Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
2023-01-22HuairuiWangYaoChengXueZhangYingpingWangHuiZhao
Huairui Wang, Yao Cheng, Xue Zhang, Yingping Wang*, Hui Zhao*
a State Local Joint Engineering Research Center of Ginseng Breeding and Application, College of Chinese Medicinal Materials, Jilin Agricultural University, Changchun 130033, China
b School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
c School of Pharmaceutical Science and Technology, Health Sciences Platform, Tianjin University, Tianjin 300072, China
Keywords:Ginseng Color Microstructure Ginsenosides α-Amylase
A B S T R A C T
1. Introduction
Ginseng (Panax ginsengMeyer, Araliaceae) is one of the most famous Chinese herbal medicines officially listed in theChinese Pharmacopoeia. It has been widely used in traditional medicine due to its various health-promoting properties. In herbal market, ginseng is consumed mainly after two types of processing, that is white and red ginseng [1]. White ginseng is produced by drying the fresh ginseng in sunlight. According to the Traditional Chinese Medicine(TCM) theory, white ginseng has been used to treat diabetes [3] and enhance immunity [4]. Red ginseng is manufactured by steaming the fresh ginseng at 95–100 °C for 2–3 h, then drying until the moisture content is less than 15% [2]. As for the effects of pharmacological,red ginseng has the effects of treatment of diabetes [5], anticancer [6],antioxidant [7], anti-inflammatory effects and fewer side effects than white ginseng [8], also has a better regulating metabolism effect, can be used to treat hyperlipidemia, obesity [9].
However, the differences induced by processing between white ginseng and red ginseng have rarely been reported. Whether red ginseng really has good health benefits as Chinese folk says, which requires us to further discuss the differences induced by processing.Thus, the characterization of chemical profiles of white ginseng and red ginseng should be helpful to explaining the variations of composition caused by processing and further recognizing the mechanisms such as its hypoglycemic and regulating metabolism effects, and try to carry out the comparative experiment of anti-α-amylase.Additionally, the characterization of color and microstructure of white ginseng and red ginseng could also be useful to illustrating the physical variations caused by processing among them.
In this research, quantifying the bioactive components of ginseng during processing could investigate chemical constituent alteration.Several analytical methods, such as high performance liquid chromatography-ultraviolet (HPLC-UV) [10], liquid chromatograph mass spectrometer (LC-MS) [11-13], and HPLC- evaporative lightscattering detector (ELSD) [14] have been used to evaluate the quality of ginseng. However, the application of these analytical methods is often restricted by the availability of reference substances and the high costs involved. Thus, the objectives of this study were to develop a simple, accurate, and reliable HPLC method for the simultaneous determination of eight bioactive ginsenosides (Fig. 1),including ginsenoside Rf, ginsenoside Rb1, ginsenoside Rg2,ginsenoside Rh1, ginsenoside Rc, ginsenoside Rb2, ginsenoside Rb3 and ginsenoside Rd; and compare the content of differences induced by processing. Remarkably, the occurrence of diabetes is increasing around the world and is now identified as one of the foremost threats to human health [15-17]. Sinceα-amylase was one of the therapeutic targets for diabetes [18,19]. Thus, the anti-α-amylase capacity was also determined to evaluate the differences of hypoglycemic activity between white and red ginseng. Molecular docking was used to screen the most promising anti-α-amylase inhibitors. Moreover,color parameters and scanning electron microscope were determined to elucidate the differences of ginseng color and microstructure induced by processing procedure. To our knowledge, this is the first systematic study on the composition, structure, physicochemical properties and anti-α-amylase function between white and red ginseng. This study provides detailed information could be helpful to explaining the differences induced by processing and recognizing the processing mechanism. In addition, the present study can also lead to the further research on its application value in human health and medical wellness.
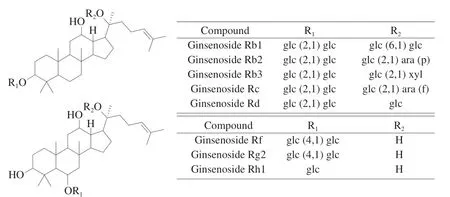
Fig. 1 Chemical structures of eight major ginsenosides of ginseng.
2. Materials and methods
2.1 Chemicals and materials
2.1.1 Materials
Thirty-six species of white ginseng and red ginseng were collected from Changbai Mountain, Jingyu and Tonghua (Tables 1 and 2), Jilin.White ginseng and red ginseng samples were crushed by shredder and sieved by 40-meshes. The powders were stored at –20 °C till extraction.
2.1.2 Chemicals and reagents
Acetonitrile (ACN) and methanol were purchased from Tianjin Kangde Technology Co., Ltd., China.n-Butanol was obtained from Tianjin Fuyn Fine Chemical Co., Ltd., China. Eight ginsenosides,which purity is reaching HPLC-grade and higher than 98%,including ginsenoside Rb1, Rb2, Rb3, Rc, Rd, Rf, Rh1 and Rg2 were purchased from Shanghai Yuanye Biotechnology Co., Ltd., China.3,5-Dinitrosalicylic acid (DNS) was obtained from Shanghai Macklin Biotechnology Co., Ltd., China. Sodium hydroxide was purchased from Tianjin Jiangtian Chemical Co., Ltd., China. Potassium sodium tartrate and anhydrous sodium sulfite were obtained from Tianjin Damao Chemical Reagent Factory of China. Alpha amylase (Bacillus subtilis) was purchased from Shanghai Yuanye Biotechnology Co.,Ltd., China. Soluble starch was obtained from Beijing Solarbio Science & Technology Co., Ltd., China. Ultrapure water was produced by milli-Q system.
