the synthesis of primary amines from alcohols at low NH3/alcohol ratios is still an unsolved challenge.In our previous work,we prepared carbon deposited Co catalysts and found that the positive effect on the primary amine selectivity stems from inhibiting the adsorption of bulky secondary imines due to steric hindrance[24].Zeolites are classical micro porous mineral materials,which have been widely used for shape-selective reactions.From the catalytic perspective,confinement of noble metal nanoparticles in a zeolite is a promising way to prepare shape-selective catalysts.Recently,methods of preparing metal@zeolite catalysts have been developed rapidly[28-31].These materials have zeolite micropores with different pore sizes to sterically control substrate molecule adsorption and diffusion on the external surface of metal nanoparticles.These features lead to a significant variation of product distributions in various metal-catalyzed reactions,outperforming traditional supported metal catalysts[32-34].
Herein,we report the first example of controlling product distributions in alcohol amination by zeolite crystals.Ru nanoparticles encapsulated inside microporous LTA zeolites have been employed for the direct amination of 1-octanol with NH3into octylamine at low NH3/alcohol ratios(2.1-2.4).This approach can be further extended to the selective synthesis of other highly valuable primary amines.
1 Experimental
1.1 Catalyst preparation
Ru@LTA zeolites were prepared as previously described[35].Typically,a mixture of NaAlO2(8.5 g)and Na2SiO3(28.1 g)were dissolved in 50 mL deionized water and stirred thoroughly for 2 h.Then,a predetermined amount of RuCl3in 25.0 mL of deionized water was added dropwise to the gel and stirred thoroughly for 2 h.After thorough homogenization,the gel was transferred into a 180 mL Teflon-lined stainless steel autoclave and heated at 120℃for 24 h.The resulting product was filtered off,washed with deionized water and dried before calcination at 400℃in air for 2 h.The 5Ru@KA and 5Ru@Ca A samples were prepared by ion-exchange of the 5Ru@Na A sample three times with KNO3and Ca(NO3)2solution,respectively.Then,the solids were washed and dried before calcination at 400℃in air for 2 h.
To prepare 5Ru@Na A-HF,a certain amount of 5Ru@Na A sample was added to 5wt% HF solution.After stirring at room temperature for 30 min,the mixture was centrifuged,washed with a large amount of deionized water,and dried at 100℃overnight to obtain final product.
Ru/Na A pre pared by traditional impregnation method(denoted as 5Ru/Na A)was employed as the reference catalyst .Commercial Ru/C and Ru/Al2O3catalysts were also employed as reference catalysts(denoted as 5Ru/C and 5Ru/Al2O3).
1.2 Catalyst characterization
The crystalline structures were analyzed by X-ray diffraction(XRD)on a Bruker D8 Advance diffractometer equipped with Cu Kαradiation(40 kV and 40 mA).The textural characteristics were determined by nitrogen adsorption-desorption using a Quantachrome AUTOSORB-IQ instrument.The morphology was obtained by field emission transmission electron microscope(TEM)on a JEOL JEM-2011 microscope,operating at an acceleration voltage of 200 kV.The chemical composition was determined by X-ray fluorescence(XRF)with a Bruker-AXS spectrometer.The X-ray photoelectron spectroscopy(XPS)measurements were recorded on KRATOS Axis Ultra DLD equipped with a monochromatic X-ray source.Prior to the above characterizations,the samples were pre-reduced in a H2flow at 350℃for 2 h.The redox properties were evaluated by H2temperature-programmed reduction(TPR)using a Micromeritics AutoChemⅡ2 920.The samples were heated from 50 to 900 ℃with a ramping rate of 10℃/min.O2titration was also performed on a Micromeritics AutoChemⅡ2 920.A 0.05 g sample was pre-reduced with a H2flow at 200℃for 1 h and then cooled to room temperature.The chemisorption of oxygen on the samples was performed by 0.5 mL pulses of 5% O2in He until full saturation was achieved.
