Composition analysis of acid hydrolysates from Cucurbita moschata Duch. polysaccharides and their effect on oxidative stress resistance of Caenorhabditis elegans
2023-01-23WenJingYkunZhngJingyingZengJingYoAoxueLuZhiyuFngGeWngWeiminWngYongjunZhng
Wen Jing, Ykun Zhng, Jingying Zeng, Jing Yo, Aoxue Lu,Zhiyu Fng, Ge Wng, Weimin Wng, Yongjun Zhng,*
a Key Laboratory of Specialty Agri-Product Quality and Hazard Controlling Technology of Zhejiang Province, College of Life Sciences, China Jiliang University, Hangzhou 310018, China
b College of Modern Science and Technology, China Jiliang University, Hangzhou 310018, China
Keywords:Chinese traditional starter Fermentation characteristics Thermal processing Allergenicity Wheat matrix
A B S T R A C T Pumpkin polysaccharides (PPe) have a variety of bioactive effects and our previous research showed the acid hydrolysate (PPe-S, a mixture) from PPe had an antioxidative capacity both in vitro and in viro. The aim of this study was to purify PPe-S and investigate the antioxidant stress effects of 2 purif ied components(PPe-S-1 and PPe-S-2) using Caenorhabditis elegans as model organism. The results showed that PPe-S-2 had a notable antioxidant effect, and could significantly enhance the activities of antioxidant enzymes including superoxide dismutase (SOD) (P < 0.01), catalase (CAT) (P < 0.01) and glutathione reductase (GR)(P < 0.05), and increase the level of glutathione (GSH) (P < 0.01), and decreased the content of malondialdehyde (MDA) (P < 0.05). PPe-S-2 could significantly extend the survival time of C. elegans(P < 0.01), which were stress-induced by hydrogen peroxide and methyl viologen. PPe-S-2 was a heteropolys accharide composed of glucose, arabinose, rhamnose and galactose with the molar ratio of 1.00:0.03:0.02:0.14.The molecular weight of PPe-S-2 was 0.73 kDa detected by high performance liquid chromatography. These studies demonstrated that PPe-S-2 obtained by the acid hydrolysis of PPe had a prominent protective effect to the damage induced by the intracellular free radical generating agents.
1. Introduction
Oxidative stress refers to the imbalance of cellular redox between reactive oxygen species (ROS) level and endogenous antioxidant capacity, the relative def iciency of antioxidant capacity. The relative increase of intracellular ROS, which leads to the attack of biological macromolecules and the damage of cells and tissues. Almost all organs of the human body will be injured by oxidative stress, such as fatigue, general weakness, muscle and joint pain, dyspepsia, anxiety,skin itching, headache, diff iculty in concentration, infection and so on.The most common diseases induced by elevated levels of oxidative stress are cardiovascular disease, cancer, diabetes, osteoarthritis and neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, etc. Therefore, reducing the level of oxidative stress in the body is one of the most valuable research f ields in current medicine and health product research.
Pumpkin (Cucurbita moschataDuch.) is an annual herb of Cucurbitaceae vegetable, which is cultivated and consumed around the world. It has a very high nutritional value and health care value,and contains a large number of benef icial trace elements, carotenoids,dietary f iber and other substances. The polysaccharides are considered to be a kind of important biological macromolecules with a variety of biological activities, such as hypoglycemic [1] and hypolipidemic effect [2]. Pumpkin polysaccharides (PPe) have recently been found to have the antioxidant capability bothin vitroandin vivo[3-5],and have great potential for applications in food and pharmaceutical industry as an antioxidant. Some literature and our own previous studies have found that the antioxidant activity of the hydrolysates obtained by further acid hydrolysis of PPe was stronger than that of PPe [6,7], indicated that there might be one or several active fragments of small molecules in PPe, just like enzyme molecules, and these small molecule active fragments had the characteristics of high bioavailability and easy absorption in organisms.
