Au@Ag-labeled SERS lateral f low assay for highly sensitive detection of allergens in milk
2023-01-23JingLiJiXuYiPnYongzhiZhuYunfengWngShouhuiChenXinlinWei
Jing Li, Ji Xu, Yi Pn, Yongzhi Zhu, Yunfeng Wng, Shouhui Chen, Xinlin Wei,*
a Department of Food Science and Technology, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China
b Institute of Food Engineering, College of Life Science, Shanghai Normal University, Shanghai 200234, China
Keywords:Casein α-Lactalbumin Au@Ag NPs SERS-LFA
A B S T R A C T Casein and α-lactalbumin (α-LA) are the main allergens in cow’s milk, which can affect the skin, respiratory system, and gastrointestinal tract, even cause anaphylactic shock. Developing a rapid and sensitive detection method of casein and α-LA is still pursued. Herein, a surface-enhanced Raman scattering based lateral f low assay (SERS-LFA) method for rapid and highly sensitive detection of milk allergens in food was established for effectively preventing allergic symptoms. Gold@silver nanoparticles (Au@Ag NPs) were synthesized as SERS active substrate to prepare the antibody-modif ied SERS Probe and SERS-LFA strips toward casein and α-LA were assembled according to the sandwich mode. The detection results were calculated according to colorimetric and Raman signal. The introduction of SRES signal significantly increased the sensitivity of detection with the limit of detection (LOD) of 0.19 ng/mL and 1.74 pg/mL, and exhibited an excellent linear relationship within the range of 0.55-791.50 ng/mL and 0.1 pg/mL-100.0 ng/mL for casein and α-LA,respectively. Furthermore, SERS-LFA strips was highly specif ic with the recovery rates for corresponding 80.36%-105.12% and 85.73%-118.22% for casein and α-LA, respectively. Therefore, the SERS-LFA could be in great potential to develop a unique allergen detection method.
1. Introduction
Milk allergy, the third largest food allergy after peanuts and nuts,accounts for about 10%-19% of food allergies, and is mainly mediated by immunoglobulin IgE [1]. The symptoms of milk allergy including skin symptoms (erythema, itching, and rash), respiratory diseases (wheezing,coughing, and gastrointestinal damage) and even multiple organ systemic allergic reactions usually appear within 1 h after intake [2].Therefore, establishing a highly sensitive and rapid method to detect the main allergens in milk to correctly evaluate the allergic risk and effectively reduce the allergic properties is urgent.
Casein andα-lactalbumin (α-LA) are the main allergens in milk [3].Casein accounts for about 80% of all milk proteins and is divided into multiple types (αs1-casein,αs2-casein,κ-casein andβ-casein) [4,5].Due to the different proportions of hydrophobic residues, polar residues and charged residues, the molecular structure of casein is not consistent. Additionally, the genetic polymorphisms of casein can lead to changes in high degrees of heterogeneity, glycosylation,and hydrolysis. Consequently, the allergy properties of casein could be greatly affected [6].α-LA is the second highest protein in whey accounting for 20% [6]. Epitope inα-LA structure has aff inity with IgE, which makes it potentially allergenic, and the incidence of allergic reaction is 27.6%-62.8% [7]. Therefore, developing a simple,fast and effective approach for detection is still in demand.
The detection methods for milk allergens include mass spectrometry (MS) [8,9], real-time PCR [10], and loop-mediated isothermal amplification (LAMP) [11,12]. They have been powerful techniques for protein analysis with high accuracy, sensitivity,specificity, reproducibility and no cross-reactivity problem due to the independence of the reaction with the antibody [13,14]. But these methods are not suitable for the food industry with the expensive instrument of great performance, complex separation technology and professional operators [15,16]. Immunoassays based on allergenantibody interactions are the common methods for allergens detection,including enzyme-linked immunosorbent assay (ELISA) [17],fluorescence-linked immunosorbent assay (FLISA) [18,19],biosensors [20] and lateral flow immunoassays (LFIAs) [21,22],which create specific and repeatable systems. However, it is timeconsuming for ELISA or FLISA. Biosensors need to prevent false positive or false negative results [13].