1.3 Catalytic reactions
The catalytic tests were conducted in a stainless steel high-pressure autoclave(30 mL).The catalysts were pre-reduced under a flow of H2(30 mL/min)at 350 ℃for 2 h.Typically,100 mg of catalyst and 1.0 mL of 1-octanol were added to the autoclave.Afterward,the reactor was pressurized with 5 bar of NH3and 5 bar of H2.The reaction temperature was 180 ℃and the stirring rate was 800 rpm.The liquid products were analyzed on an Agilent 7 890 B gas chromatograph equipped with a HP-5 column and a FID detector using biphenyl as the external standard.The hydrogenation of 12-tricosanone was also conducted in a stainless steel high-pressure autoclave(30 mL).Typically,10 mg of catalyst and 339 mg of 12-tricosanone were added to the autoclave and then the reactor was pressurized with 40 bar of H2.The reaction was conducted at 140℃for 3 h.
2 Results and Discussion
Fig.1 shows the XRD patterns of Na A,5Ru/NaA and 5Ru@LTA(5Ru@Ca A,5Ru@NaA and 5Ru@KA)catalysts.The 5Ru@LTA and 5Ru/Na A exhibit XRD peaks associated with a typical LTA zeolite structure[36].5Ru@LTA catalysts exhibit lower crystallinity in comparison with Na A,which means the addition of RuCl3during crystallizing process has a negative effect on zeolite crystallinity.The 5Ru/Na A catalyst shows XRD patterns characteristic of metallic Ru at 2θ=38.4°and 44.0°.On the other hand,it is difficult to observe the same peaks over 5Ru@LTA zeolites,indicating that the Ru nanoparticles may be too small or encapsulated in the zeolites.

Fig.1 XRD patterns of 5Ru@Na A,5Ru@KA,5Ru@Ca A,5Ru/Na A and Na A
Fig.2 shows TEM images of 5Ru@NaA and 5Ru/Na A.In 5Ru@Na A,Ru nanoparticles were not observed on the external surface of zeolite crystals,suggesting that the Ru nanoparticles are probably encapsulated within the zeolite crystals as core-shell structures.By counting more than 100 nanoparticles(Fig.3),it was found that the size of Ru nanoparticles in 5Ru@Na A ranged from 1 nm to 6 nm.The narrow distribution of particle sizes could be reasonably assigned to the unique core-shell structure of 5Ru@LTA zeolites,where the zeolite shell prevents the aggregation and sintering of Ru nanoparticles.In the sectioned view of 5Ru/Na A,the Ru nanoparticles are found only on the external surface of LTA zeolite crystals and the size of most Ru nanoparticles is above 100 nm,indicating that the Ru nanoparticles are located on the external surface of the LTA zeolite crystals.

Fig.2 TEM and STEM images of 5Ru@Na A(a,b)and 5Ru/Na A(c,d)
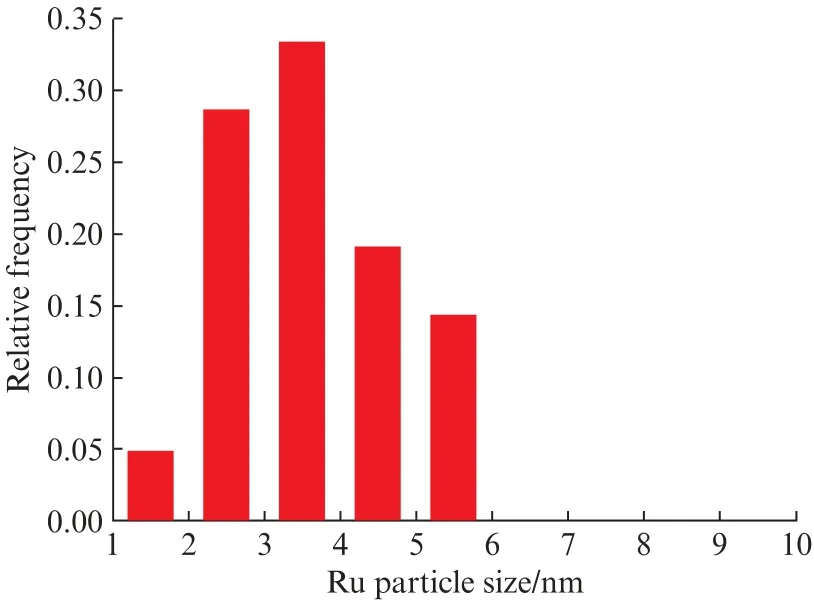
Fig.3 Histograms of the Ru particle size in 5Ru@Na A
The Ru 3p XPS spectra(Fig.4)of 5Ru/Na A and 5Ru/C clearly indicate the presence of metallic Ru.However,no obvious signal could be observed on 5Ru@Na A,5Ru@KA and 5Ru@Ca A,which have the same Ru loading as 5Ru/Na A and 5Ru/C.It proves indirectly that the Ru nanoparticles may be encapsulated in the zeolites.

