Effect of lipid peroxidation on the allergenicity and functional properties of soybean β-conglycinin (7S) and glycinin (11S)
2023-01-23LiyingYeLingtoXioLinKnHeXioqunYngZhiliWnLizhongLiuHiqinWuShojunXingXuliWu
Liying Ye, Lingto Lü, Xio Lin, Kn He, Xioqun Yng, Zhili Wn,Lizhong Liu, Hiqin Wu, Shojun Xing*, Xuli Wu,*
a School of Public Health, Health Science Center, Shenzhen University, Shenzhen 518060, China
b School of Food Science and Technology, South China University of Technology, Guangzhou 510640, China
c School of Basic Medical Science, Health Science Center, Shenzhen University, Shenzhen 518060, China
Keywords:β-Conglycinin Glycinin Lipid peroxidation Allergenicity Functional properties
A B S T R A C T This study aimed to analyze the effect of lipid peroxidation on the allergenicity and functional properties of soybean β-conglycinin (7S) and glycinin (11S). Oxidation complexes were determined using the lipid peroxidation method. Functional properties were analyzed based on emulsifying and foaming properties.The potential allergenicity was evaluated by in vitro and in vivo methods. The results found that oxidation altered structures of the proteins and resulted in the formation of cross-linked protein polymers. The emulsion and foaming properties of the proteins were improved after oxidation. The IgE-binding capacity of 7S and 11S reduced after oxidation. KU812 cell assays showed that both histamine and IL-4 release decreased after oxidation treatment. A mouse model showed that oxidation reduced the IgE, IgG, and IgG1 levels, as well as reduced histamine and mMCP-1 release in serum, which might suppress the allergic reaction. In conclusion,the lipid peroxidation treatment likely causes changes to the functional properties of soybean, decreasing the potential allergenicity of 7S and 11S.
1. Introduction
Soybean is a common source of protein globally because of its nutrients and properties, making it popular in food manufacturing industries. However, soybean protein is a confirmed major food allergen [1]. Some researchers reported that around 2.7% of children and around 0.27% of adults suffer from soybean allergy [2,3].Current research shows that the main allergenic proteins of soybean predominate inβ-conglycinin (7S) and glycinin (11S), which account for more than 70% of total content [4]. 7S is a trimeric glycoprotein combined of three subunits:α,α’, andβ, with molecular masses of 57-68, 57-72, 45-52 kDa, respectively [5]. 11S contains two subunits of different sizes linked via disulfide bonds. There are six acidic subunits (35-37 kDa) and six basic subunits (20 kDa) [6].
The high allergenicity of soybean could limit its application in daily life. In particular, the allergenicity of soybean could be inf luenced during food processing, including heat denaturation [7], high pressure [8], polysaccharide conjugation [9], and fermentation [10].However, the allergenicity and functional properties of soybean protein could be influenced, due to a large number of chemical reactions during food processing. Soybean also contains abundant unsaturated fatty acids, which easily occurred to oxidation during processing and preservation [11].
Proteins and unsaturated fatty acids were interdependent in food [12-14]. The previous study showed that lipid peroxidation(including free radicals and reactive aldehyde derivatives) can modify proteins [15]. Wu et al. [16] found that a solution containing 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH) induces oxidative changes in soybean protein due to the generation of peroxyl radicals under aerobic conditions. Uchida [17] observed that acrolein(CH2=CH-CHO) was formed due to lipid peroxidation that existed extensively in food systems. These oxidative modifications may cause changes to the structure and multiple functional properties of soybeans by peroxyl radicals and acrolein. Therefore, it was hypothesized that lipid peroxidation could influence the structural and functional properties of 7S and 11S, including the allergenicity.
In the study, the changes to the structure, functional properties and allergenicity of soybean 7S and 11S via lipid peroxidation(AAPH and acrolein) treatment were investigated. Structural changes were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), intrinsic fluorescence, and analysis of free amino and thiol groups. Functional properties were analyzed based on foam properties and emulsifying activity. The IgE binding capacity was determined by western blot and ELISA with sera from patients with soybean allergies. The allergenicity of 7S and 11S treated via lipid peroxidation was also evaluated byin vitroandin vivoassay. The study aimed to understand how the functional properties and allergenicity of soybean 7S and 11S changed by lipid oxidation during processing and preservation.
