磁粒子成像示踪剂的研究进展
2023-01-25张庆鹏关国强刘慧怡宋国胜
张庆鹏,关国强,刘慧怡,陆 畅,周 颖,宋国胜
(湖南大学化学化工学院,长沙 410082)
1 Introduction
The development of biomedical imaging technology is of great significance,helping people to explore and study living systems,structures and physiological processes,and helping to diagnose and treat various diseases in clinical practice[1].Although a variety of biomedical imaging technologies have been developed,including various optical imaging,magnetic resonance imaging(MRI),ultrasound imaging(US),X-ray computed tomography(CT)and positron emission tomography(PET),etc.,all of these imaging technologies have certain disadvantages[2,3].In 2005,Gleich and Weizenecker[4]introduced a novel and promising imaging mode called magnetic particle imaging(MPI).
MPI has the advantages of positive contrast signal,low tissue background,unlimited tissue penetration depth,non-invasive imaging and no ionizing radiation.Comparing to MRI,MPI can directly detect changes in the responsiveness of nanoparticles in the magnetic field,and has a higher signal-to-noise ratio[5—8].Around the characteristics of MPI,superparamagnetic iron oxide nanoparticles(SPIONs),the most commonly used MRI contrast agent,were first used as MPI tracers.Examples include Ferucarbotran(Resovist,Bayer Healthcare)and Feridex(Ferumoxides,Berlex Laboratories)[9].However,according to current studies,MPI is inferior to MRI in spatial resolution and CT in sensitivity,and currently there are only a few kinds of nanoparticles that can be used as MPI tracers.Table 1 compares MPI and various medical imaging techniques in different ways[10].
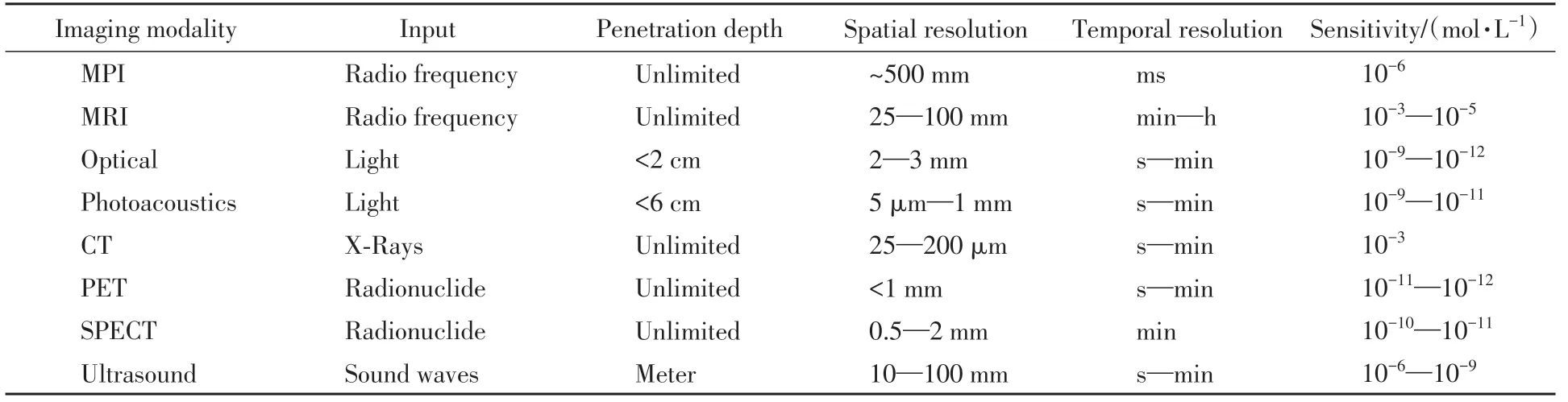
Table 1 Comparison of different imaging modalities
The sensitivity and resolution of MPI are highly dependent on the performance of the nanoparticle tracer itself,so current research is mainly focused on the design and synthesis of nanoparticle tracers.In this review,the latest progress of MPI tracers in recent years is summarized,and the types,synthesis methods,properties and biomedical applications of magnetic nanoparticles that can be used as MPI tracers are discussed.
2 The Fundamentals of MPI
MPI is a tracer method for detecting the spatial distribution of MNPs based on functional and tomography imaging techniques.The imaging system includes magnetic field excitation part,signal acquisition part and computer image processing part.The image processing part is mainly responsible for processing the magnetic field signal through reconstruction algorithm,to obtain MNPs concentration distribution information,which is the core part of the whole imaging system[11].