LFIAs are widely applied in food industry for routine cleaning tests and product tests due to convenience, efficiency, lower cost and no need for professional operations, and therefore is in the potential to be applied in the Hazard Analysis Critical Control Point(HACCP) system [23,24]. While LFIAs can only realize a detection with qualitative or semiquantitative results. Galan-Malo et al. [25]developed an LFIA test strip with two test lines to simultaneously detect casein andβ-lactoglobulin (β-LG). However, the detection limits of these two proteins only reached 1-5 mg/L. SERS refers to the phenomenon that the Raman signal excited by surface plasmon resonance is significantly enhanced when the sample is adsorbed on a solid substrate with metal nanostructures on the surface, or in a colloid of metal nanoparticles [26]. SERS enhancement mechanisms include electromagnetic enhancement and chemical enhancement.In the electromagnetic enhancement mechanism, the local surface plasma concentrates the local electric field in the “hot spots” near the metal surface, and therefore the Raman enhancement factor can reach 108-1011. In chemical enhancement, charge transfer occurs between the metal surface and the target analyte with the Raman enhancement factor of 102-103. However, due to the complexity of a specific SERS system, analytes are affected by both mechanisms [27]. SERS has attracted considerable interest for imaging applications, bioanalytical and environmental monitoring as a selective and ultrasensitive analytical technique, and is widely used in the identification and detection of DNA fragments, proteins, drugs and other analytes due to its high detection efficiency, rich information spectral characteristics and non-destructive testing [28]. The SERS material components and the metal nanostructure play important roles in generating strong SERS signals. Su et al. [29] used covalent organic frameworks (COFs)containing Au NPs to simulate nitroreductase to label the detection antibody, and 4-aminothiophenol (4-ATP) was used as a Raman reporter to connect with COFs, thereby generating Raman “hot spots”.On this basis, a nano-enzyme-linked immunosorbent assay (NELISA)method for the detection ofβ-LG was established, and the limit of detection (LOD) was 0.01 ng/mL. This method with high accuracy and low cost provided a new idea for the trace analysis of SERS technology.
In this work, five kinds of Au@Ag NPs with different particle sizes were prepared by the seed mediated growth method, and then the most suitable of these Au@Ag NPs with a strong Raman signal was selected as the active substrate to perform SERS probes.SERS-LFA strips for detecting casein andα-LA according to the sandwich mode were assembled, and the accuracy and applicability were evaluated. Finally, the SERS-LFA strips were applied to the detection of milk allergens in food samples (milk, biscuits, chocolate,milk powder, et al.). The rapid and highly sensitive detection of SERS-LFA is realized.
2. Materials and methods
2.1 Materials
Casein (CN, purity 91%) standard, chloroauric acid, silver nitrate,concentrated hydrochloric acid, concentrated nitric acid, potassium dichromate and trehalose were purchased from Sinopharm Chemical Reagent Co., Ltd.α-LA (purity 98.40%) andβ-LG (purity 96.03%)standard were purchased from Sigma-Aldrich Co., Ltd. BSA, gelatin,Tween-20 and polyvinylpyrrolidone (PVP) K-30 were commercially available from Solarbio Co., Ltd. Citric acid monohydrate and ammonia water were purchased from Aladdin Co., Ltd. Dipotassium hydrogen phosphate, disodium hydrogen phosphate dodecahydrate,sodium chloride, potassium chloride, sodium bicarbonate, sodium carbonate, ovalbumin, sodium dodecyl sulfate (SDS) and soy protein isolate (SPI) were purchased from Macklin Co., Ltd.4-Mercaptobenzoic acid (4-MBA) was obtained from Qiaoyi Reagent Co., Ltd. Tris-HCl buffer was purchased from Shanghai Titan Scientific Co., Ltd. Running buffer of casein andα-LA were provided by Hangzhou Nankai Biotech Co., Ltd. Milk, cookies, chocolate, O bubble fruit milk, Jelly drink were purchased from Shanghai Wumart;BEBA hydrolyzed milk powder (Nestlé) was obtained online;monoclonal antibodies (mAb) and polyclonal antibodies (pAb) of casein andα-LA were all provided by our laboratory.
2.2 Synthesis of Au@Ag nano particles
Au-Ag core-shell NPs were synthesized via a seed mediated growth method according to previously study with minor modification [30-32]. The reduction of silver on Au NPs with trisodium citrate was performed here. Firstly, 100 mL of 0.01% (m/V)HAuCl4solution was heated to boiling, then 2 mL of 1% (m/V)trisodium citrate solution was quickly added under stirring for 10 min.After 100 mL water and 3 mL 1% (m/V) trisodium citrate solution were added, varying amounts (0, 1, 2, 3, 4, 5 mL) of AgNO3solution was added into the solution (0.2 mL/min) and heated at 110 °C for 1 h to prepare Au NPs, Au@Ag1NPs, Au@Ag2NPs, Au@Ag3NPs,Au@Ag4NPs, Au@Ag5NPs, respectively. Finally, samples were cooled to room temperature. The microstructures of Au NPs and Au@Ag NPs were observed using transmission electron microscope(TEM, FEI Talos L120C G2, FEI Co., USA). Energy dispersive spectroscopy (EDS) and high-angle annular dark field image(HAADF) were characterized with Field Emission TEM (Talos F200X, FEI Co., USA). UV-vis spectra were collected using UVVis Spectrophotometer (T6, Beijing Purkinje General Instrument Co.,Ltd., China) over the range from 200 nm to 800 nm.