Fig.4 Ru 3p XPS spectra of 5Ru@Na A,5Ru@KA,5Ru@Ca A,5Ru/NaA and 5Ru/C
Furthermore,the hydrogenation of 12-tricosanone was used as a probe reaction to assess the encapsulation of Ru nanoparticles.It was found that the conversion of 12-tricosanone was zero on 5Ru@Ca A,5Ru@Na A,and 5Ru@KA,but 32% on 5Ru/Na A under the same condition.The fact that 5Ru@LTA zeolites are completely inactive to 12-tricosanone hydrogenation is consistent with the encapsulation of Ru particles inside LTA zeolites,preventing bulky molecules such as 12-tricosanone from accessing the active metallic sites[35].On the contrary,5Ru/NaA is active for the hydrogenation of 12-tricosanone,because the Ru nano-particles on the external surface of LTA crystals can be easily accessed by 12-tricosanone molecules.The combined evidence from 12-tricosanone hydrogenation and TEM images indicate the successful fixing of Ru nanoparticles into the LTA crystals.
The physiochemical properties of 5Ru@LTA zeolites and 5Ru/NaA are provided in Tab.1.The Ru loadings are 4.87%,4.75% and 4.67% for 5Ru@NaA,5Ru@KA and 5Ru@CaA,respectively.For comparison,5Ru/Na A shows a similar Ru loading of 5.13%.All the samples have high surface areas(380-425 m2/g)and large pore volumes(0.17-0.21 cm3/g),indicating the successful formation of LTA zeolite crystals,which is in good agreement with the XRD results in Fig.1.The H2-TPR profiles of 5Ru@LTA zeolites and 5Ru/Na A are presented in Fig.5.The 5Ru/Na A displays one characteristic peak in the temperature range of 150-250℃,which is attributed to the one-step reduction of Ru O2to metallic Ru.The reduction behavior of 5Ru@LTA zeolites shows a significant difference,with extra peaks at high temperature between 250℃and 650℃.The observed high-temperature peaks are most likely linked to the reduction of RuO2inside the zeolites,which was delayed to higher temperature due to the nature of RuO2with stronger interaction with zeolites(Ru silicates)[37-38].
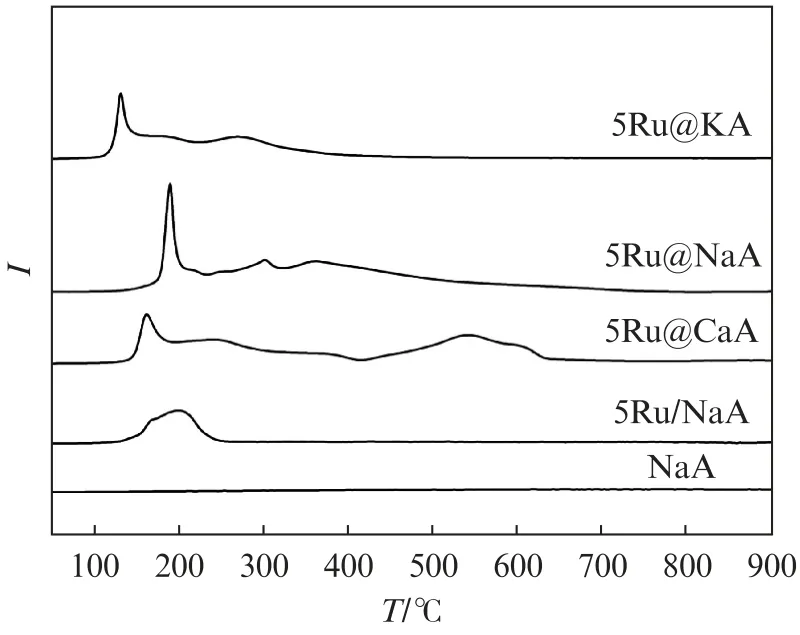
Fig.5 H2-TPR profiles of 5Ru@LTA zeolites and 5Ru/NaA

Tab.1 Physiochemical properties of 5Ru@LTA zeolites and 5Ru/NaA
The catalytic activity and product distribution for the direct amination of 1-octanol with NH3at low NH3/alcohol ratios(2.1-2.4)are shown in Fig.6.The main products include octylamine(OA),dioctylamine(DOA)and trioctylamine(TOA).The commercial catalysts(5Ru/C and 5Ru/Al2O3)are very active for alcohol amination,reaching a conversion of 56% and 59% in just two hours,respectively;however,the octylamine selectivity is quite low(32% and 37%,respectively)due to the formation of DOA.When the reaction was carried out over 5Ru@LTA zeolites at similar conversions,all of them show higher octylamine selectivity.The selectivity to octylamine on 5Ru@KA is 76%,much higher than those of commercial supported Ru catalysts.In contrast,the 5Ru/Na A shows similar conversion for the same reaction time,but very low octylamine selectivity(26%).When the NH3/alcohol ratio was increased from 2 to 4,the octylamine selectivity can reach 100% over 5Ru@KA at the expense of a lower conversion(~20%).