Caenorhabditis eleganshas a short life cycle, small individuals and easy to cultivate [8], and is an excellent model organism for studying oxidative stress and an attractive model for studying the mechanism of stress response [9]. Our previous studies suggest that PPe and theiracid-hydrolysis (a mixture) could enhance the activities of the antioxidant enzymes (catalase (CAT) and glutathione reductase(GR)) and increase the level of glutathione (GSH) in type 2 diabetes mellitus rats [7], and ameliorate the oxidative stress level and exhibit the ability on stress resistance inC. elegans[10]. However, the exact molecular weight and molecular composition of the hydrolysate with antioxidant activity are not clear after the hydrolysis of PPe.The current study was to prepare the acid hydrolysate (PPe-S) from PPe based on our previous study. PPe-S were purified by Sephadex G-50 and two main component named PPe-S-1 and PPe-S-2 was obtained, their monosaccharide compositions and molecular weights were determined and compared with gas chromatography-mass spectrometry (GC-MS) and high performance liquid chromatography(HPLC), respectively, and then antioxidant potentials the two hydrolysates were assesse dusingC. elegansasmodel animals. This study is helpful to find the active center in PPe.
2. Materials and methods
2.1 Materials and chemicals
Fresh and uniformly shaped pumpkins (Cucurbita moschataDuch.) were purchased from local farmers’ market (Hangzhou, China).The pumpkin was peeled, removed the seeds and sliced into filaments(0.25 cm × 0.25 cm × 4.0 cm), and dried in a hot air circulation drying oven (DHG-9240A, China) at 60 °C, then pulverised to a particle diameter of 0.15 mm.C. elegansstrains N2 (wild-type) andEscherichia colistrain OP50 were given by College of Animal Sciences, Zhejiang University (China); The total superoxide dismutase (T-SOD) assay kit (hydroxylamine method), CAT assay kit (visible light), GR assay kit (ultraviolet colorimetry), GSH assay kit (colorimetric method) and malondialdehyde (MDA)assay kit (thiobarbituric acid (TBA) method) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemical reagents were of analytical or chromatographic purity and were obtained from commercial sources. Deionized water was used throughout the experiment.
2.2 Preparation of the acid hydrolysate from PPe
Extraction of PPe was performed according to our previous method [10]. In brief, pumpkin powders were extracted with hot water and alcohol (95%,V/V), respectively, the tow extracts were combined and isolated by chloroform andn-butanol successively. Then, alcohol was added to make a final 80% alcohol concentration at 4 °C more than 12 h and centrifuged at 3 000 r/min for 5 min. The precipitates were re-dissolved in water, and alcohol (30%, 50%, 70%, and 90%,V/V, respectively) were used to fractionate further. Then 70%of alcohol precipitation was purified by Sephadex G-50 and the main fraction (PPe) was obtained. The preparation of PPe-S was based on the previous method of our laboratory [7], that is, under the optimal hydrolysis conditions obtained by response surface optimization(hydrolysis temperature 60 °C, hydrolysis time 85 min, sulfuric acid concentration 1.80 mol/L), then neutralized, and dialyzed 24 h with dialysis membrane pores of 1.0 kDa. PPe-S were mainly composed of sugars ((87.03 ± 1.21)%) and contained a certain amount of uronic acid ((37.61 ± 0.97)%), protein ((1.25 ± 0.78)%) and sulfur groups((0.14 ± 0.04)%), and were a heteropolys accharide composed of glucose, arabinose, rhamnose and galactose [7,10]. The hydrolyzates were purified by Sephadex G-50 and two component named PPe-S-1 and PPe-S-2 obtained (Fig. 1).

Fig. 1 Elution curve of PPe-S by Sephadex G-50.
2.3 Antioxidant activities of PPe-S-1 and PPe-S-2 in vivo
2.3.1 Oxidative stress resistance to hydrogen peroxide and methyl viologen
C. elegansculture was performed in accordance with our previous method [10]. The L4 stage synchronized worms were divided into 7 experimental groups as follow: negative control group, positive control group (vitamin C, metformin hydrochloride), PPe-S-1 group (0.5 and 1.0 mg/mL) and PPe-S-2 group (0.5 and 1.0 mg/mL). The worms were cultured at 20 °C for 1 day, then PPe-S-1, PPe-S-2, vitamin C(1.0 mg/mL), metformin hydrochloride (1.0 mg/mL) or M9 buffer with 5-fluorouracil (12.5 mg/mL, preventing adults from generating newborn progenies) were added to NGM, and continue to cultivate for 48 h at 20 °C with triplication (n= 3), and each NGM plate was coated with inactivated OP50 (50 µL) by boiling water bath. Next,C. eleganswere transferred to 96 well plates with hydrogen peroxide(200 µL, 0.001 mol/L) or methyl viologen (200 µL, 83.33 mg/mL)and incubated at 20 °C. The death number ofC. eleganswas recorded every 40 min or 1 h. WhenC. elegansfailed to respond to the gentle touch of platinum wire, they were recorded as dead [11].