2.1 The Signal Generation of MPI
The signal generation of MPI is based on langevin’s nonlinear magnetization curve law[5].MPI determines the spatial distribution of the MNPs by measuring the magnetization change of the MNPs in the timevarying external magnetic field.The magnetization behavior of MNPs is described by Langevin’s theory[12].The theory assumes that the magnetization of particles is always consistent with the external magnetic field and there is no hysteresis effect.The relationship between the external magnetic field and magnetization is nonlinear,so when the excitation field passes through the MNPs,the received signal is a harmonic signal[Fig.1(A)].To determine the position of the detected signal,an additional static gradient field,called the selection field,is introduced into the spatial coding,usually consisting of two magnets arranged in a Maxwell structure[Fig.1(B)].MPI’s static gradient magnetic field,or selection field,causes MNPs at different positions to produce differentiated signals.The selected field has a corresponding field vector at each spatial position,and the field vector at the central position is 0,which is called the field-free point(FFP)and the magnetic field intensity is 0.When FFP passes through a region containing MNPs,particles farther away from FFP reach magnetic saturation,do not react to changes in the total magnetic field,and the receiving coil does not detect the signal.On the contrary,the particles in the region near FFP have not reached magnetic saturation,and the magnetization of MNPs changes in size and direction.The signal voltage is detected in the receiving coil,and the voltage signal is allocated to each position of FFP for reconstruction,so that the spatial distribution information of magnetic particles,namely MPI signal,can be obtained.Due to the diamagnetism of body tissues,no interfering signal is generated,so MPI does not display anatomical structure[13].

Fig.1 Schematic diagram of MPI signal generation[13]
2.2 The Factors Affecting MPI Imaging Quality
MNPs are subjected to an oscillating magnetic field,resulting in nonlinear magnetization.After magnetization,MNPs relax according to Brownian and Néel rotations[Fig.2(A)][14].Brownian rotations are rotations of physical particles,and Neel rotations are rotations of internal magnetic moments.The Brownian and Néel rotations time depend strongly on size,anisotropy constant and fluidity of MNPs.Shorter relaxation time can make the magnetization behavior of MNPs respond faster under time-varying external magnetic field,obtain higher MPI signal,and improve the imaging effect[5].
Based on MPI signal acquisition,MPI image is output through X-space reconstruction,and its imaging effect depends on the diffusion function(PSF)[15,16].PSF is determined by the gradient magnetic field(G)and the derived function of the tracer magnetization curve(dM/dH)[12].The gradient magnetic field(G)is the intrinsic parameter of the instrument,so we can improve the imaging sensitivity and spatial resolution by increasing the peak height of dM/dH or decreasing the half-peak width(FWHM)[Fig.2(B)and(C)].Importantly,the dM/dH height and FWHM depend largely on the intrinsic magnetic properties of the MPI tracer,including saturation magnetization(Ms),susceptibility(χ),magnetic relaxation time(τ),and coercivity(Hc).

Fig.2 Schematic diagram of magnetic relaxation mechanism and X-space image reconstruction technique[16]
Based on relaxation theory and X-space theory,the imaging effect of MPI depends on the magnetism and relaxation time of MNPs.The magnetic properties and relaxation time of MNPs are affected by the composition,size and morphology,etc.Table 2 compares MPI performance of different MNPs.