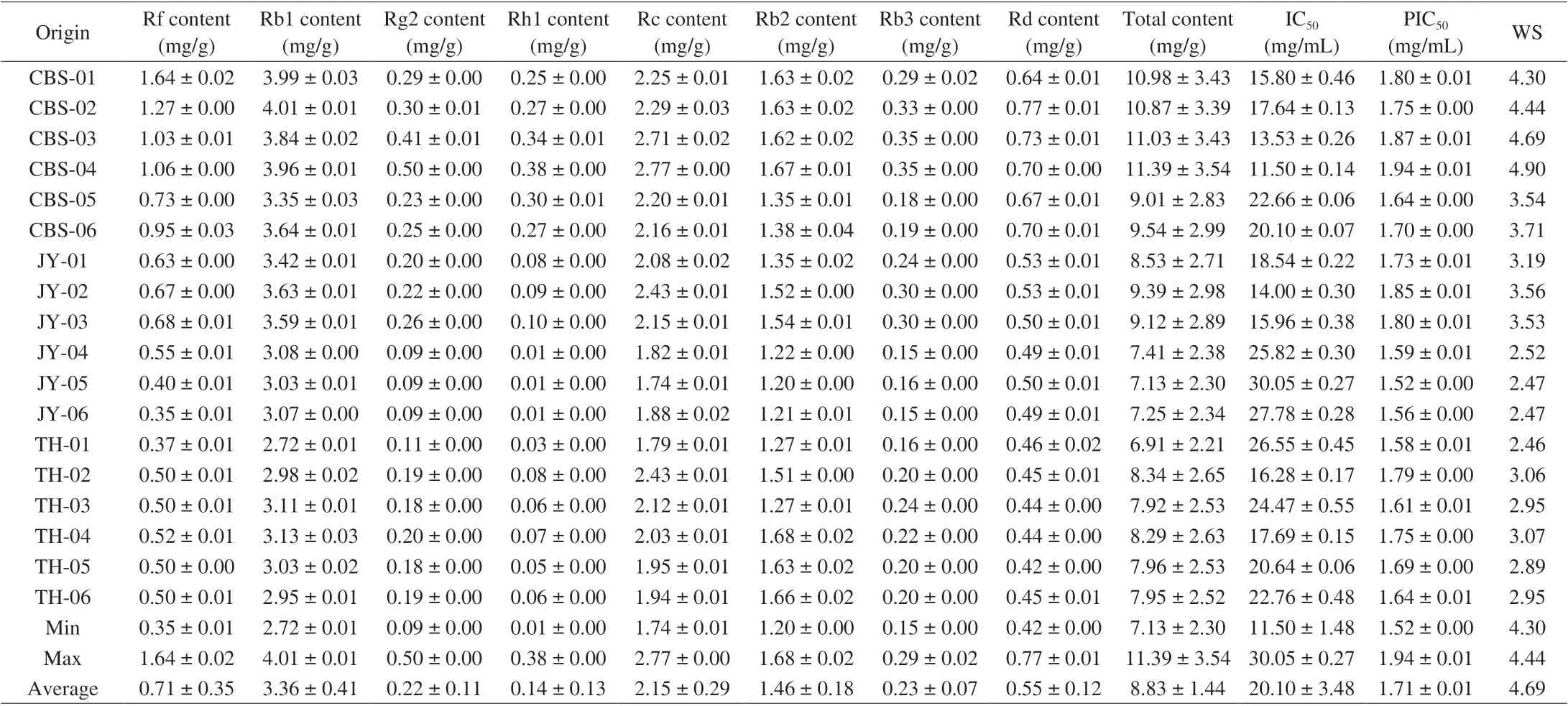
Table 1 Eight ginsenosides yields, α-amylase inhibitory activities, and IC50 of white ginseng extracts.
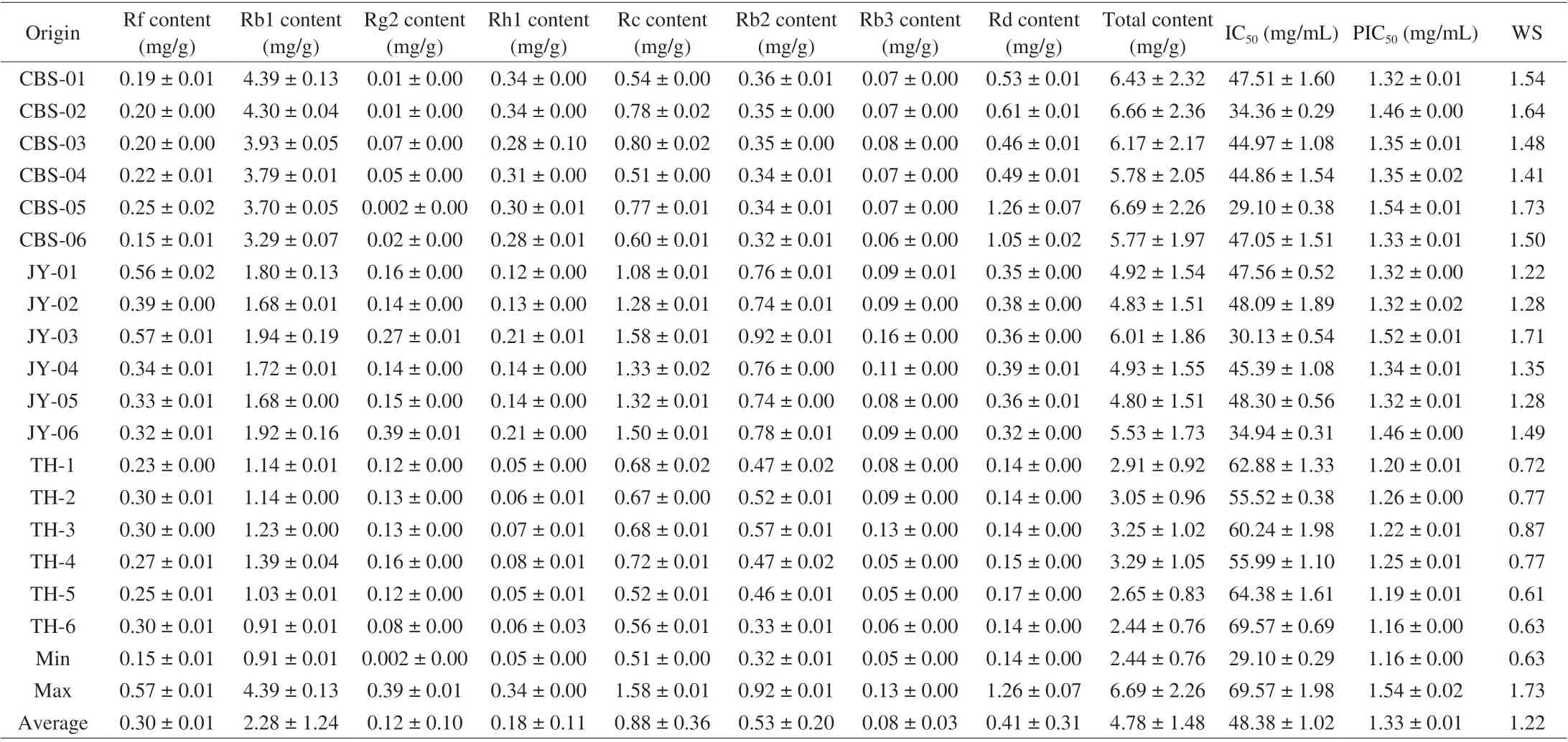
Table 2 Eight ginsenosides yields, α-amylase inhibitory activities, and IC50 of red ginseng extracts.
2.2 HPLC method
2.2.1 Instruments and conditions
Analyses were performed on an Shimadzu i-Series HPLC system that made up of a binary pump, an automatic injector and a photodiode array (PDA) detector, and liquid chromatographic separation was achieved via a Kromasil C18(4.6 mm × 250 mm,100 Å, 5 μm). The mobile phases were consisted of ultrapure water(A), acetonitrile (B), the flow rate was 1 mL/min, elution procedure:0–5 min, 5% B; 20–70 min, 32% B, the detection wavelength was 203 nm, the injection volume was 10 μL, and the column temperature was maintained at 25 °C.
2.2.2 Preparation of sample solutions
All the thirty-six batches of samples were crushed until uniformly mixed and passed through a 40-mesh sieve. To avoid the misleading results due to the extraction efficiency difference, the sample was prepared as follows: an aliquot (1 g) was accurately weighed. Then sample powders (1 g) were extracted with methanol (m/V= 1:40)by water bath shaking extraction for 2 h at 60 °C. After extraction and centrifugation for three times, the supernatants were combined and dryness by water bath. Then the extract was dissolved in 10 mL ultrapure water (m/V= 1:10), and extracted overnight with water saturated withn-butanol (m/V= 1:20). Then-butanol layer was collected and dried by rotary evaporation, the extract was diluted to 1 mL with methanol after desiccating. The solutions were filtered with a 0.22 μm millipore membrane filter before HPLC analysis.
2.2.3 Preparation of standard solutions
The concentrations of ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Rf,Rh1 and Rg2 in the mixed working stock standard solution (Marked as S1) were, separately, 4.8 mg/mL in methanol. To make calibration curves, eight series concentrations were then separately prepared by diluting the working stock solution S1 with 0.83 fold difference. That is, 4 mL of former standard solution and 0.8 mL of methanol were taken to make 4.8 mL of standard solution. The standard solution was preserved in a –20 °C refrigerator prior to analysis.
2.2.4 Analytical curves: limits of detection (LOD) and quantification (LOQ)
The series of standard solutions containing compounds at eight different concentrations were analyzed in triplicate by the described method with injection volumes of 10 μL. The calibration curve for each compound was constructed by plotting the peak area (y) against the corresponding concentration of the standard solutions (x, mg/mL).
2.2.5 Precision, repeatability, stability, and recovery
The precisions of the method were determined by analyzing eight consecutive injections using standard solutions prepared at three different concentration levels (low, medium, and high).
The repeatability of the analysis method was evaluated based on six replicates of samples from the same source using the method described above.
The stability of the sample solutions was tested by injecting the same sample solutions at 0, 4, 8, 12, 24, and 48 h after preparation using the developed method.
To determine the recovery rates, a known amount of the mixed standard solution was added to 1.0 g of the samples quantified previously, and the spiked samples were analyzed three times using the described method.
2.2.6 Relative correcting factor
The RCF was calculated according to previous studies [20].Owing to ginsenoside Rb2 was readily available, high abundance and separation easily from other components in the sample, the ginsenoside Rb2 was selected as the internal standard, and a formula (1) was used to calculate the RCFs for other components.

WhereAsis the peak area of the internal standard,Csis the concentration of the internal standard,Aiis the peak area of compound i, andCiis the concentration of compound i in the sample solution,through formula (1), we can deduce formula (2).

The concentration of each compound in the sample solution can be calculated by formula (2).
2.3 Ginseng quality analysis
2.3.1 Color evaluation
The color of the white ginseng and red ginseng samples were determined using the CM-5 colorimeter (konica minolta, inc. Japan),which measures the Hunter’s colourL*,a* andb* values.L*represents a light-dark spectrum with a range from 0 (black) to 100(white), whilea* shows a red-green spectrum with a range from –60(green) to +60 (red), andb* indicates a yellow-blue spectrum with a range from –60 (blue) to +60 (yellow). Prior to use, the instrument was calibrated using black and white tile standards that came with the instrument. The data were obtained by measuring the same positions on the samples to obtain representative values. Three replicate measurements were taken, and the results were averaged.
2.3.2 Scanning electron microscope
The microstructures of white and red ginseng samples were measured by the scanning electron microscope (ZEISS Gemini SEM 500) following the method described by Ju et al. [21]. For SEM observations, the samples were placed on aluminum stubs with double-sided adhesive tape. Then the samples were sputter-coated(SC 7640, Quorum Technologies Ltd., Newhaven, UK) for 5 min with gold in order to stabilize the structure. Duplicate specimens were viewed at different magnifications, and the representative areas images were saved for experimental analysis.
2.3.3 Weighted equation and rules
According to published article [22] with a slightly modification,weighted equation and rules were established. In inhibitory effect of the extracts of different ginseng onα-amylase activity, each ginsenosides have their own weights to influence theα-amylase activities.
Acquire the different correlation (R2) from various scoring functions, PIC50as well as total content of ginsenoside to obtain their own weights. Weighted equation was as follows:

2.3.4 Inhibitory effect of the extracts of different ginseng on α-amylase activity
The method ofα-amylase inhibitory activity was determined according to previous research [23] with a revision slightly. Theα-amylase activity was assessed using soluble starch (1%) as a substrate in PBS buffer (pH 6.8). The method of operation: firstly,200 µL ofα-amylase solution (20 U/mL) was mixed with 20.0 mL of PBS buffer (pH 6.8) and 200 µL of the extracts of ginseng that dissolved in PBS (5–50 mg/mL) (reaction system A). After incubation at 25 °C for 10 min, 200 µL of the starch solution (1%) was added and the mixture re-incubated at 25 °C for 10 min. The reaction was terminated by adding 400 µL of DNS solution and boiled for 5 min in a boiling water bath; 200 µL of the extract of ginseng that dissolved in PBS was mixed with 200 µL of PBS buffer (pH 6.8) (reaction system B); 200 µL ofα-amylase solution was mixed with 200 µL of PBS buffer (pH 6.8) (reaction system C); 200 µL of PBS was mixed with 200 µL of PBS buffer (pH 6.8) (reaction system D). The mixture of 50 µL reaction solution and 150 µL ultrapure water was added into 96 plates, and detected at 540 nm. Acarbose was adopted as a positive control. The assay was performed in triplicate. Inhibition (%) was calculated via the formula (4).
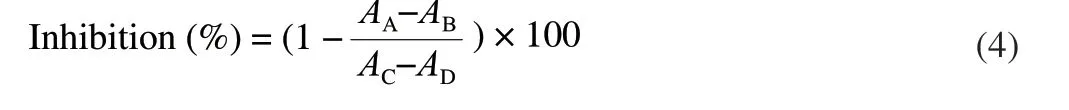
WhereAA–ADrepresent the absorbance of reaction system mentioned above.
2.4 Molecular docking
To investigate the interaction between target protein and studied compounds, molecular docking experiments against theα-amylase and ginsenoside were carried out by using AutoDock Vina program [24].The structures of ligands were achieved from the PubChem database(http://pubchem.ncbi.nlm.nih.gov). The structure ofα-amylase (PDB code: 5KEZ) was obtained from the protein data bank (http://www.rcsb.org/pdb). Receptor preparation includes: firstly, removing water molecules and impurity ions; secondly, extraction of co-crystallized ligand; thirdly, adding polar hydrogen atoms. Docking calculations were performed based on the Lamarckian genetic algorithm (LGA).Most of the parameters for the docking calculations were set to the default values. The predicted binding energy (kcal/mol) was calculated. From the docking results, the best scoring (i.e., with the lowest free energy of binding) docked model of a compound was chosen to represent its most favorable binding mode.
2.5 Statistical analysis of data
All data were preserved as mean ± standard deviation (SD). The mean value and SD of experiment data were calculated by Microsoft Office Excel 2010 software (Microsoft, USA), Origin Pro 9 software(Origin Lab, USA) was used for drawing.