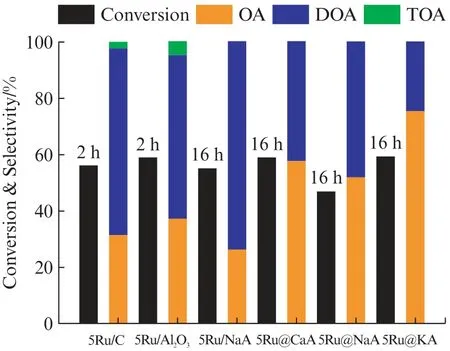
Fig.6 Catalytic performances of all catalysts
It is generally accepted that dioctylamine is produced by the self-coupling reaction of octylamine[25].Octylamine is first dehydrogenated to the corresponding imine,which reacts with another octylamine to form dioctylamine and release NH3.Dioctylamine could further react with the imine to form trioctylamine[39].According to the mechanism,these bulky molecules(dioctylamine and trioctylamine)could be formed on the exposed Ru surface of conventional supported Ru catalysts,and the selectivity to octylamine will be markedly reduced.However,it is difficult to form dioctylamine in the zeolite micropores with sizes of 0.3-0.5 nm over 5Ru@LTA zeolites.The positive effect on the octylamine selectivity stems from the steric hindrance in the hydrogenation of the bulky secondary imine.Considering that the 5Ru@LTA zeolites and supported Ru catalysts have similar Ru loadings,it is reasonable to believe that the high octylamine selectivity over the 5Ru@LTA zeolites is directly attributed to the coreshell structure.In order to demonstrate the importance of the LTA zeolite as a shell for controlling the product selectivity in alcohol amination over the Ru@LTA zeolites,5Ru@Na A-HF was tested.After partial destruction of the LTA zeolite framework in the 5Ru@NaA,an octylamine selectivity of 4% was obtained after 16 h,significantly lower than the 47% selectivity over the original 5Ru@Na A.
In addition,5Ru@LTA zeolites show relatively low activity,which are due to the complexity of diffusion pathways,lower diffusion rate and fewer accessible Ru atoms[40].O2pulse titration was used to estimate accessible Ru atoms.Assuming that one Ru atom adsorbs one O atom,the accessible Ru values for 5Ru/Al2O3,5Ru/C,5Ru@Na A,5Ru@Ca A,5Ru@KA and 5Ru/Na A are 28%,27%,7%,5%,4% and 0.5%,respectively.All the Ru@LTA catalysts have fewer accessible Ru atoms due to zeolites coating.5Ru/Na A exhibits the minimum value,which is consistent with the large size of Ru particles(100-200 nm)in this catalyst.
We report a novel strategy to control the selectivity to primary amines in the direct amination of 1-octanol with ammonia by encapsulating Ru nanoparticles inside LTA zeolites.The Ru@LTA zeolites exert excellent shape-selectivity of zeolite micropores,giving an octylamine selectivity as high as 76% at low NH3/alcohol ratios(2.1-2.4).The positive effect on the primary amine selectivity stems from the steric hindrance in the hydrogenation of bulky secondary imines,which are intermediates to secondary amines.This method provides a simple and atom-efficient method for the synthesis of primary amines.