2.3.2 Determination of the SOD, CAT, GR activities and the GSH and MDA levels
Worms with 3 independent experiments were treated with samples (PPe-S-1 or PPe-S-2, 0.5, 1.0 mg/mL), vitamin C(1.0 mg/mL) or M9 buffer for 2 days as described above, and washed 3 times with M9 buffer and diluted to 10% (V/V). Then, the activity of SOD, CAT and GR was measured following the instructions of the assay kits (T-SOD: A001-1-1, CAT: A007-2, GR: A062). The level of GSH or MDA was detected following the instructions of the assay kits (GSH: A006-2, MDA: A003-1).
2.4 Monosaccharide compositions analysis
The monosaccharide compositions of PPe-S-1 and PPe-S-2 were analysed according to the reported method with minor modification [12].The monosaccharide, including mannose, xylose, arabinose,rhamnose, glucose and galactose, was used as a standard. An Agilent 7890A gas chromatography instrument coupled to an Agilent 5975C mass spectrometer was used to analyze the monosaccharide compositions.
2.5 Molecular weights determination
The Agilent 1100 HPLC system equipped with a Waters Ultrahydrogel Linear column (7.8 mm × 300 mm, 10 µm, USA) and a differential refractive index detector was used to detect the molecular weight. The mobile phase was phosphoric acid buffer, the flow rate was 0.5 mL/min, and the injection volume was 20 µL. Five dextran standards with molecular weights of 505, 1 200, 4 300, 20 100 and 91 100 Da were used for calibration. The standard equation showed a linear relationship between retention time (tR) and logarithm (MW),and the molecular weight of the sample was calculated bytR.
2.6 Statistical analysis
Data were performed as mean ± SD of 3 replicate values. A least significant difference (LSD) as post-hoc test was utilized to determine the difference among samples and control groups with SPSS 22.0(IBM, USA).
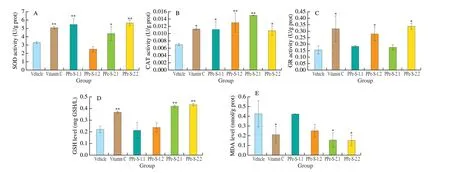
Fig. 2 Effects of PPe-S-1 and PPe-S-2 on SOD (A), CAT (B) and GR (C) activities and GSH (D) and MDA (E) levels in C. elegans.The number of worms used per experiment was 100 with triplicate (n = 3). *P < 0.05, significant difference compared with the control group;**P < 0.01, highly significant difference compared with the control group.
3. Results and discussion
3.1 Antioxidative potentials of PPe-S-1 and PPe-S-2 in C. elegans
3.1.1 Enhanced antioxidant enzyme activity of C. elegans
Antioxidant enzyme system plays an essential role in balancing redox homeostasis and reducing free radicals mainly caused by oxidative stress [13,14]. SOD, CAT and GR are the main part comprising antioxidant enzyme system. SOD can convert superoxide anion into hydrogen peroxide, while CAT catalyzes the conversion of hydrogen peroxide into water and oxygen, reducing the level of intracellular ROS [15]. Therefore, the enzyme defense system makes an essential effect on the response to excessive ROS.
As shown in Fig. 2A, certain concentrations of PPe-S-1 and PPe-S-2 could significantly improve the SOD activity in worms(P< 0.05 orP< 0.01). Compared with the vehicle group, the SOD activity of PPe-S-1 in worms was enhanced by 65.96% at 0.5 mg/mL, and the SOD activity of PPe-S-2 was enhanced by 32.78% and 71.68% at 0.5 and 1.0 mg/mL, respectively. It could be seen that for PPe-S-2, the higher the concentration of PPe-S-2,the better the effect in a certain range. But for PPe-S-1, the effect of low concentration was better than that of high concentration. It was speculated that the molecular weight of PPe-S-1 was higher than that of PPe-S-2, and the high concentration of PPe-S-1 would produce high osmotic pressure. SOD is the first line of defense against ROS.The acid hydrolysate of PPe, especially PPe-S-2, could improve the SOD activity in worms and had a good effect on reducing superoxide anion free radicals.