2. Materials and methods
2.1 Materials and reagents
7S and 11S proteins were gifted by the South China University of Technology. AAPH, acrolein, and 3,3’,5,5’-tetramethylbenzidine(TMB) were acquired from Sigma Chemical Co. (St. Louis, MO,USA). Horseradish peroxidase (HRP)-labeled goat anti-human IgE antibody was purchased from Kirkegaard & Perry Laboratories(Gaithersburg, MD, USA). ELISA kits of histamine, IL-4 and mouse mast-cell protease-1 (mMCP-1) were purchased from R&D Systems (Minneapoils, MN, USA). All other chemicals were of analytical grade.
2.2 Patients’ sera
Serum samples from soybean-allergic patients were generously acquired by Shenzhen Children’s Hospital (Shenzhen, China). All the patients provided signed informed consent. The specific IgE levels to patients’ sera were evaluated by an ImmunoCAP system (Phadia AB, Uppsala, Sweden). When the specific IgE levels were > 10 kU/L,serum samples were and collected and kept at -40 °C.
2.3 Sample preparation
7S and 11S proteins (dispersed fully in phosphate buffer, pH 7.4)from soybean were mixed with AAPH and acrolein solution,respectively. The AAPH solution was adjusted to 1, 5, and 25 mmol/L.The acrolein solution was adjusted to 0.1, 1, and 10 mmol/L. The final samples were incubated for 24 h at 37 °C. And then the reaction was stopped by immediately cooling the samples to 4 °C in an ice bath. After dialysis, all the samples were obtained by lyophilization and kept at -40 °C.
2.4 Characterization of oxidized samples
2.4.1 Electrophoretic analysis
The molecular weight of 7S and 11S after oxidation was determined by SDS-PAGE. The sample solution was mixed with loading buffer (4:1,V:V), and then heated for 7 min at 100 °C. Later,the mixture was loaded into separate wells with SDS-PAGE gel. After finishing, the gel was stained with Coomassie Brilliant Blue R-250 for 120 min, and then destained with deionized water. The result was acquired by a Tanon-4200SF (Tanon Science & Technology Co., Ltd.Shanghai, China).
2.4.2 Free amino and thiol groups
Free amino groups were determined using an established method previously [18]. All the sample solutions were mixed with 4 mL OPA reagent for 2 min. Optical densities were read at 340 nm. The content of sulfhydryl thiol groups was determined as previously described [19].Samples (dissolved in Tris-Gly buffer, pH 8.0) were reacted with DTNB for 1 h. Thiol groups were measured at a wavelength of 412 nm.
2.4.3 Intrinsic fluorescence analysis
All the samples (0.1 mg/mL) were dissolved in phosphate buffer solution (PBS, pH 7.4). Fluorescence measurements were performed on a Jasco-810 spectropolarimeter (Jasco, Tokyo, Japan) at room temperature.The fluorescence spectra were collected over a 290-400 nm range.
2.5 Determination of functional properties
2.5.1 Emulsifying properties
The treated 7S and 11S samples were determined to analyze emulsifying properties following the method of Ullah et al. [20]. The emulsifying activity index (EAI) was analyzed with the following equation:

whereA0represented the absorbance at 500 nm; DF represented the dilution factor;ρis concentration (g/mL) of samples before emulsification;φis the oil volume fraction (V/V) of the emulsion.
2.5.2 Foaming properties
The treated 7S and 11S samples were determined to analyze the foaming properties with the method of Yue et al. [21]. In brief, an aliquot (10 mL) of 5 mg/mL protein was homogenized at 10 000 r/min for 2 min at room temperature. The foaming capacity (FC) and foaming stability (FS) were calculated with the following equation:

whereV0andV10are the foam volumes at 0 and 10 min,respectively, after homogenization.