3. Results and discussion
3.1 Analytical method validation
3.1.1 Linearity, LOD and LOQ
The linearity, limit of detection, and limit of quantification of the analyses were studied to validate the proposed method. The chromatograms of standard compounds and typical chromatogram of sample were depicted in Fig. 2. The correlation coefficients of the eight components were > 0.998 9, indicating satisfactory linearity at their appropriate range of concentration, and the calibration curve was considered effective for quantitative analysis within the test concentration ranges. The LOD and LOQ were determined as the concentrations of a compound with a signal-to-noise ratio (RS/N) of 3:1 and 10:1, respectively (Table 3).
3.1.2 Precision, repeatability, stability and recovery
In the precision validation, the RSD values of the intraday precisions as well as interday precisions ranged from 0.13% to 0.38%and from 0.15% to 2.96%, respectively (Tables 4 and 5). Recovery was used to further evaluate the accuracy of the method. A known amount of the mixed standard solution was added to known amounts of the samples quantified previously, and the spiked samples were analyzed three times using the described method. Recovery of the eight components (Table 6) averaged from 98.130% to 103.25%(RSD ≤ 3.692%). For confirming the repeatability, six different working solutions prepared from the same source were analyzed in parallel by the method described above. The RSDs of the analytes were within the range of 0.19%–2.11%, which revealed high repeatability of the method (Table 7). The stability of the sample solutions was tested by injecting the same sample solutions at 0, 4,8, 12, 24, and 48 h after preparation using the developed method.The RSD values of the six analytes were all less than 2.41%, which demonstrated that the sample solutions remained stable within 48 h(Table 8). All the above-mentioned results indicated that the present method has good precision, recovery, repeatability, and stability for the eight components.
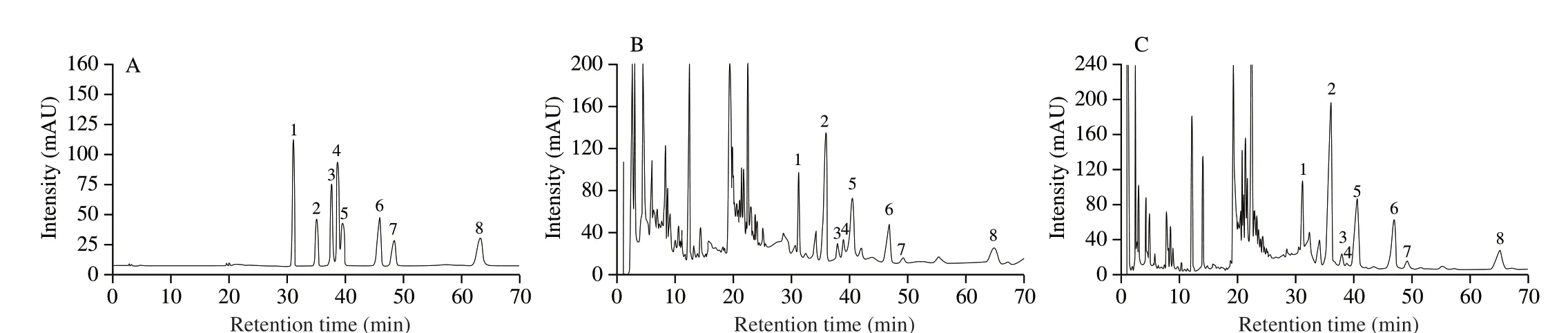
Fig. 2 Representative HPLC chromatograms of the ginsenoside standards (A) and the extract of red ginseng samples (B) as well as the extract of white ginseng samples (C). Peak identification: 1, ginsenoside Rf; 2, ginsenoside Rb1; 3, ginsenoside Rg2; 4, ginsenoside Rh1; 5, ginsenoside Rc; 6, ginsenoside Rb2; 7,ginsenoside Rb3; 8, ginsenoside Rd.

Table 3 HPLC Calibration curve data for reference standards of eight compounds (n = 3).
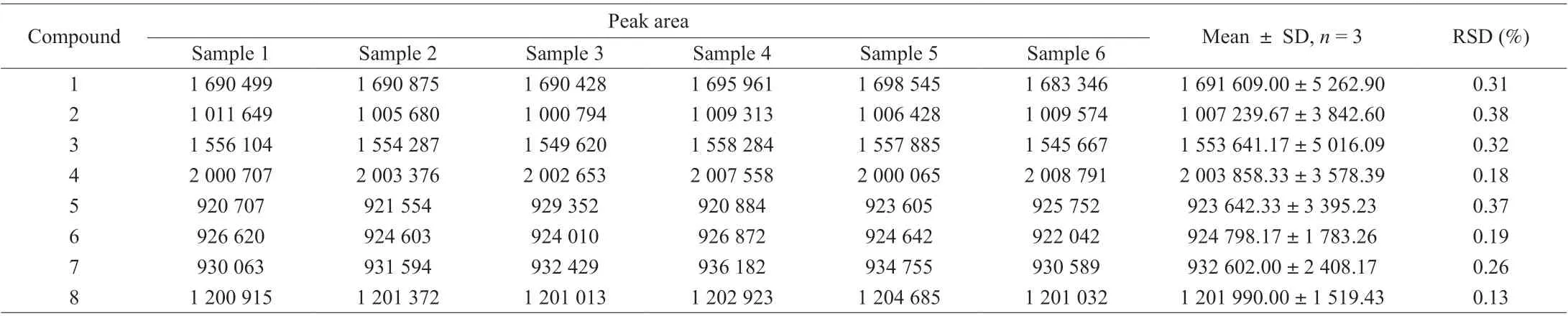
Table 4 Intraday precision test results of quantitative analysis of multi-components by a single-marker method.
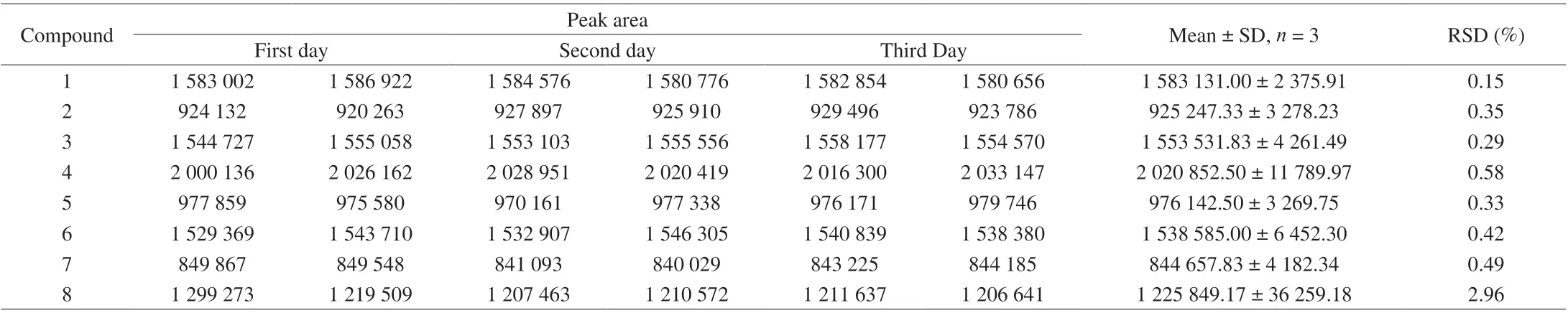
Table 5 Inter day precision test results of quantitative analysis of multi-components by a single-marker method.

Table 6 Sample recovery of quantitative analysis of multi-components by a single-marker method.
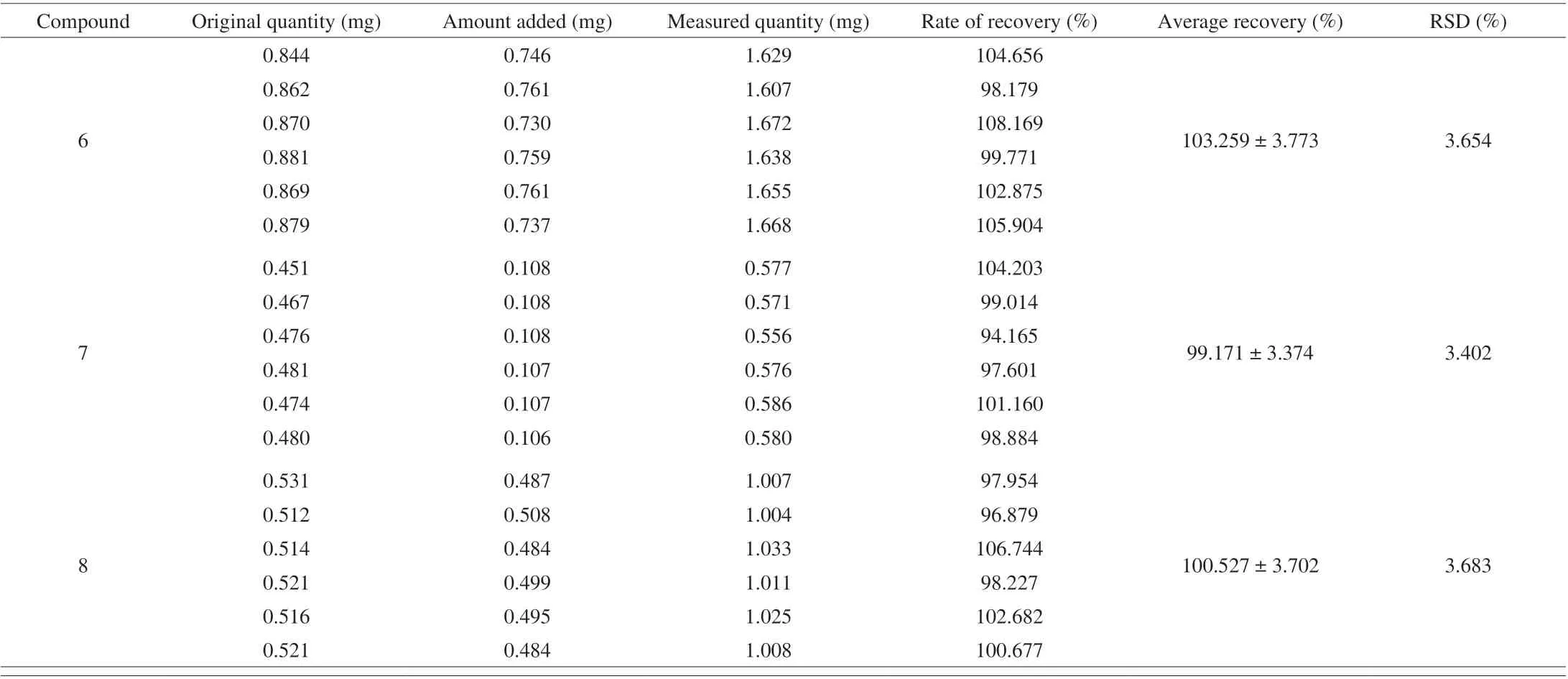
Table 6 (Continued)

Table 7 Repeatability test results of quantitative analysis of multi-components by a single-marker method.
Table 8 Stability test results of quantitative analysis of multi-components by a single-marker method.
3.1.3 Calculation of relative correcting factor (RCF)
Since relative correcting factor (RCF) is a key parameter in the application of QAMS method for the quality control, to choose the appropriate internal standard takes on added importance. Ginsenoside Rb2 was obtained easily, abundance highly and good separation from other components in the sample, ginsenoside Rb2 was selected as the internal reference. QAMS was adopted to simultaneously qualify the content of the eight ginsenosides, RCFs were detected via formula (1).The results of RCFs of eight ginsenosides are shown in Table 9.
3.1.4 Method assessment
Simultaneous determination of eight components in thirty-sixsamples from different regions was performed by both the external standard method (ESM) and QAMS, and the results are listed in supporting information Table 10. The content of ginsenoside Rf,ginsenoside Rb1, ginsenoside Rd, ginsenoside Rc and ginsenoside Rb2 were higher (> 0.69 mg/g), while the contents of ginsenoside Rg2, ginsenoside Rh1 and ginsenoside Rb3 were relatively low(< 0.34 mg/g), respectively.
Table 9 RCF values of eight components of ginseng samples (n = 3).

Table 10 Contents of the eight components in samples detected by ESM and QAMS methods (n = 3).
On comparing QAMS and ESM, it was observed that there was no significant difference between the two methods, the values of RSD ranged from 1.41% to 5.76% (Table 10). The results demonstrate that it is feasible to simultaneously determine eight components in white and red ginseng using QAMS.
3.2 Color variance
Color is one of the most important appearance attributes which influences consumer’s acceptability [25,26]. Undesirable changes in color of processed ginseng may lead to a poor consumer acceptance as well as lowering its market value. Color is an indicator of heattreatment severity and can be used to predict the corresponding quality deterioration resulting from heat exposure [27]. Generally the color of the ginseng changed from yellowish-white to light reddish brown, then to reddish brown, and finally to dark reddish brown during the processing. The changes in theL*,a* andb* parameters of white and red ginseng during processing are shown in Table 11,and the color with significant changes. The values ofa* andb* of red ginseng increased during processing, varying from 1.88 ± 0.80 to 7.62 ± 1.59 fora* and 19.90 ± 1.96 to 29.38 ± 1.22 forb*. Whereasthe values ofL* decreased, varying from 85.06 ± 1.16 to 69.09 ± 0.88 forL*. The color alteration underlying food processing was found to be related to transformation in the composition of the components in the foods [28]. In previously reported [27] that the color change of red ginseng is a result of a chemical reaction named Maillard reaction,which is a chemical reaction between reducing sugars and amino acids resulting in glycosylamines and or ketosamines [29]. In addition, the fact that when the moisture content decreased, the ginseng became more opaque and the starch reflection of light became stronger [27],which was also one of taking responsibility for color change.
Table 11 Color parameters of powder of ginseng from different habitats.
3.3 SEM microstructure analysis
The micro morphologies of white ginseng and red ginseng were shown in Fig. 3. The surface of white ginseng showed a more loosely packed microstructure with more cracks (Figs. 3A and 3C). In contrast,the red ginseng samples displayed a compact structure and presented of a gel layer on the surface due to processing (Figs. 3B and 3D). Such a microstructure could lower water penetration during rehydration [30,31].It was seen that processing had a significant effect on the microstructure of the ginseng sample. The loosely starch kernels and uneven pore distribution could be observed in the microstructure of white ginseng. However, steaming processing of ginseng at 90–100 °C for 2 h resulted in destruction of cell membranes and some starch gelatinisation due to the physical impact of high temperature, the surface of red ginseng became a more compact and filled structure and displayed a gel layer. So in terms of the microstructure of ginseng samples of scanning electron microscope, the processing treatment promotes case hardening and leads to the internal structure tightly.Additionally, the microstructure of processing ginseng may also beneficial to keep internal quality of red ginseng.
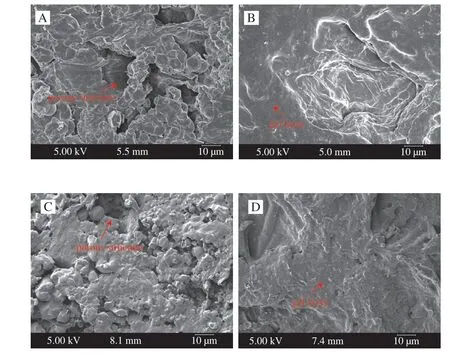
Fig. 3 Microstructure of white ginseng (A, C) and red ginseng (B, D).
3.4 Variation of bioactive ginsenosides and its correlation with anti-α-amylase activity and weighed
The validated method was applied to analyze 36 batches of samples collected from different habitats. The chromatograms of sample are depicted in Figs. 4 and 5. The QAMS that was based on HPLC-PDA was used to quantitatively analyze eight compounds in the sample at the same time. The results are shown in Tables 1 and 2.White ginseng samples with the highest content of total ginsenosides((11.39 ± 3.54) mg/g) were from Changbai Mountain area, and Tonghua ((6.91 ± 2.21) mg/g) was oppositely. Red ginseng samples with the highest content of total saponins ((6.69 ± 2.26) mg/g), which cultivated from Changbai Mountain area, and the samples with the lowest content ((2.44 ± 0.76) mg/g) were grown in Tonghua region.Moreover, we found that the levels of protopanaxatriol-type (PPTtype) ginsenosides (Rf, Rg2) and protopanaxadiol-type (PPD-Type)ginsenosides (Rb1, Rb2, Rb3, Rc, Rd) were reduced with steaming,while the levels of Rh1 of PPT-type ginsenosides were increased after processing. What’s more, the content of ginsenoside Rb1 was higher in both white ginseng and red ginseng than other components (Fig. 6 and Fig. 7). Studies have shown that the major ingredients of white ginseng are primary ginsenosides, such as Rf was PPT-type primary ginsenosides, and Rb1, Rc, Rb2, and Rd are PPD-type primary ginsenosides [32]. In general, PPD-type ginsenosides (Rb2, Rb3, Rc,Rd) as well as PPT-type ginsenosides (Rf, 20S-Rg2) are relatively more abundant in white ginseng [33]. Conversely, ginsenosides Rg3,Rh1, and Rh2 are considered to be characteristic components of red ginseng [34,35]. Our experimental results were consistent to previous studies and also validating the past research.

Fig. 4 The chromatogram of white ginseng samples. 1, The chromatograms of eight standard compounds; 2–7, White ginseng samples from Changbai Mountain region of Jilin Province; 8–13, White ginseng samples from Jingyu region of Jilin Province; 14–19, White ginseng samples from Tonghua region of Jilin Province.
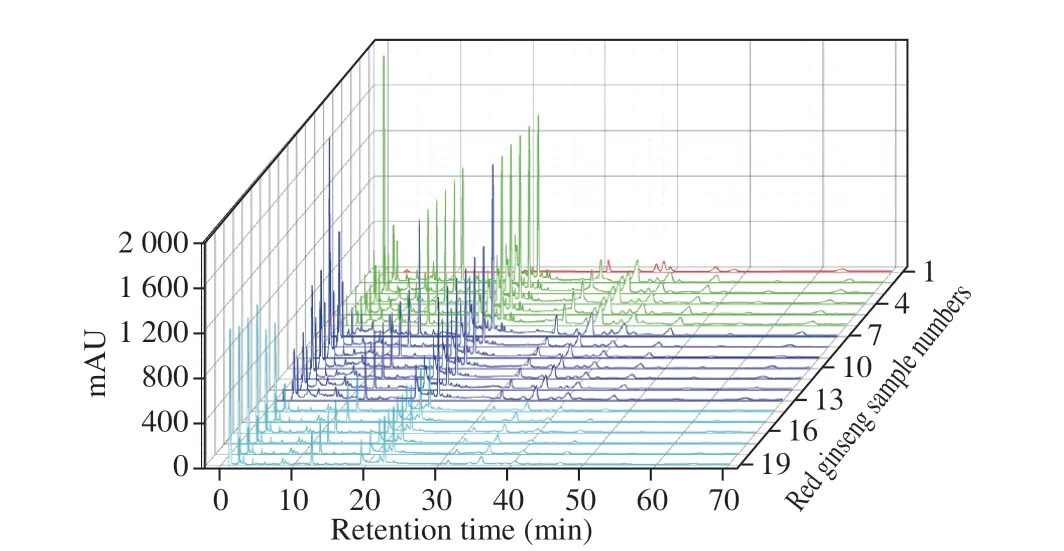
Fig. 5 The chromatogram of red ginseng samples. 1, The chromatograms of eight standard compounds; 2–7, Red ginseng samples from Changbai Mountain region of Jilin Province; 8–13, Red ginseng samples from Jingyu region of Jilin Province; 14–19, Red ginseng samples from Tonghua region of Jilin Province.
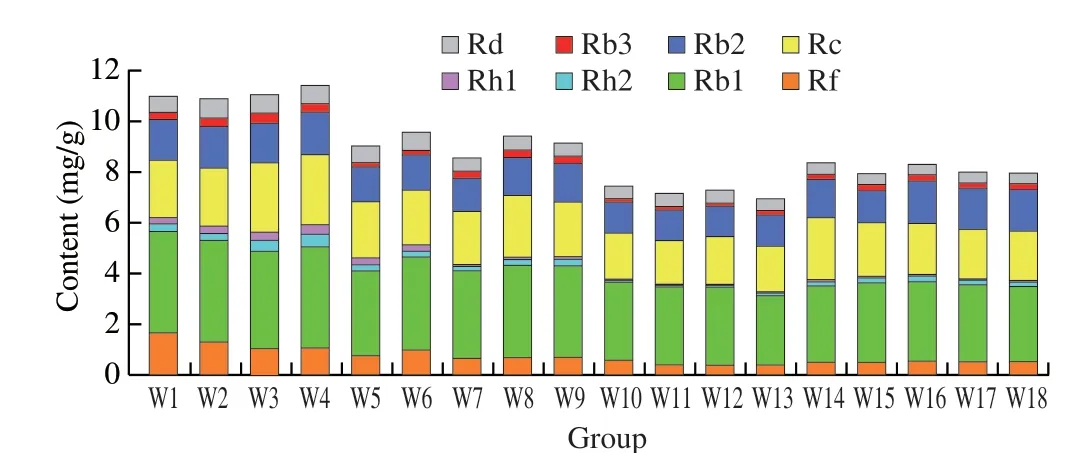
Fig. 6 Comparison of eight investigated components in white ginseng samples (W1–W18) from different habitats.

Fig. 7 Comparison of eight investigated components in red ginseng samples(R1–R18) from different habitat.
The red ginseng processing procedure consists of two main steps: steaming, followed by drying [36]. The steaming would supply sufficient water and heat which was needed in the hydrolysis reaction, and that was the major procedure in processing of ginseng.The possible pathway of chemical transformation is shown in Fig. 8. During the steaming, originally 2-1-glycosidic bond at C-6 of the ginsenosides Rf and Rg2 were destroyed, leading to the partial release of the glycosyl, and generating ginsenoside Rh1. This might be the reason for the increase of the content of ginsenoside Rh1 after processing in red ginseng. In addition, the steaming treatment were also believed to result in the partial release of the glycosyl moieties that are originally attached at the hydroxyls at C-20 and C-3 of the major ginsenosides via (2,1) or (6,1)-glycoside bond destroying,which might also be the reason for the decrease of the content of these ginsenoside in red ginseng such as Rb1, Rb2, Rb3, Rc, Rd and Rf.The six ginsenosides would yield intermediate ginsenosides, such as Rh2, F2, Rg3, eventually converting to the corresponding aglycones during the red ginseng processing procedure. The drying process made the moisture contents of red ginseng drops to 15% or 18% [37],and presents good moisturizing, delicate surface, bright and clean,yellow and red inside, which could increase consumers’ acceptance of red ginseng.
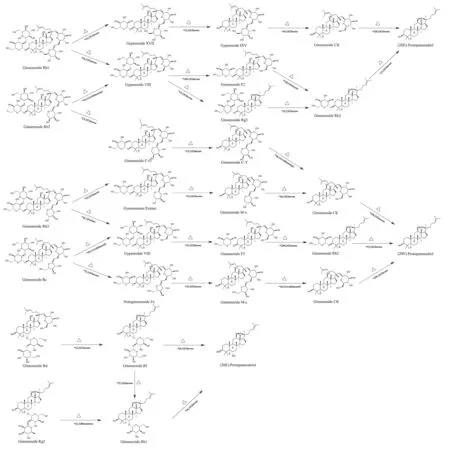
Fig. 8 Pathways of chemical changes of ginsenosides during red ginseng processing.
What is more, we also compared the anti-α-amylase activity of the samplesin vitro. The results are shown in Tables 1 and 2. Based on the mean IC50of samples, white ginseng ((20.10 ± 3.48) mg/mL) had the greater inhibitory activity than red ginseng ((48.38 ± 1.02) mg/mL),and the major reason for the decrease ofα-amylase activity was that the significant amount of ginsenosides was decreased in red ginseng.
It should be pointed out that processing exerted a great influence on the compact microstructure of red ginseng. The results of sections 3.2 and 3.4 indicated that theL* value and the content of ginsenoside were significantly decreased during processing. Subsequently, the activity of anti-α-amylase was declined due to the decreased of sapnions content.
Weighted theory was used to investigate the latent relationships of the eight bioactive ginsenosides and anti-α-amylase activity during processing; TheR2correlation coefficients were calculated based on the quantities of the contents of ginsenosides and the values of PIC50. TheR2correlation analysis is shown in Fig. 9.Although both the ginsenosides contents and the anti-α-amylase activity of sample extracts were decreased after processing, the eight bioactive ginsenosides correlated with the anti-α-amylase activity in different ways. According to the weighted rules, theR2was substituted into weighted equation as the weight. The sample(CBS-04, CBS-05 in Tables 1 and 2) which exhibits the highest PIC50was chosen as the control sample to normalize the weighted equation, respectively. By substituting the weight (R2) and the value of ingredients of sample into equation (3), the equation (5)and (6) was obtained as follow.
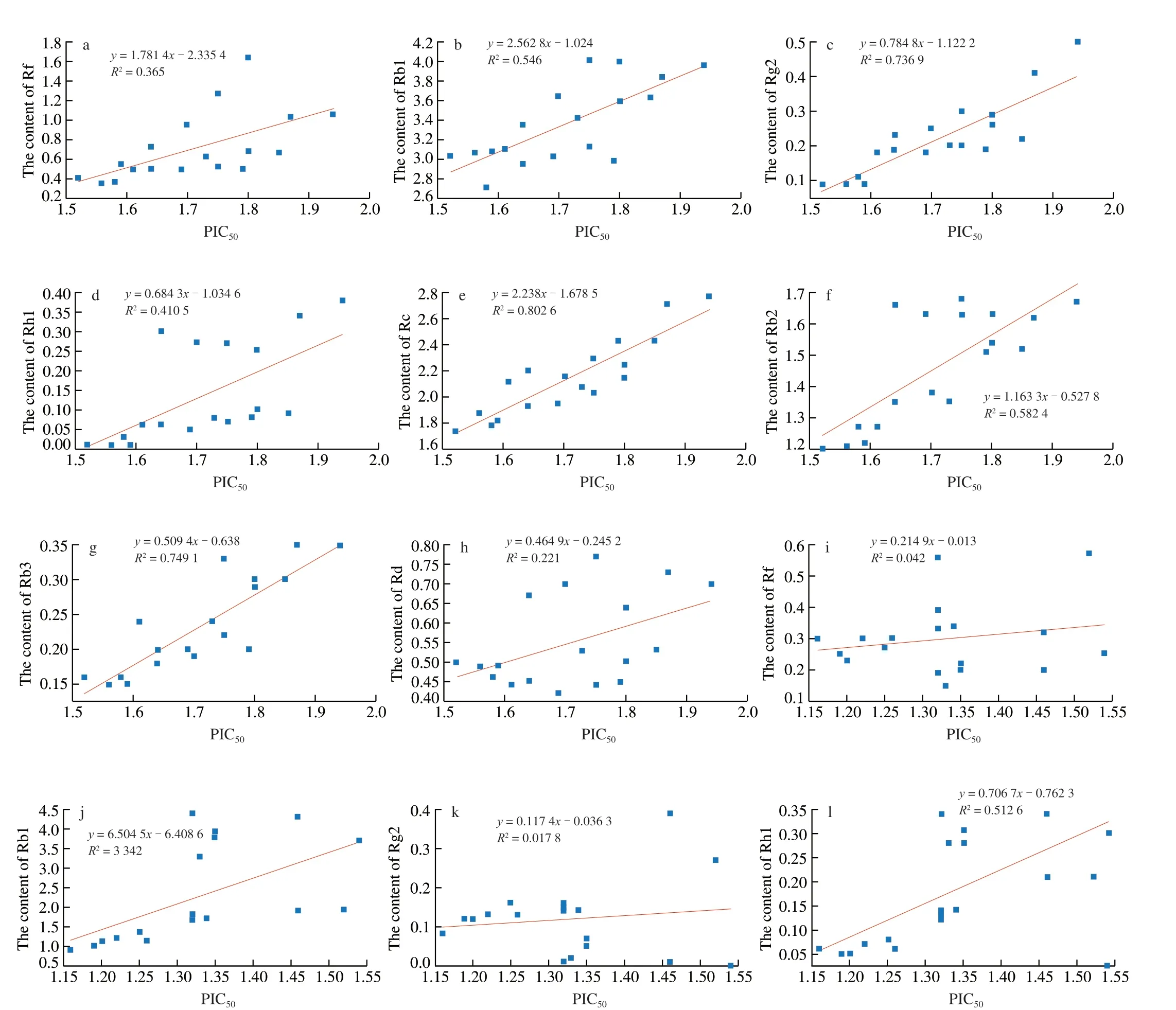
Fig. 9 The value of ginsenosides versus PIC50 are plotted with various R2. a–h, The value of eight ginsenosides in white ginseng versus PIC50 are plotted with various R2; i–p, The value of eight ginsenosides in red ginseng versus PIC50 are plotted with various R2; q, The value of WS in white ginseng versus PIC50 are plotted with various R2; r, The value of WS in red ginseng versus PIC50 are plotted with various R2.