From Fig. 2B, different concentrations of PPe-S-1 and PPe-S-2 could significantly improve the CAT activity in worms (P< 0.05 orP< 0.01), and there was no significant difference between the samples(PPe-S-1 and PPe-S-2) group and vitamin C group. Compared with the vehicle group, the CAT activity of PPe-S-1 in worms was enhanced by 59.73% and 82.18% at 0.5 and 1.0 mg/mL, and the CAT activity of PPe-S-2 in worms was enhanced by 115.03% and 55.08%at 0.5 and 1.0 mg/mL, respectively. CAT can catalyze peroxidase reaction, the acid hydrolysate of PPe (both PPe-S-1 and PPe-S-2)could enhance the CAT activity and play an important role in cell defense against oxidative damage.
GR is one of the most essential enzymes in human redox system, which can reduce the oxidized glutathione disulfide (GSSG)to regenerate GSH in the presence of NADPH (H+), acting as a central role in preventing the oxidation. As shown in Fig. 2C, high concentration of PPe-S-1 and PPe-S-2 could significantly increase the GR activity in worms (P< 0.05), while low concentration of them could increase the GR activity sightly without significant level.Compared with the vehicle group, the GR activity of PPe-S-1 in worms was enhanced by 18.08% and 80.55% at 0.5 and 1.0 mg/mL,and the GR activity of PPe-S-2 in worms was enhanced by 13.45%and 118.51% at 0.5 and 1.0 mg/mL, respectively. Accordingly, PPe-S-1 and PPe-S-2 at the high concentration (1.0 mg/mL) could significantly enhance the activity of GR, and there was no difference compared with positive control group (P> 0.05), indicating that there was a dosedependent relationship for PPe-S-1 and PPe-S-2 to the activity of GR.
3.1.2 Enhanced the level of GSH and reduced the level of MDA in C. elegans
GSH, as a part of non-enzymatic defense system, is the central intracellular thiol-containing compound, which plays a pivotal role in scavenging free radicals and couples with GSSG to maintain the intracellular redox homeostasis. As shown in Fig. 2D, both of the different concentrations of PPe-S-2 could significantly increase the level of GSH in worms (P< 0.01), and there was no difference between PPe-S-2 group and vitamin C group (P> 0.05). Compared with the vehicle group, the GSH level of PPe-S-2 in worms was increased by 90.21% and 95.87% at 0.5 and 1.0 mg/mL. While two different concentrations of PPe-S-1 had no effect on the level of GSH in worms, and there was no significant difference compared with the control group (P> 0.05). GSH, as the main compound determining the redox state of cells, can scavenge O2-·, H2O2and lipid hydroperoxide (LOOH). PPe-S-2 could improvethe level of GSH, and reduce organic hydroperoxide to water and corresponding ethanol,then remove the active oxygen speciesin vivo.
The production of cellular ROS results in the damage to lipid,MDA is the secondary end-product of lipid peroxidation (LPO),which is a marker of oxidative damage in cellular [16]. As shown in Fig. 2E, compared with the control group, low and high concentration of PPe-S-2 could significantly decrease the level of MDA in worms by 170.21% (P <0.05) and 178.38% (P< 0.05) at 0.5 and 1.0 mg/mL,respectively. What’s more, the MDA level in worms with the treatment in both low and high dose of PPe-S-2 was lower than vitamin C, indicating that PPe-S-2 had remarkable effect on the reduction of MDA level and better than positive control (vitamin C).Two different concentrations of PPe-S-1 had no effect on the level of MDA in worms, and there was no significant difference compared with the vehicle group (P> 0.05).This results was consistent with the result of GSH level in worms, and indicate that PPe-S-2 could reduce the level of oxide in worms, improve the antioxidant defense ability,then reduce DNA damage and direct inhibition of protein expression.
3.1.3 Improved the oxidative stress resistance of C. elegans
Oxidative stress exerts many negative effects on organism via highly reactive free radicals, initiating damage of cell, organ and body viaLPO, protein peroxidation and DNA damage [17,18]. As a result,the lifespan of organism will be influenced by the factors mentioned above, but the antioxidants could prolong the longevity [19,20].