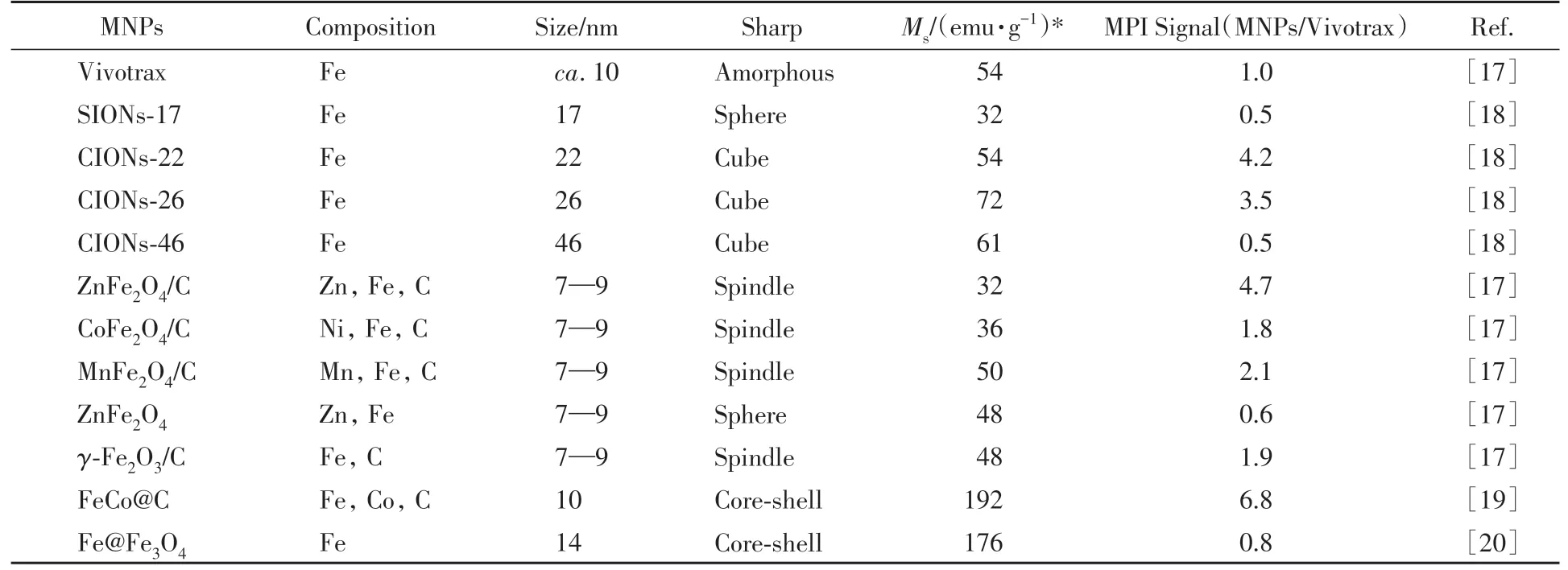
Table 2 Comparison of MPI performance of different MNPs
3 Nanoparticle Tracers for MPI
At present,the reported tracers that can be used in MPI are mainly magnetic nanoparticles with superparamagnetic properties,including iron oxide nanoparticles(IONPs)and magnetic heterostructure nanoparticles.Here,the synthesis methods of these magnetic nanoparticles and their performance in MPI imaging are summarized.In addition,the influencing factors of MPI signal of these nanoparticle tracers and the methods to improve their MPI signal are discussed,to promote the future development of nanoparticle tracers for MPI.
3.1 Iron Oxide
IONPs are the most studied and widely used tracers in MPI.They are benign,non-toxic,biodegradable and have adjustable magnetic properties.In a certain size range,IONPs exhibit superparamagnetism and high MPI signal[21].At present,there are many reported synthesis methods of IONPs,including coprecipitation,hydrothermal process,thermal decomposition process,etc.[22].Previous researches show that there are many factors affecting the properties of IONPs,including size,shape,composition,aggregation[23,24].
Wanget al.[18]developed a special MPI tracer based on cubic IONPs with an edge length of 22 nm(CIONs-22)by controlling the size and shape of MNPs in accordance with MPI physics theory.They found that the disordered surface of CIONs-22 has a lower ratio of spin and a significantly higher saturation magnetization than that of the sphere.Compared with the larger size CIONs,their saturation magnetization is not much different,but it has a lower coercivity[Fig.3(A)and(B)].Due to these special magnetic properties,CIONs-22 has significant advantages as a dedicated tracer for MPI with high sensitivity and resolution.Their data showed that,at the same iron concentration,the MPI signal of CIONs-22 was significantly stronger than that of Version and larger CIONs,and the MPI signal intensity generated by CIONs-22 was 4.15 times higher than that of the commercial Vivotrax[Fig.3(C)and(D)].

Fig.3 IONPs of different sizes and shapes act as MPI tracers[18]
Based on the principle that carbon loading and metal doping can improve the MPI property of IONPs,Jianget al.[17]designed and synthesized a series of new MPI tracers MFe2O4/C(M=Mn,Co,or Zn)andγ-Fe2O3/C.Using MIL-88A as raw material,MFe2O4andγ-Fe2O3nanoparticles loaded with carbon were prepared by thermal decomposition in 450℃and coated with polydopamine(PDA)to improve the biocompatibility of the nanoparticles[Fig.4(A)].The nonlinear magnetization curves of all nanoparticles were measured at 25℃using a vibrating sample magnetometer(VSM),and the superparamagnetism and saturation susceptibility of various nanoparticles were obtained with aMs value of 32.06 emu/g for ZnFe2O4/C[Fig.4(B)].Inin vitroMPI test,it was found that the MPI signal intensity of various nanoparticles at the same concentration was in the order of ZnFe2O4/C@PDA>MnFe2O4/C@PDA>γ-Fe2O3/C@PDA>CoFe2O4/C@PDA>Vivotrax>ZnFe2O4.The MPI signal of ZnFe2O4/C@PDA was 1787.7,4.7 times that of the commercial Vivotrax(383.2)[Fig.4(C)—(E)].
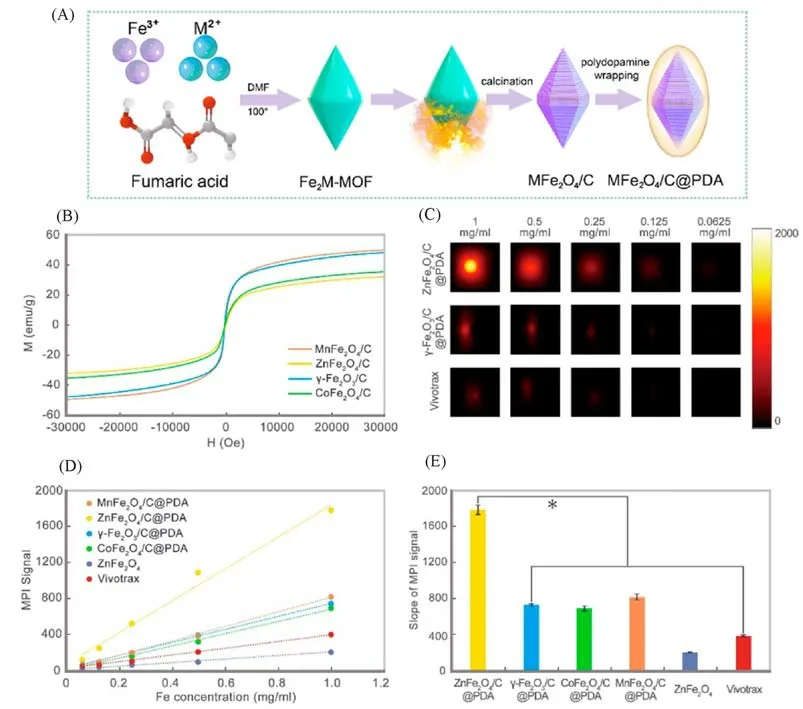
Fig.4 Carbon loading and metal doping improve the performance of MPI tracers[17]
Existing nanoparticles have mild S-type magnetization responses that limit resolution and sensitivity.Tayet al.[25]developed a chain of superferromagnetic iron oxide nanoparticles(SFMIO)to achieve an ideal stepped magnetization response,thereby improving image distinguishability and SNR by more than ten times that of conventional MPI.The basic mechanism relies on dynamic magnetized MPI excitation with square hysteresis loops in response to 20 kHz and 15 KAM-1,and the nanoparticles are assembled into chains under an external magnetic field.The“one-dimensional avalanche”dipole inversion of each nanoparticle in the chain occurs when the applied field overcomes the dynamic coercivity threshold from the dipole-dipole field of adjacent nanoparticles in the chain[Fig.5(A)].The event produces a strong sensing signal,which causes a sharp signal spike.SFMIOs did achieve its goal of improving MPI image property by switching between application areas of approximately 1 mT.Compared with the existing MPI nanoparticle Vivotrax,SFMIOs has a 40-fold improvement in SNR and a 10-fold improvement in spatial distinguishability[Fig.5(B)—(D)].