2.6 In vitro assay
2.6.1 Western blot analysis
Western blot was considered to analyze the IgE binding capacity of the treated 7S and 11S samples. After SDS-PAGE, the samples were transferred to polyvinylidene fluoride (PVDF) membranes.And then the membranes were blocked for 1.5 h. After washing with PBS containing 0.05% Tween -20 (PBST). The membranes were then incubated with soybean-allergic patients’ sera (dilution 1:50)overnight. They were then incubated with HRP-labeled mouse anti-human IgE antibody (dilution 1:2 000) for 1 h at room temperature. Finally, protein bands were determined by ECL Reagent(GE Healthcare, Buckinghamshire, UK).
2.6.2 ELISA analysis
The sample solution was dissolved in sodium carbonate buffer(50 mmol/L, pH 9.6) and incubated (100 µL, 1 mg/mL) at 4 °C overnight in a 96-well plate. After washing the plate in PBST three times, PBST containing 5% BSA was used to block it at 37 °C for 60 min. After blocking, washing was performed with PBST and then incubated with 100 µL pooled sera (dilution 1:50). After washing, the plate was incubated with 100 µL goat anti-human IgE antibody (dilution 1:4 000)at 37 °C for 1.5 h. Finally, TMB substrate solution (50 µL) was added.And then sulfuric acid (2 mol/L) was added to terminate the reaction.The absorbance value was immediately measured at 450 nm.
2.6.3 KU812 cells assay
KU812 cells were cultured following a previously reported method with slight modifications [22]. KU812 cells (1 × 106cells/well)were seeded in a 48-wells plate, and sensitized with soybean-allergic patients’ sera for 24 h. After incubation, allergic activation was induced by incubating with 10 µL treated samples (5 mg/mL) for 4 h.KU812 cells were centrifuged, and the supernatant was acquired to detect histamine and IL-4 content with ELISA kits.
2.7 In vivo assay
2.7.1 Mice
Seventy BALB/c mice (five-week-old) were approved by SPF(Guangdong) Biotechnology. The mice were acclimated for at least 1 week before immunization. All mice were separated into 7 groups(n= 10/group): control, 7S, 7S-AAPH, 7S-acrolein, 11S, 11S-AAPH,and 11S-acrolein group.
2.7.2 Sensitization and challenge experiment
The sensitization experiment was considered to follow the method of Ren et al. [23]. In the negative control group, mice were intragastrically immunized with 300 µL PBS solution with 15 μg cholera toxin. The mice were then challenged with 500 µL PBS on day 28. In the immunized group, mice were sensitized by an intragastric gavage of 500 µL PBS containing native 7S or treated 7S(5 mg/mouse) and cholera toxin (15 μg/ mouse) on day 0, 7, 14, 21.On day 28, all mice received a challenge of 20 mg 7S, 7S-AAPH,or 7S-acrolein complexes in 500 µL PBS, respectively. After 1 h,anaphylactic symptoms were evaluated using an established scoring system (Table 1). Blood samples were collected to measure the levels of IgE, IgG, IgG1, histamine and mMCP-1 by ELISA kits. Oral sensitization testing was also analyzed for 11S.

Table 1 Anaphylactic symptom scoring.
2.7.3 Histological assessment of duodenal tissue
All the mice were euthanized after 1 h challenged with 7S,7S-AAPH, or 7S-acrolein, and 11S, 11S-AAPH, or 11S-acrolein.The duodenum was immediately collected from the mice. It was then immediately fixed in 4% formalin. Fixed tissues were processed using standard histological techniques. Paraffin sections were deparaffinized in xylene, rehydrated through an ethanol gradient to water, and stained with eosin and hematoxylin. Finally, the structure of the duodenum was observed by a light microscope (Nikon Ti Microscope, Japan).
2.8 Statistical analysis
All the samples were evaluated in triplicate. All the results were exhibited as mean ± SD. Differences were analyzed by ANOVA with Tukey’s post hot test.P-values of less than 0.05 were considered significant.
3. Results and discussion
3. 1 Characterization of oxidized samples
The results of SDS-PAGE showed that the molecular weight of 7S and 11S changed after oxidation. Compared with native 7S and 11S, the intensity of 7S and 11S bands shrank with increasing concentration of AAPH and acrolein (Fig. 1A). However, the aggregation bands were much stronger. Thus, oxidized 7S and 11S results were related to AAPH and acrolein concentration.Electrophoretic patterns of the higher molecular bands indicated that AAPH and acrolein caused aggregations to form via free-radical chain polymerization. Our findings support those of Wu et al. [24],who described the oxidative modification of soy protein. The contents of both the free amino and thiol groups decreased with increasing concentration of AAPH and acrolein (Fig. 1B, C). Thus,AAPH and acrolein might combine with some free amino groups in 7S and 11S.

Fig. 1 (A) SDS-PAGE, (B) free amino group, (C) thiol group, and (D) fluorescence analysis of 7S (A1, B1, C1, D1) and 11S (A2, B2, C2, D2) after treatment with AAPH and acrolein. 0, Control (untreated); 1, 1 mmol/l AAPH; 2, 5 mmol/L AAPH; 3, 25 mmol/L AAPH; 4, 0.1 mmol/L acrolein; 5, 1 mmol/L acrolein;6, 10 mmol/L acrolein. *P < 0.05, **P < 0.01 vs Control group.
The fluorescence intensity of 7S and 11S oxidation complexes was lower compared to that of purified protein sample (Fig. 1D).Oxidation might cause a blue shift in the spectrum of the maximum emission wavelength. For both 7S and 11S, fluorescence intensity was lowest (about 10% and 8% of native 7S and 11S) at 25 mmol/L AAPH with blue shifts of 20.7 and 13.3 nm, respectively. The fluorescence intensity and blue shift of 7S and 11S with acrolein treatment showed a similar trend. The decrease in intrinsic fluorescence intensity might be attributed to the conversion of tryptophan (Trp) residues of the protein. The blue shift indicated aggregation after oxidation and decreased exposure of Trp residues.Trp residues that were previously located outside the molecule in a polar environment were transferred to an internal non-polar environment of 7S and 11S after oxidations [25]. Thus, oxidation could cause structural changes to 7S and 11S.
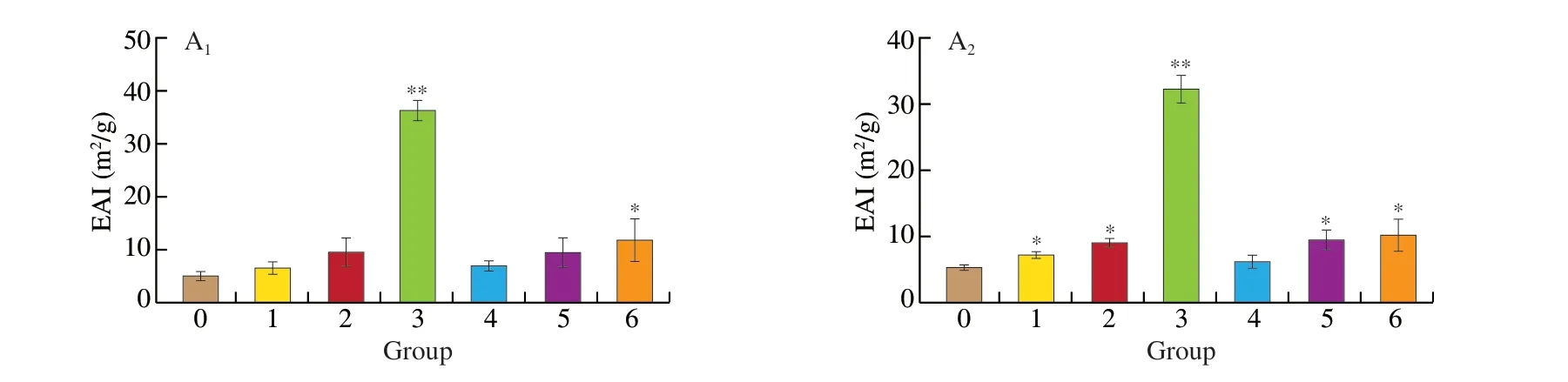
Fig. 2 (A) Emulsifying activities and (B) foam properties of 7S (A1, B1), and 11S (A2, B2) after treatment with AAPH and acrolein. 0, Control (untreated);1, 1 mmol/l AAPH; 2, 5 mmol/L AAPH; 3, 25 mmol/L AAPH; 4, 0.1 mmol/L acrolein; 5, 1 mmol/L acrolein; 6, 10 mmol/L acrolein. *P < 0.05,**P < 0.01 vs Control group.
3.2 Functional properties
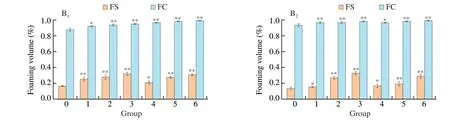
Fig. 2 (Continued)
Oxidation could influence the functional properties of soybean after AAPH and acrolein treatment. Protein conformational structure is a major factor impacting protein emulsifying properties [26].Compared to 7S, EAI values of all the samples after oxidation with AAPH (25 mmol/L) and acrolein (10 mmol/L) ranged from 7.78 to 38.52 m2/g and 12.63 m2/g, respectively (Fig. 2A1). The EAI values of 7S oxidation complexes were significantly (P< 0.05)higher compared to that of purified 7S. Moreover, both FC and FS of 7S oxidation complexes also significantly increased (Fig. 2B1).Furthermore, the EAI, FC, and FS of 11S oxidation showed a similar tendency (Fig. 2A2, B2). The conformational structure of 7S and 11S might alter with the exposure of hydrophobic domains through lipid peroxidation, thus improving the emulsifying activity of proteins.The foaming properties of protein were considered to determine their application in food systems [27]. FC might be relevant to the production of air droplets in proteins. In comparison, FS implies that protein has sufficient viscosity to keep FC stable [28]. Increased foaming properties indicate that the oxidation complexes with protein enhance protein-protein interactions at the air-water interface. This result supported that of Tan et al. [29]. Furthermore, the structure of 7S and 11S after oxidation was fully extended, becoming loose when the concentration of AAPH and acrolein increased. This phenomenon indicated that oxidation could accelerate the spread of proteins on the water-air interface.

Fig. 3 IgE binding ability of 7S (A1, B1) and 11S (A2, B2) after treatment with AAPH and acrolein detected by (A) Western blot and (B) ELISA. (C) Basophil histamine release and (D) IL-4 release with KU812 cell degranulation of 7S (C1, D1) and 11S (C2, D2) after treatment with AAPH and acrolein.0, Control (untreated); 1, 1 mmol/l AAPH; 2, 5 mmol/L AAPH; 3, 25 mmol/L AAPH; 4, 0.1 mmol/L acrolein; 5, 1 mmol/L acrolein; 6, 10 mmol/L acrolein.*P < 0.05, **P < 0.01 vs Control group.
3.3 In vitro assessment of allergenicity
Changes to structural and functional properties of soybean protein could influence its allergenicity. To determine whether the lipid peroxidation could affect the allergenicity of soybean protein,western blot was considered to determine the IgE binding capacity of 7S and 11S after AAPH and acrolein treatment (Fig. 3A). And the result showed that the IgE binding capacity of 7S and 11S decreased with increasing the concentrations of AAPH and acrolein. Moreover,the aggregation bands were also observed after lipid peroxidation.The western blot results of 7S and 11S corresponded well with the SDS-PAGE results, confirming the oxidative modification of protein.Indirect ELISA was further carried out to evaluate the IgE binding capacity of the protein samples (Fig. 3B). Interestingly, the IgE binding capacity of 7S and 11S also significantly (P< 0.05) decreased after lipid peroxidation by ELISA analysis. AAPH and acrolein might generate cross-linked polymers to arise, potentially causing linear IgE epitopes to prevent IgE binding. However, both western blot and ELISA could not be considered to predict the ability of allergens to trigger the degranulation of effector cells. Therefore, changes to the potential allergenicity of 7S and 11S after oxidation were assayed by the KU812 cell model. IgE-mediated KU812 cells usually were considered to determine type I allergic responses. The severity of allergic symptoms mainly depended on the secretion of cellular components. Consequently, their measurements are meaningful for examining the state of the body in an allergic state [30]. The release of histamine, IL-4, and other cytokines is an important characteristic of basophils. Compared with native 7S and 11S, the oxidation complexes had lower release of histamine (Fig. 3C) and IL-4 (Fig. 3D). Thus,the results showed that lipid peroxidation significantly inhibits the ability of 7S and 11S to trigger cell degranulation. Zhang et al. [22]reported that peanut allergens were less likely to trigger KU812 cell degranulation after thermal processing. Lipid peroxidation altered the conformational structures of 7S and 11S, potentially altering the antigenic epitopes by intra/intermolecular interactions. The response of KU812 cells might be attributed to oxidation that covered the IgE epitopes and other concomitant structural changes that hindered the degranulation of mast cells and basophils.
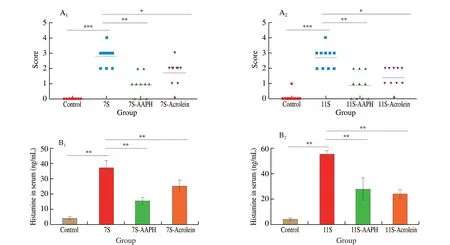
Fig. 4 Hypersensitivity symptoms of BALB/c mice with 7S or 7S-AAPH or 7S-acrolein complexes (A1) and 11S, 11S-AAPH, or 11S-acrolein complexes (A2)were scored on a scale from 0 (no symptoms) to 5 (death) (see Table 1 for details). Levels of plasma histamine in serum from mice with 7S or 7S-AAPH or 7S-acrolein complexes (B1) and 11S, 11S-AAPH, or 11S-acrolein complexes (B2). Results are expressed as mean ± SD. ***P < 0.001, **P < 0.01, *P < 0.05 compared with oxidation complexes group.
3.4 In vivo assessment of allergenicity
The allergenicity of 7S and 11S and their oxidation complexes were further assayed by BALB/c mice model. Compared with native 7S and 11S, anaphylactic shock symptoms following 7S and 11S oxidation complexes were less severe (Fig. 4A1, 4A2). The level of plasma histamine is correlated with allergy symptoms. The plasma histamine content in oxidation complexes groups was also lower than that in the native proteins groups (Fig. 4B1, 4B2), which support the finds of anaphylactic shock symptoms. Moreover, the result of histopathological analysis of duodenal tissue is listed in Fig. 5.Compared with oxidation complexes groups, the intestines were more seriously damaged in mice sensitized with 7S, 11S, indicating that oxidation complexes create a weaker immune reaction than the native proteins. Thus, the allergenicity was lowerin vivowith 7S and 11S oxidation complexes compared to native proteins.
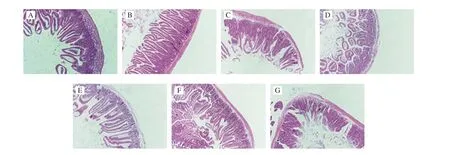
Fig. 5 Effects of control (A), 7S (B), 7S-AAPH (C), 7S-acrolein (D), 11S (E), 11S-AAPH (F), and 11S-acrolein (G) treatment on the morphological structure of duodenum in mice.

Fig. 6 Levels of mMCP-1 (A), IgG (B), IgG1 (C) and IgE (D) in serum from mice with 7S, 7S-AAPH, or 7S-acrolein complexes (A1, B1, C1 and D1) and 11S,11S-AAPH, or 11S-acrolein complexes (A2, B2, C2 and D2). Results are expressed as mean ± SD. **P < 0.01, *P < 0.05 compared with oxidation complexes group.
The levels of IgE, IgG, IgG1 and mMCP-1 in mouse sera were detected to further evaluate the potential allergenicity of 7S and 11S after lipid peroxidation with AAPH and acrolein. Compared with 7S and 11S alone, 7S and 11S oxidation complexes showed reduced lower levels of mMCP-1 (Fig. 6A), IgB (Fig. 6B), IgG1(Fig. 6C), and IgE (Fig. 6D). The results of the allergen-specific immunoglobulin levels are similar to the findings in a previous study that fermentation could reduce the potential allergenicity of soybean proteins [31]. Food allergy is mostly a characteristic type I hypersensitivity, which is almost mediated through IgE antibodies. Lots of IgE antibodies are created when food allergen first enters the body,and these antibodies bind to the target cells. When the same allergen enters the body again, the allergen can bind to the IgE on the target cells to cause the release of inflammatory mediators, resulting in allergy symptoms [32]. Moreover, IgG1 produced in the sensitization period in mice may also cause anaphylactic reactions [33]. Mast cell plays an important role in inflammatory responses. The serum mMCP-1 concentration can reflect the degranulation of mucosal mast cells in mice after being challenged [32]. The increasing level of mMCP-1 can improve the permeability of the epithelium to induce Th2-type immune response,which might cause allergic reactions [32]. Therefore, in this study, the levels of IgE, IgG1 and mMCP-1 in the oxidation complexes groups were lower than that in the native groups, which might be responsible for the lighter hypersensitivity symptoms in the oxidation complexes groups.
The epitope on the allergen plays a decisive role in the immune response. The epitope can react with either antibody or be recognized by the T-cell receptor to trigger the immune response. T-cell receptor recognized epitopes are linear peptides, while antibody recognized epitopes can be either conformational epitopes or linear conformational epitopes [34]. Understanding the changes of epitopes in the allergen might reveal the mechanism of reducing/eliminating the allergenicity of food allergen by food processing methods. The native 7S and 11S groups exhibited more severe allergic symptoms, compared to 7S and 11S oxidation complexes groups, which might be due to the fact that lipid peroxidation modifications. Lipid peroxidation modifications might result from the following: (1) the protein structure was changed after lipid peroxidation, thereby affecting the conformational epitopes of the proteins; (2) lipid peroxidation modified the amino acid residues on the surface space of proteins to affect linear epitopes. Thus, lipid peroxidation might prevent the accessibility of the epitopes to the IgE and T-cell receptors. Thein vitroandin vivostudies confirmed that the allergenicity of 7S and 11S was reduced by lipid peroxidation.Potential mechanisms are likely the inactivation or destruction of antigenic epitopes without denaturing the protein. Comparison of protein denaturation by heat treatment and hydrolysis showed that lipid peroxidation could reduce the allergenicity of 7S and 11S without destroying, or even improving, emulsifying and foaming properties. These results indicate a new possibility of reducing the allergenicity of soybean while improving protein functionality.However, whether the 7S and 11S lipid peroxidation products are safe needs investigation.
4. Conclusion
In conclusion, lipid peroxidation altered the conformational structure of 7S and 11S and improved functional properties of 7S and 11S. Moreover, the oxidation complexes had lower allergenicityin vitroandin vivoassays. Lipid peroxidation decreased the ability for IgE binding, triggering the cell degranulation of 7S and 11Sin vitro. Moreover, the levels of specific IgE, IgG1, IgG, histamine, and mMCP-1 were suppressed, which, in turn, might suppress the allergic reaction, respectively. Therefore, the oxidation of 7S and 11S with AAPH and acrolein both could reduce the allergenicity. The results were expected to understand the change in the functional and allergic properties of soybean during processing and preservation.
Declaration of competing interest
All authors declare that no conflict of the interest exist.
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (32172311), Key-Area Research and Development Program of Guangdong Province (2019B020213001),Guangdong Basic and Applied Basic Research Foundation(2021A1515012413). The authors also thank the support from the Instrumental Analysis Center of Shenzhen University (Xili Campus).
杂志排行
食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard