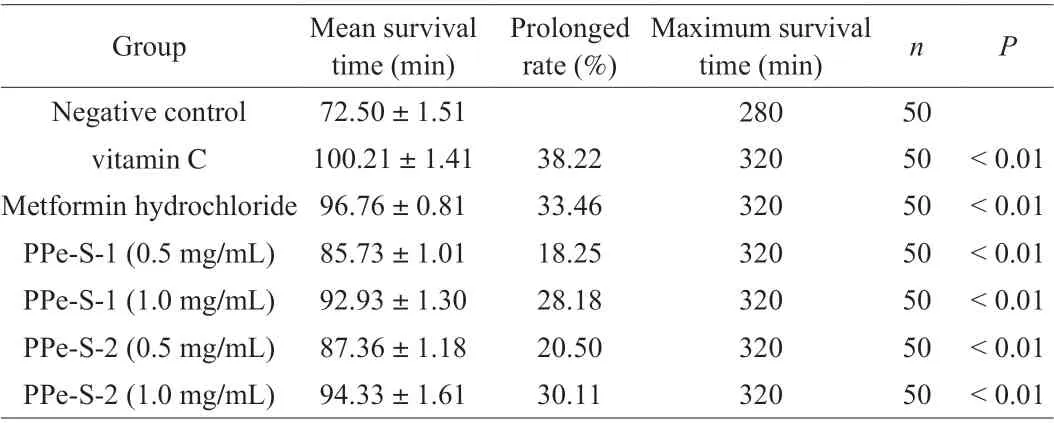
Table 1 The effects of PPe-S-1 and PPe-S-2 on the survival time of C. elegans induced by hydrogen peroxide.
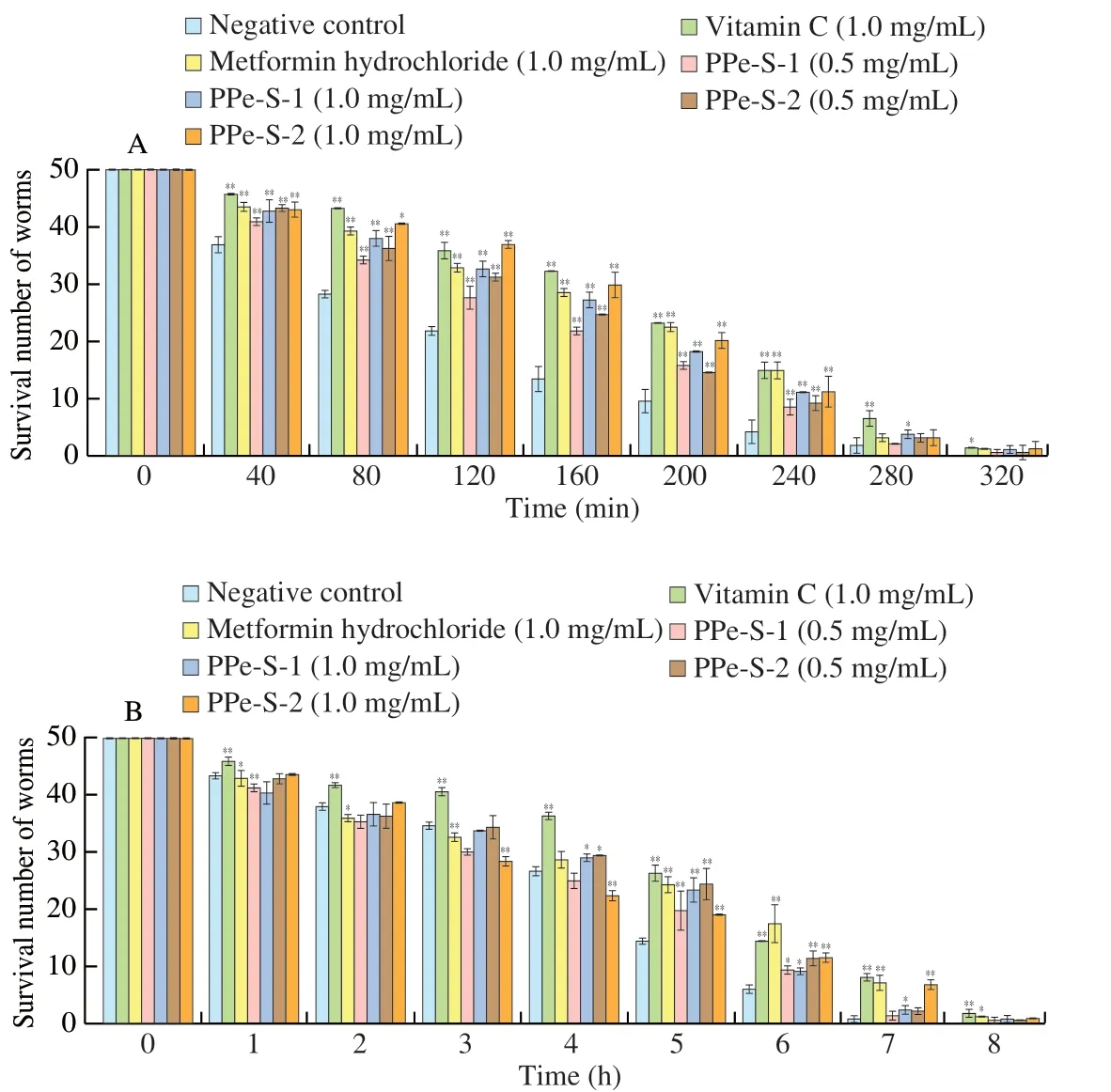
Fig. 3 The effects of PPe-S-1 and PPe-S-2 on resistance to hydrogen peroxide (A) and methyl viologen (B) induced oxidative stress.
As shown in Fig. 3A and Table 1, metformin hydrochloride and vitamin C could significantly enhance the resistance ofC. elegansto hydrogen peroxide-induced oxidative stress compared with the negative control group (P< 0.01). Similarly, both low and high concentration of PPe-S-1 and PPev-S-2 could significantly extended the lifespan of the worm and there were right-shifted survival curves of PPe-S-1 and PPe-S-2 compared with the negative control group (P< 0.01).The maximum survival time and mean survival time ofC. elegansin negative control group were 280 min and (72.50 ± 1.51) min,respectively. The two indexes ofC. eleganstreated with low and high concentrations of PPe-S-1 and PPe-S-2 were higher than those in the negative control group. In addition, the prolonged rate of average survival time of PPe-S-1 and PPe-S-2 at 1.0 mg/mL was higher than that of 0.5 mg/mL, indicating that the higher dose had better life-extension ability. Hydrogen peroxide can cause DNA damage and gene mutation of human genetic material, and can enter the free radical reaction chain, which is closely related to the occurrence of various diseases. Numerous reports have confirmed that polysaccharides