2.3 Preparation of antibody-modified SERS probe
4-MBA was modified on the surface of Au@Ag NPs according to previously reported methods with minor modification [33]. Typically,2.5 µL of 4-MBA solution (1 mmol/L, ethanol as solvent) was added to 2 mL of Au@Ag NPs solution under slight stirring at room temperature for 12 h. Then Au@Ag@MBA conjugation was formed and washed twice with filtered water by centrifugation. Raman spectra of Au@Ag@MBA were performed on Confocal Raman Microscope (invia qontor, Renishaw, UK) for analyzing the Raman single enhancement of these Au@Ag NPs. A 50 × objective lens with the numerical aperture of 0.75 was used with a laser of 785 nm and acquisition time of 10 s. After Raman enhancement analysis, the most suitable nanoparticles were selected as the active substrate.
The antibody was conjugated to Au@Ag@MBA to obtain a SERS probe. 2 mL of Au@Ag@MBA was adjusted to pH 8.5 with 0.1 mol/L K2CO3solution. Subsequently, 10 µL of detection antibody against casein (2 mg/mL) and 10 µL of detection antibody againstα-LA (2 mg/mL) were respectively reacted with the activated Au@Ag@MBA at 25 °C for 2 h. After the reaction, 30 µL BSA(10 mg/mL) was added to block at 4 °C and kept overnight. The unconjugated antibody was removed by centrifugation (8 000 ×g,10 min) at 4 °C, and then the antibody-conjugated Au@Ag@MBA were resuspended in 20 µL re-suspending solution (0.01% (m/V)BSA, 0.3% (m/V)trehalose in 0.01 mol/L PBS buffer), which can achieve efficient diffusion in SERS-LFA strip. The final SERS probes were obtained and stored at 4 °C for further use.
2.4 Preparation of SERS-LFA strips
The final SERS probes of casein andα-LA were fixed on the conjugate pad at a jetting rate of 4 µL/cm, and then dried at 37 °C for 30 min. Capture reagents (capture antibody against casein or capture antibody againstα-LA) and goat anti-rabbit IgG (2.0-2.5 mg/mL)were separately spotted onto the nitrocellulose (NC) membrane(Millipore 135) at a dispense rate of 1 µL/cm to generate test (T) line and control (C) line. The NC membranes were then dried at 37 °C for 2 h. Finally, the sample pad, conjugated pad, NC membrane and absorbent pad were attached to the backing pad in turn, and the ends of each component overlapped to ensure the chromatographed of solution by capillary action. After being assembled, strips were cut into 3 mm in width and stored in a sealed bag until use.
Raman spectra of the test line on SERS-LFA strips was measured on a portable Raman spectrometer (BWS465-785, B&W Tek Inc.,USA). A 785 nm laser line was used for all the measurements and power was 0.5%. Colorimetric measurement of the test line on SERSLFA strips was performed on an immunoassay quantitative analyzer(HG-98, Shanghai Huguo Scientific Instruments Co., Ltd., China).
2.5 Analysis of food sample
The sample pretreatment was carried out with minor modifications as follows. The liquid sample was centrifuged at 9 000 ×gfor 15 min and diluted with the running buffer after taking the supernatant [34].Cookies and chocolate melted at 58 °C were homogenized, then 1 g of the homogenized sample was added to 20 mL 20 mmol/L Tris-HCl(2% Tween-20, pH 8.0) [3,35]. The mixture was stirred overnight(16 h) and then centrifuged at 8 000 ×gfor 20 min. The aqueous layer was obtained and then diluted 20 times with Tris-HCl buffer for further use. 1 g of milk powder was dissolved in 10 mL PBS buffer (0.01 mol/L), and then centrifuged at 8 000 ×gfor 15 min to remove fat [17].
3. Results and discussion
3.1 Principle of the SERS-LFA strips
The SERS-based LFA strips was composed of five parts: sample pad, conjugated pad, NC membrane (coated with T line and C line),absorption pad and bottom plate. The general principle of the strips for the quantitative detection of casein orα-LA was shown in Fig. 1.When the sample solution was pipetted onto the sample pad and then move to the conjugated pad with capillary action. The target protein in samples would be recognized and conjugated by the casein orα-LA detection antibody labeled Au@Ag@MBA (SERS probe) immobilized on the conjugated pad. Then the conjugate was captured by the mAb fixed on the T line with capillary action,forming sandwich immune complexes of capture antibody-casein orα-LA-detection antibody. The extra SERS probe would continue to flow, and be combined with the goat anti-rabbit antibody on the C line. Consequently, the T line and C line turned to orange due to the accumulation of Au@Ag NPs when casein orα-LA was present.Only the C line developed color change in the absence of the protein.According to the T line, quantitative detection could be achieved by colorimetric measurement or Raman signal at 785 nm.
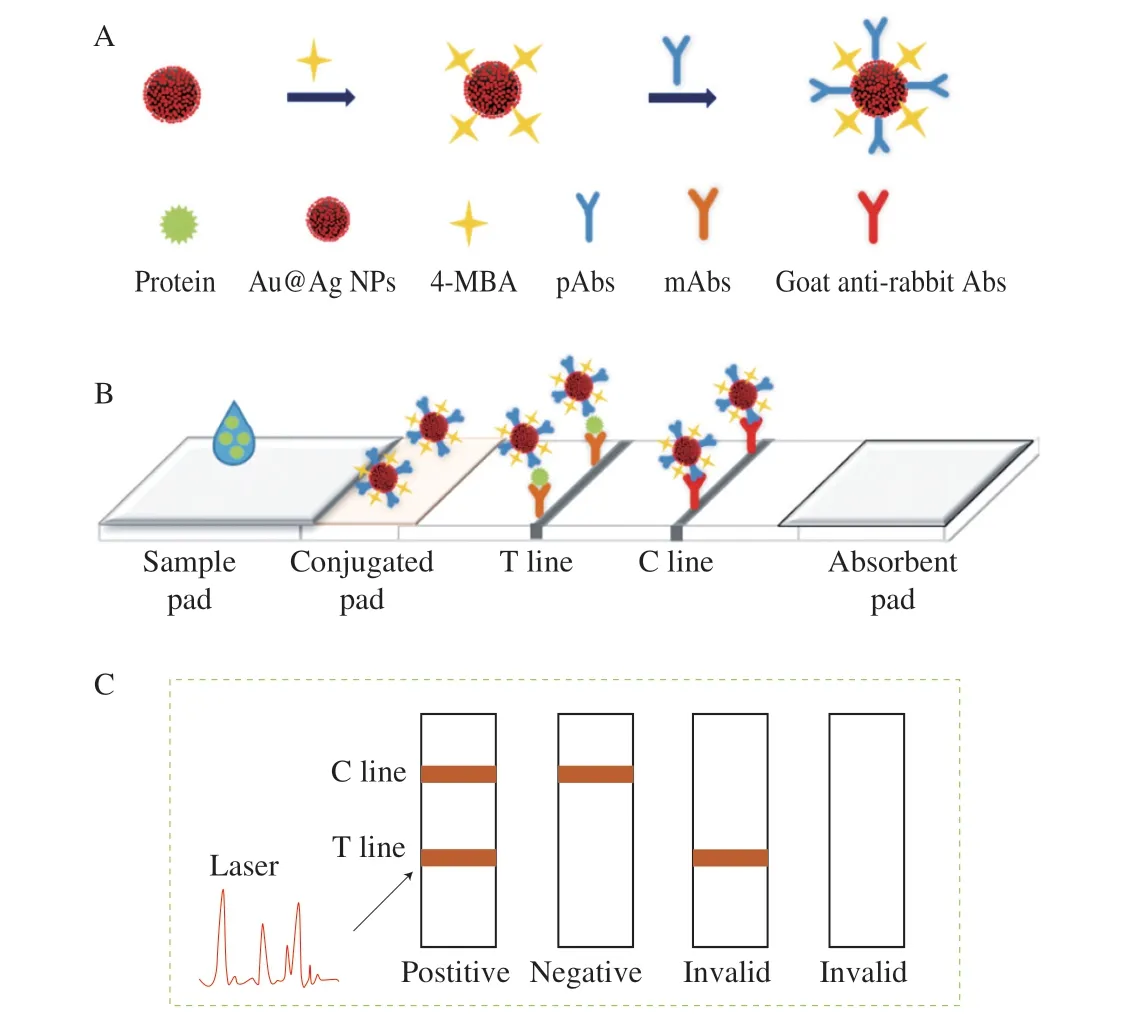
Fig. 1 Schematic illustration of the Au@Ag -based SERS lateral flow assay.
3.2 Characterization of Au@Ag NPs
The morphology and structure of Au NPs and Au@Ag NPs were characterized by TEM. Fig. 2A portrays Au NPs were nearly spherical with an average diameter of approximately 17 nm. From Au@Ag1NPs to Au@Ag5NPs, the thickness of the reduced Ag shell gradually increased, as well as the particle size. The average diameter of Au@Ag1NPs was 21.96 nm, which was nearly 4.95 nm larger than Au NPs.Au@Ag1NPs were characterized by field emission TEM (Fig. 2B).In the HADDF image, since the brightness is proportional to the square of the atomic number (Z2), the image of Au atoms (Z= 79)produced was brighter than that of Ag atoms (Z= 47) [36].In the EDS mapping image, the Au element only appeared in a small radius, and the surface of the Au microsphere was covered by the Ag shell. These proved that Au and Ag formed a core-shell structure.The inset in Fig. 2C shows Au NPs were red and Au@Ag NPs were orange. The maximum UV absorption peak of Au NPs was at 518 nm,and the UV absorption peak blue shifted to 384 nm gradually with the thickness of the Ag-shell increased (Fig. 2C). When adding enough AgNO3solution, the single-mode plasmon resonance of Ag appeared.On the one hand, the Au core was covered by Ag shell in the Au@Ag NPs, and the optical effect of Au was completely shielded. On the other hand, the content of Ag was greater than Au, therefore the light absorption of Au was masked by the light absorption of Ag [36,37].In general, the characterization results of UV absorption spectrum were consistent with TEM observation.