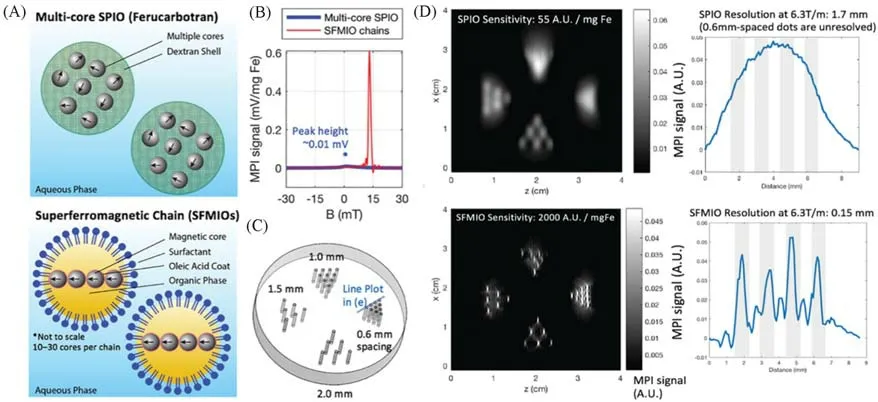
Fig.5 Order-of-magnitude mass sensitivity and resolution gains using superferro magnetic chains(SFMIOs)[25]
3.2 Heterostructured Nanoparticles
3.2.1 Iron-base Alloy The sensitivity and distinguishability of MPI are highly dependent on the performance of nanoparticles tracer,and the magnetic properties of nanoparticles depend on its nonlinear magnetization curve[4].With the increase of magnetic field intensity,magnetization tends to saturate,and high saturation magnetization usually has high MPI signal.Among various MNPs,the saturation susceptibility of iron-based alloys,such as PtFe(100 emu/g),Fe5C2(125 emu/g),and FeCo(215 emu/g),is better than that of Fe3O4(21—80 emu/g)[26—29].Therefore,the development of iron-based alloy as MPI tracers has a great prospect.
Owing to the instability of uncoated FeCo nanoparticles in air,Songet al.[19]introduced graphite carbon shell protection and prepared carbon-coated FeCo nanoparticles(FeCo@C)by methane chemical vapor deposition(CVD)as a novel MPI tracer.They modified the amphiphilic PEG-grafted poly(maleicanhydridealt-1-octadecene)onto the surface of FeCo@C through hydrophobicity and obtained biocompatible nanoparticles(FeCo@C-PEG).They investigated the effects of metal core composition and particle size on the MPI signal strength of FeCo@C-PEG nanoparticles.The best nuclear mole ratio of FeCo@C-PEG is Fe to Co=1∶1.Using the method of density gradient rate separation,the synthesized FeCo@C-PEG nanoparticles were centrifuged into gradient layers,and five kinds of nanoparticles with different sizes were collected manually[Fig.6(A)—(G)].The study showed that under the condition of the same nuclear molar concentration,The MPI signal produced by FeCo@C nanoparticles with a 10 nm nuclear diameter was 6.08 times that of VivoTrax(a commercial MPI tracer),and 14.91 times that of Feraheme[Fig.6(H)].
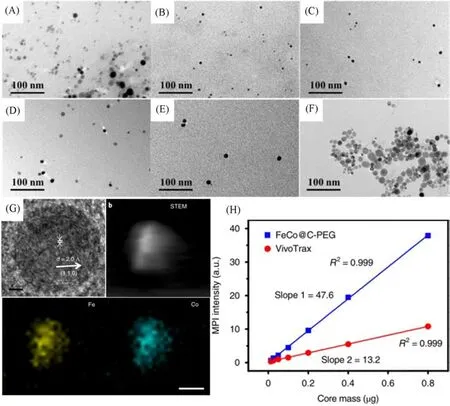
Fig.6 Representation and MPI performance of FeCo@C-PEG[19]
3.2.2 Core-shell Nanoparticles The size of core-shell magnetic nanoparticles is regulated by core diameter and shell thickness,and the direct contact between core and shell leads to strong exchange coupling[30].Research on magnetic nanoparticles with core-shell structure is progressing[31].
For example,Chaiet al.[32]developed a one-pot organic phase synthesis method to prepare nanoparticles with magnetic core-shell structure.In this method,they prepared core-shell nanoparticles with uniform morphology,high crystallinity and tunable composition,such as FePt@Mn-oxide,FePt@Mn-oxide,FePt@Cooxide and FePt@Ni-oxide,and studied their magnetic properties.Their data show that core-shell nanoparticles have stronger saturation susceptibility.
Gloaget al.[20]developed a<15 nm magnetic nanoparticle composed of a zero-valent iron core and an iron oxide shell for MPI tracers.They found that magnetic nanoparticles with small,highly magnetic zero-valent cores,which are half the diameter of Vivotrax,the best commercial MPI tracer,have similar MPI signal size and resolution.Therefore,the strong magnetism of zero-valent iron enables the smaller nano core to produce stronger MPI signals.Compared to the commercial VivoTrax nanopore tracer,the core coated with the 11-nm polymer achieved more than 40%of the MPI signal at the same iron content and more than 80%at 14 nm(Fig.7).

Fig.7 Representation and MPI performance of zero valent iron core-iron oxide shell nanoparticles[20]
The study of these core-shell magnetic nanoparticles enriches MNPs that can be used for MPI.Their unique magnetic properties allow them to be used in MPI with higher sensitivity,resolution and more possibilities.Therefore,magnetic core-shell nanoparticles have broad application prospects in MPI field.
3.2.3 Janus Nanoparticles We know that a certain imaging method has more or less shortcomings,and in the process of disease diagnosis and treatment,a variety of imaging methods are usually used to obtain more accurate information[33].Therefore,the development of nanoparticles with multi-mode imaging function has great potential.Janus nanoparticles with regulable asymmetric structures provide two different nanoparticle territories for multi-mode imaging.The two different territories of Janus nanoparticles can fully retain the characteristics of the two domains,and there is no steric hindrance between each other,which can carry out the functions of drug loading or target[34].
Songet al.[35]obtained Janus Fe3O4@PFODBT nanoparticles by wrapping the synthesized IONPs in a fluorescent semiconductor polymer.Each Janus particle contained two or more Fe3O4nanoparticles,but they showed the same MPI signal strength as uncoated Fe3O4nanoparticles at the same iron concentration(Fig.8).Therefore,the Janus nanoparticles developed in this study have MPI,MRI and fluorescence imaging multimode imaging capabilities.

Fig.8 Synthesis and MPI performance of Janus MNPs[35]
4 Biomedical Applications of MPI
To date,MPI research has focused on machine hardware,technical improvements,and the design and development of MPI tracers to improve performance such as temporal and spatial resolution and sensitivity.Now MPI has entered the pre-clinical stage,exploring its application in biomedicine is the focus of future research.This review summarizes the recent research on the biomedical applications of MPI.
4.1 MPI Tracers for Quantification of Body Fat Uptake
Lipids are the primary origin of energy for most biological tissues,so their absorption and stockpile are important for the energy balance in cellular tissues.Until now,the imaging quantification of body fat uptake has largely relied on radioisotope labeling,but this radiolabeling method exposes human or laboratory animals to ionization radiation[36].The quantitative detection of MPI by non-radiative high sensitivity method is more concerned.
Chylous microparticles are the main carriers of dietary fat.Hildebrandet al.[37]used MPI and magnetic particle spectroscopy(MPS)to quantifyin vivouptake of chylous microparticles in metabolically active tissues.They loaded artificial chylomere(ACM)with IONPs to enable fast and highly sensitivein situdetection of lipid uptake by MPS[Fig.9(A)—(C)].They then synthesized ACM with MPI tracer properties by using highly magnetic zinc-doped iron oxide nanoparticles(ZnMNPs),replacing the current standard Resovist,allowing quantitative intake of fat from whole-animal scans.They used acute cold exposure as a means of inducing chylomicron uptakein vivo.MPI signals in the interscapular region were significantly increased in mice injected with ZnMNPs-ACM under cold conditions[Fig.9(D)and(E)].
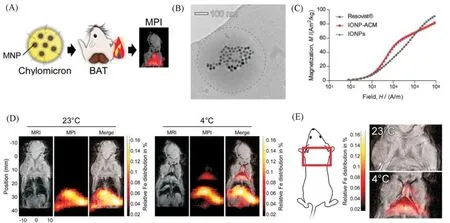
Fig.9 MPI tracers for quantification of lipoprotein uptake in vivo[37]
4.2 MPI Tracers for Cell Tracing
Cell tracer can help people understand the distribution of transplanted cellsin vivo,which is of great significance to the research of using cells as vectors.There are many imaging methods for cell tracing,such as MRI,SPECT,PET,and optical imaging methods[35].However,PET and SPECT tracers have poor biocompatibility and are not suitable for long-term follow-up[38,39].In addition,radioactive labeling methods may affect cell activity and hinder cell-based studies[40].The optical probe labeling method is limited by the light penetration ability,while the MRI tracking method also has the problem of signal artifact,loss and so on[41—44].Therefore,the application of the emerging MPI technology in cell tracing has attracted much attention.
The multifunctional Janus MNPs developed by Songet al.[35]has been used to label and imaging HeLa cellsin vivoorin vitro.In this study,the excellent performance of MPI for cell tracing was obtained by comparing Janus MNPs labeled cells in three modes including MPI,MRI and fluorescence.In vivo,MPI can clearly display 250 labeled HeLa cells in mouse model,but fluorescence imaging has low contrast due to the limitation of depth of tissue penetration and high background signal.Magnetic resonance imaging of labeled cancer cells,on the other hand,could not clearly show labeled cells because IONPs produced abscissive signals similar to dark signals at the air-tissue interface.For example,the negative contrast of 2500 labeled cells is difficult to distinguish inT2-weighted MRI[Fig.10(A)—(E)].They also used MPI to track tumor growth in mice after hypodermic implantation of Janus MNPs labeled HeLa cells[Fig.10(F)and(G)].
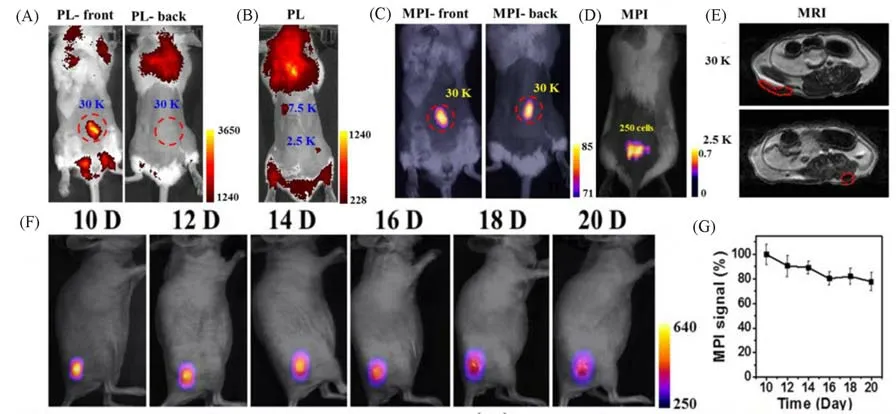
Fig.10 MPI tracers for cellular tracking[35]
4.3 MPI Tracers for Cancer Imaging and Therapy
High sensitivity tumor imaging is of great significance for early detection and staging of cancer.Generally,MNPs can be retained and enriched at the tumor site through EPR effect.Based on this principle,MPI tracers are widely used in tumor imaging[35].Compared with other imaging methods,MPI has more exciting performance.Concurrently,MNPs-mediated magnetothermal therapy is hopeful to be a new advance in cancer treatment[45].Based on the fundamental by which ferromagnetic/ferromagnetic MFNPs are injected into the tumor site for heating under an alternating magnetic field(AMF),MFNPs-mediated hyperthermia can rapidly and directly kill the local tumor without affecting adjacent healthy tissues[46].
At present,previous studies show that intratumoral injection is the best route for MNPs-mediated hyperthermia.In this way,high concentration of MNPs can be injected at the objective targets,while avoiding the off-target virulence of general systemic administration to normal tissues[47,48].In order to distribute administered MNPs evenly in tumors and to generate heat equably in the tumor,the usual tactics is to inject multiple doses of MNPs at different locations in the tumor[49].The emergence of MPI provides a new method to accurately quantify the number and distribution of nanoparticles in solid tumors.Unlike MRI,which can detect iron oxide indirectly,MPI can directly image the biological distribution of MNP with special sensitivity and specificity.Owing to MPI immediately detects MNPs,MPI images can be applied to monitoring the absorptivity dose of the target site to assure security and effectiveness of magnetic hyperthermia(MHT).
Duet al.[50]combined MPI guidance with peptides that target tumors to improve consistency of hyperthermia for solid tumors.They developed CREKA-modified IONPs for dual-mode MRI/MPI precision imaging and guided magnetic hyperthermia in 4T1 breast tumor mouse models.IONPs modified with CREKA polypeptide(IOs-CREKA)can target the highly expressed fibronectin in tumor microenvironment(TME).Data from their study showed that IOs-CREKA showed a higher and more uniform MPI signal across the tumor area 4 h after injection than non-peptide-modified IONPs[Fig.11(A)and(B)].T2-weighted MRI also shows the same trend.However,T2-weighted MRI often leads to imaging artifactsin some situations,such as calcification,bleeding or metal deposition.According to infrared thermography,the temperature of the mice tumor which injected with IOs-CREKA nanoparticles rose toca.43℃when exposed to AMF,effectively killing tumor cells[Fig.11(C)].BLI is used to dynamically and accurately monitor outcomes across treatment groups.In comparison,IOs-mediated hyperthermia had a good therapeutic effect at incipient stage of caner treatment,but some vestigial tumor cells at the tumor margin were gradually rebuilt,while IOs-CREKA NPs mediated hyperthermia almost disappeared the tumor and no bioluminescent light signal was detected.The improved targeting and administration uniformity makes cancer ablationviamagnetothermal therapy more effective than other equivalent untargeted IONPs[Fig.11(D)—(F)].

Fig.11 MPI tracers for cancer imaging and MHT[50]
4.4 MPI Tracers for Real-time Monitoring of Intracranial Hemorrhage
Ludewiget al.[51]demonstrated the feasibility of applying MPI to cerebral perfusion imaging,which can intuitively display the blood flow and tissue irrigation volume during cerebral ischemia.However,no studies have shown whether MPI can be used to detect intracranial hemorrhage and the delay of detection.The application of MPI to detect bleeding will be more challenging than cerebral perfusion imaging,which requires rapid accumulation of MPI tracers at the bleeding site in order to show the difference between the bleeding site and normal tissue on MPI images.MPI will have more clinical significance if it can detect bleeding as early as possible.Exciting is that the rapid development of MPI makes it have high sensitivity and excellent temporal resolution and high spatial resolution.Recent studies have shown the MPI can through the accumulation of tracer in the region of the different viscosity condition to realize visualization blood clotting[52,53],combined with the cerebral perfusion imaging,are likely to determine whether the bleeding has stopped or is still continuing,to realize real-time monitoring of bleeding.
Szwargulskiet al.[54]investigated the ability of MPI to detect intracranial hemorrhage in a mouse model.Intracranial hemorrhage was induced by injection of collagenase into C57BL/6 mice.Intracranial hemorrhage was detected in less than 3 min after intravenous injection of a long-cycle MPI tracer consisting of SPIONPs,and hematoma enlargement was monitored in real time(Fig.12).
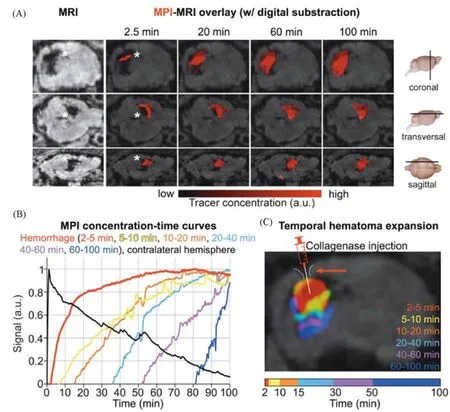
Fig.12 Rapid detection of intracranial hemorrhage with MPI[54]
5 Summary and Outlook
This review introduces the basic principle of MPI and summarizes the research progress of MPI in recent years.According to the relaxation theory and X-space theory of MPI,the magnetic properties and relaxation time of MNPs greatly affect the imaging effect of MPI.The property of MNPs is the key,so recent studies focus on the development of high performance MPI tracers.We summarize the types of MNPs available for MPI,including iron oxide,magnetic heterostructure nanoparticles(iron based alloy,core-shell nanoparticles and Janus nanoparticles).The study of IONPs mainly focused on carbon loading,metal doping,surface modification,size controling and synthesis conditions to enhance IONPs MPI signal,as well as research in and under the action of magnetic field,IONPs assembled into SFMIO nanoparticles with chain to enhance the performance of MPI.The research on magnetic heterostructure nanoparticles has developed a batch of MNPs with good magnetic properties and multi-functional,which makes them a hopeful development platform.MPI has the advantages of non-invasive,high sensitivity and non-radiation,and modified MNPs have good biocompatibility and ability to load drugs,making them widely used in a variety of biomedical applications.MPI has demonstrated superior performance in cell labeling,quantitative detectionin vivo,real-time imaging monitoring,tumor imaging and guided therapy.However,there are still some challenges before it can be translated into the clinic,such as the potential toxicity of high reticuloendothelial system(RES)uptake,the difficulty of developing responsive MPI tracers,and the need for further research on the factors influencing the performance of MPI tracers.