derived from natural plant had antioxidant effects on against hydrogen peroxide-induced oxidative stress by modulating antioxidative stress-related pathways [21-23]. Notably, PPe-S-1 and PPe-S-2 had protective effect on worms under oxidative stress induced by hydrogen peroxide.
From Fig. 3B and Table 2, it was obviously showed that both low and high concentration of PPe-S could significantly extend the lifespan of worms exposed to methyl viologen compared with the negative control (P< 0.01). The maximum survival time and mean survival time ofC. elegansin negative control group were 7 h and (2.07 ± 0.01) h, respectively, and the two indexes ofC. eleganstreated with low and high concentration of PPe-S-1 and PPe-S-2 were higher than those of negative control group, indicating that PPe-S-1 and PPe-S-2 had protective effect on worms under oxidative stress induced by methyl viologen. The prolonged rate of average survival time of PPe-S-1 at 1.0 mg/mL was higher than that of 0.5 mg/mL, consistent with the results of hydrogen peroxide-induced oxidative stress. The prolonged rate of PPe-S-2 at 1.0 mg/mL was close to that of 0.5 mg/mL, it showed that the concentration dependence was not obvious. Methyl viologen is a widely used dipyridyl herbicide, which can induce oxidative stress in cells and increase ROS production, lead to mitochondrial dysfunction and then cause damage to the body. It could be seen that both PPe-S-1 and PPe-S-2 could alleviate the oxidative stress injury induced by methyl viologen.