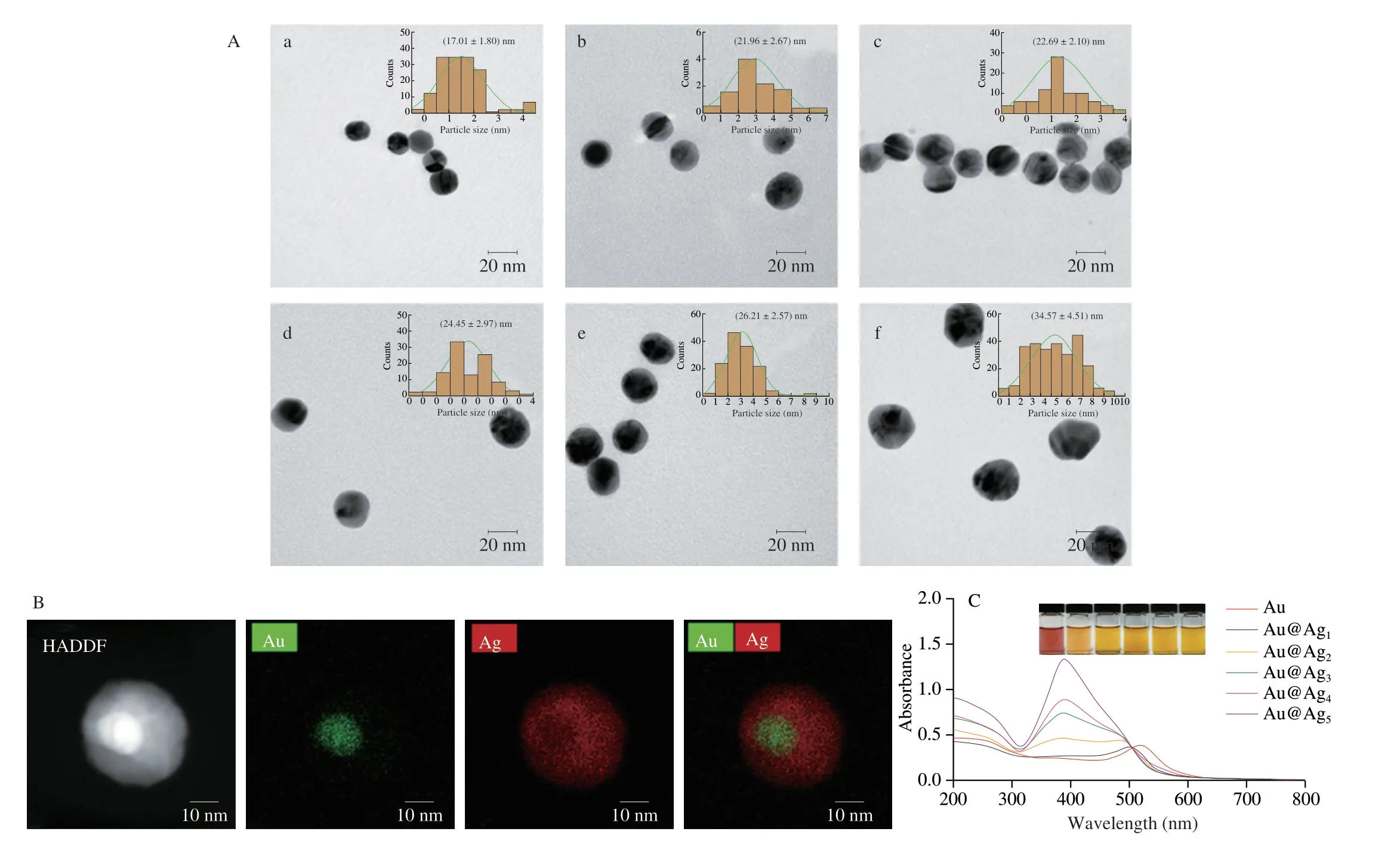
Fig. 2 (A) TEM of (a) Au NPs, (b) Au@Ag1 NPs, (c) Au@Ag2 NPs, (d) Au@Ag3 NPs, (e) Au@Ag4 NPs, and (f) Au@Ag5 NPs, the inset is the particle size distribution diagram of the corresponding sample; (B) STEM-HAADF and EDS mapping image of Au@Ag1 NPs; (C) UV spectra of different nanoparticles, the inset is a physical image.
3.3 Optimization of Au@Ag NPs
In order to optimize the performance of the SERS-LFA strips, Au@Ag NPs with different Ag shell thicknesses were prepared to obtain better Raman signal. The Raman signals of Au NPs and Au@Ag1NPs as the active substrate of 4-MBA were not obvious, while the peaks of the other four nanoparticles were prominent at 1 078 cm-1and 1 587 cm-1[32], as shown in Fig. 3A and Fig. 3B, respectively.When Au@Ag2NPs was used as a substrate, the Raman signal was higher than Au@Ag3NPs and Au@Ag4NPs. Although the signal intensity of Au@Ag5NPs was higher than Au@Ag2NPs, whereas the thickness of the Ag shell of Au@Ag5NPs was larger, referring to UV absorption spectrum characterization of Au@Ag5NPs tended to reflect the characteristics of easy polymerization and instability of Ag NPs.Additionally, nanoparticles with the diameter of 20 nm flowed more easily in the test strip. Therefore, Au@Ag2NPs were chosen as the Raman active substrate.
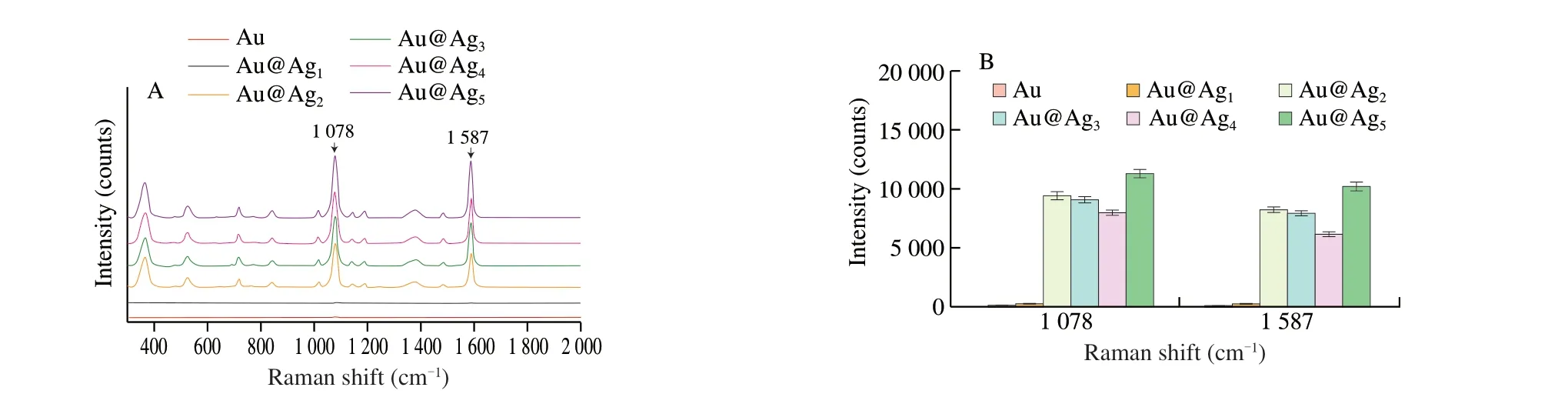
Fig. 3 (A) Raman spectra of 4-MBA under Au@Ag NPs with different particle sizes; (B) Raman intensities of Au@Ag NPs with different particle sizes at 1 078 and 1 587 cm-1.
3.4 Analytical performance of SERS-LFA
The performance was carried out according to the usage of test strips, and then the Raman single method and colorimetric method of SERS-LFA strips for detecting casein andα-LA were established respectively. With the detection concentration of casein being in the range of 0.01 ng/mL-10 μg/mL, the color of the T line gradually became lighter with the concentration of casein decreasing, and the detection line of naked eyes disappearance was 100 ng/mL(Fig. 4A). Based on the colorimetric method, the SERS-LFA strip standard curve of casein was established (Fig. 4B). The linear detection range (LDR) was 185.62-642.53 ng/mL with a regression coefficient ofR2= 0.857 6. The LOD was 3.59 ng/mL by adding 3 standard deviations to the mean of 10 blank values.The Raman spectrum at the T line of the strip was obtained. The intensity of the Raman signal at 1 611 cm-1had an obvious positive correlation with the target casein concentration (Fig. 4C). Due to the difference in the consistency of the Raman displacement obtained by different systems, the characteristic peak of the Raman reporter 4-MBA in the liquid system is 1 587 cm-1, while the 4-MBA in the strip was toward high frequency of offset with 24 cm-1. According to the analysis of SERS probe, 4-MBA coupling with Au@Ag NP to form Ag-S chemical bond, 4-MBA reacting with the amino group of the antibody to form amide bond, strip bottom plate as laser focus, all these worked together to cause molecular vibration changes, resulting in the displacement of the Raman peak. Based on the signal intensity of the Raman peak at 1 611 cm-1, a standard curve for the detection of casein by SERS-LFA strip was established (Fig. 4D). LDR was 0.55-791.50 ng/mL with a great regression coefficient ofR2= 0.991 9 and the LOD was 0.19 ng/mL.

Fig. 4 Sensitivity detection of casein SERS-LFA strip. (A) The photo of the sensitivity detection of casein SERS-LFA strip; (B) Standard curve of casein SERS-LFA strip based on colorimetric method; (C) Raman scanning spectrum at the T line of the test strip; (D) Standard curve established based on the Raman signal intensity at 1 611 cm-1.
In the SERS-LFA strips for casein detection, the sensitivity of Raman single analysis was two orders of magnitude higher than the colorimetric method and three orders of magnitude than the naked eyes. For the sandwich assay of dual-label immunosensor established by Xu et al. [34], the sensitivity of casein SERS-LFA strip by Raman single analysis established in this study was increased by more than 10 times; while in the LC-MRM/MS method Ji et al. [9] developed,the LOD of milk allergenαs1-casein was only 0.2 mg/kg.
Similarly, the sensitivity of SERS-LFA strips for detectingα-LA was measured. Fig. 5A reveals within the detection range of 0.1 pg/mL -100 ng/mL, the color of the T line became lighter gradually with theα-LA concentration decreasing, and the detection line of naked eyes disappearance was 10 ng/mL. The standard detection curve of the strip forα-LA was established by colorimetric method (Fig. 5B). LOD was 0.95 ng/mL. LDR ranged from 0.5 ng/mL to 100 ng/mL withR2= 0.954 6. Based on Raman signal detection, a standard curve forα-LA detection using SERSLFA strips was established. Fig. 5C shows that at the Raman shift of 1 611 cm-1, the intensity of Raman signal was positively correlated with the concentration ofα-LA solution. The LDR of the fitted curve was 0.1 pg/mL-100 ng/mL withR2= 0.983 1, and LOD was 1.74 pg/mL (Fig. 5D).
In the SERS-LFA strips forα-LA detection, the Raman detection method was more sensitive than the colorimetric method by two orders of magnitude and the naked eyes by four orders of magnitude. Yang et al. [38] established a competitive model FLISA based on CdSe/ZnS quantum dots, the LOD forα-LA was 0.1 ng/mL. While in our work, the sensitivity was up to pg/mL level. Ji et al. [9] detectedα-LA by MS, which only reached 0.39 mg/kg of LOD. For MS method, it needs high requirements for sample pretreatment, instrument accuracy, and professional operation, which does not have great applicability for the detection of allergens in the food industry.

Fig. 5 Sensitivity detection of α-LA SERS-LFA strip. (A) The photo of the sensitivity detection of α-LA SERS-LFA strip; (B) standard curve of α-LA SERS-LFA strip based on colorimetric method; (C) Raman scanning spectrum at the T line of the test strip; (D) standard curve established based on the Raman signal intensity at 1 611 cm-1.
3.5 Selectivity analytical performance of SERS-LFA
The Raman single analysis was used for evaluating the specificity of this study. Fig. 6A portrays the Raman intensity of casein at different concentrations was all higher than that of LOD value, which is called the cutoff value (COV). But the Raman single ofα-LA,β-LG, BSA, OVA and SPI at the three concentrations were lower than COV, indicating that they were in negative reaction. The work proved that the casein SERS-LFA strip was specific for casein detection.Similarly, the Raman intensity ofα-LA was higher than the COV,while other samples were lower at the same concentrations, indicating that theα-LA SERS test strip was specific for the detection ofα-LA (Fig. 6B).

Fig. 6 Cross-reactivity of SERS-LFA strip. (A) Casein; (B) α-LA.
3.6 Sample analysis

Table 1 Addition and recovery experiment of different samples in casein SERS-LFA strips.
The European Union has formulated specific regulations that require mandatory labeling of 14 groups of foods including milk, dairy products and other ingredients listed in the label of processed foods must be emphasized [3,39]. In order to evaluate the applicability of our SERS-LFA strips, 9 kinds of food were pretreated and the proportions of casein andα-LA were detected.Raman signal detection method was used for sample addition and recovery experiments to verify the accuracy of casein andα-LA(Tables 1 and 2). In SERS-LFA strips of casein, the recovery rate of casein was 80.36% to 105.12%, and the coefficient of variation (CV)within groups was 1.86% to 13.09%. The recovery ofα-LA in the SERS-LFA strips ofα-LA ranged from 85.73% to 118.22%, the CV ranged from 3.84% to 13.64%. These researches demonstrate that the two strips had high accuracy and were suitable for the detection of casein andα-LA in foods such as milk, biscuits, chocolate, and jelly.The SERS-LFA strip could be applied to the monitoring of allergens in the food industry. The LOD of SERS-LFA performed could achieve the level of pg/mL. And the strips were cost-saving and timesaving with getting results of 5-10 min. Therefore, in response to the rapidly growing demand for allergen detection methods, researchers can design a portable Raman spectroscopy device with advanced technology and a new software program integrating data processing,analysis and readout so that end users can easily obtain test results for SERS-LFA strips. In this way, it can provide support for its various applications.

Table 2 Addition and recovery experiment of different samples in α-LA SERS-LFA strip.
4. Conclusion
In conclusion, we developed SERS-LFA for rapid detection of casein andα-LA, respectively. Au@Ag NPs with a diameter of near 22.69 nm were prepared as the Raman enhancement active substrate to synthesize SERS probe. For casein, LODs by naked eyes, colorimetric method and Raman single detection were 100.00, 3.59 and 0.19 ng/mL, respectively, and LDRs of colorimetric method and Raman single detection were 185.62-642.53 and 0.55-791.50 ng/mL,respectively. Forα-LA, LODs by naked eyes, colorimetric method and Raman single detection were 10.00, 0.95 and 1.74 pg/mL,respectively, and LDRs of colorimetric method and Raman single detection were 0.5-100 ng/mL and 0.1 pg/mL-100 ng/mL,respectively. Accordingly, the sensitivity of Raman detection method was two orders of magnitude higher than that of the colorimetric method and 3 to 4 orders of magnitude higher than that of detected by naked eyes, which confirmed that Au@Ag NPs was an active SERS substrate to detect casein andα-LA. Importantly, the detection could be completed in 5-10 min, which greatly improved the efficiency of detection. In this study, SERS combined with lateral flow assay could establish a linear relationship between Raman signal and the concentration of target protein, so as to achieve rapid and highly sensitive detection. In the future, this developed test concept could greatly prevent the occurrence of allergies and help relevant departments to control and monitor the presence of allergens.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was supported by the Science and Technology Innovation Action Plan of Shanghai, China (17391901302) and the National Key R&D Program of China (2018YFC1604401).
杂志排行
食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard