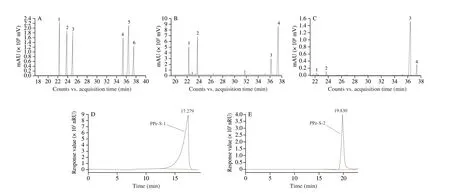
Fig. 4 GC-MS spectrum ((A) standard monosaccharide mixture: 1. arabinose; 2. rhamnose; 3. xylose; 4. mannose; 5. glucose; 6. galactose; (B) PPe-S-1;(C) PPe-S-2) and HPLC profile of PPe-S-1 (D) and PPe-S-2 (E).

Table 2 The effects of PPe-S-1 and PPe-S-2 on the survival time of C. elegans induced by methyl viologen.
3.2 Molecular composition analysis of PPe-S-1 and PPe-S-2
The monosaccharide compositions of PPe-S-1 and PPe-S-2 was analyzed by the GC-MS method. The chromatogram profiles of PPe-S-1 and PPe-S-2 was shown in Figs. 4A-C.
From Figs. 4A-C, it was concluded that both PPe-S-1 and PPe-S-2 were heteropolys accharide. By comparing the peak time of GC-MS spectrum of the two samples with that of standards, PPe-S-1 was observed to mainly contain glucose, arabinose, rhamnose and galactose, with the molar ratio of 1.00:1.58:1.13:3.90, and galactose was the predominant component. PPe-S-2 contained the same monosaccharide with the molar ratio of 1.00:0.03:0.02:0.14,and glucose was the predominant component. The monosaccharide composition of PPe-S-1 and PPe-S-2 was the same as that of PPe, but the molar ratio was different. The molar ratio of 4 monosaccharides in PPe was 1.00:5.19:1.12:3.91 [12].
3.3 Molecular weight of PPe-S-1 and PPe-S-2
β-Glucan standards were used to prepare the linear standard equation, i.e. lgMW= -0.529 5tR+ 13.362 0 (R2= 0.998 1). As shown in Figs. 4D-E, the elution curves of PPe-S-1 and PPe-S-2 were a single peak, indicating that both PPe-S-1 and PPe-S-2 were homogeneous.The molecular weight of PPe-S-1 and PPe-S-2 was calculated as 16.32 and 0.73 kDa using the equation of linear regression, respectively.
The antioxidant activities of polysaccharides are related to their molecular weight, chemical composition, and other characters [24].Studies have shown that there was a positive correlation between the number of small molecular fragments and antioxidant capacity, and the functional groups of the side chain in polysaccharides were likely to induce and increase free radical scavenging ability [25]. PPe-S-1 and PPe-S-2 had the same monosaccharide composition, different molecular weight and molar ratio of monosaccharide, but both of them made a positive effect on inhibiting oxidative injury of worms and prolonged their lifespan.
4. Conclusion
The acid hydrolysates of PPe contained PPe-S-2, which had great potential in the antioxidant defense ofC. elegansmodel system. PPe-S-2 had excellent antioxidant capacity which could enhanced intracellular antioxidant systems comprising SOD by 71.68% (P< 0.01), CAT by 55.08% (P< 0.05) and GR by 118.51% (P< 0.05), increase the level of GSH by 95.87%(P< 0.01), and reduce the level of MDA by 178.38% (P< 0.05)at the concentration of 1.0 mg/mL. In addition, PPe-S-2 showed the significant ability of longevity extension onC. elegansunder oxidative stress induced by hydrogen peroxide and methyl viologen(P< 0.01), indicating that the antioxidant effects PPe-S-2 possessed were achieved by modulation of antioxidant enzymatic system and lifespan.The molecular weight PPe-S-2 was 0.73 kDa, and was a heteropolys accharide composed of glucose, arabinose, rhamnose and galactose with the molar ratio of 1.00:0.03:0.02:0.14. Future studies can be performed to obtainthe mechanistic insights, and to provide a therapeutic support for antioxidant products.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This study was supported by the Key R & D Projects of Department of Science and Technology of Zhejiang Province(2020C02035).
杂志排行
食品科学与人类健康(英文)的其它文章
- The role of probiotics in prevention and treatment of food allergy
- Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses
- The role of gut microbiota and its metabolites short-chain fatty acids in food allergy
- Association of nutrients intake during pregnancy with the risk of allergic disease in offspring: a meta-analysis of prospective cohort studies
- Purif ication and immunoglobulin E epitopes identif ication of low molecular weight glutenin: an allergen in Chinese wheat
- Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard