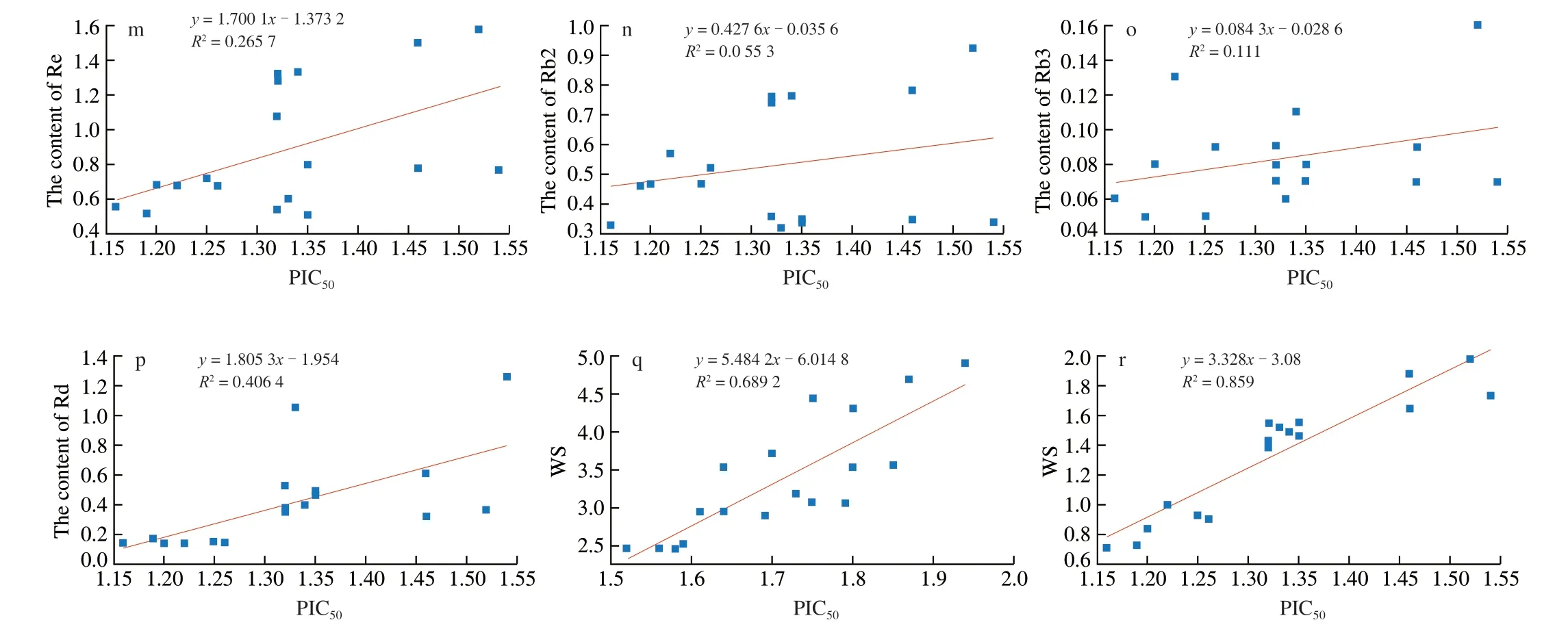
Fig. 9 (Continued)
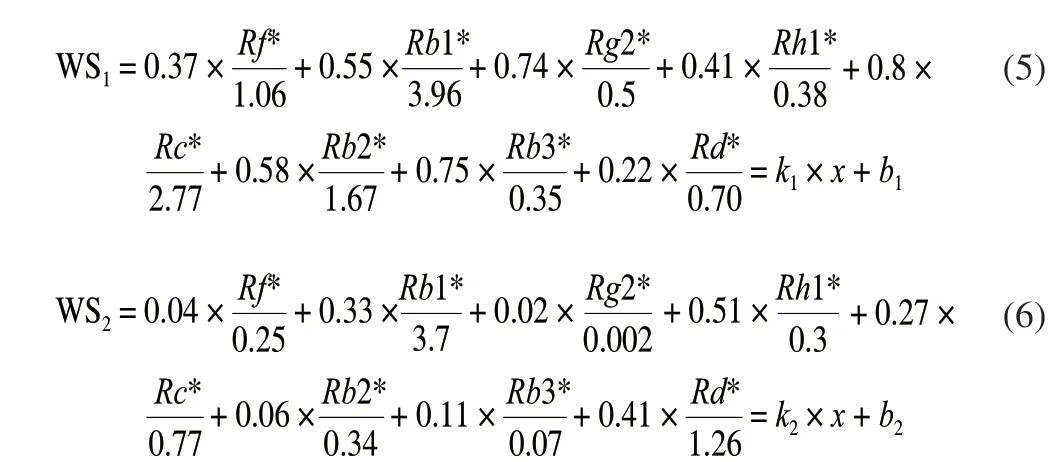
Where WS1and WS2represent white ginseng and red ginseng respectively.Rf,Rg1,Rb2, etc. indicate the content of the corresponding substances.
Through regression the PIC50and saponins, the values ofR2were taken as the weights shown in equation (5) and (6). The values of WS of PIC50with constituents were calculated by equation (5) and (6).Ginsenosides and WS were plotted as shown in Fig. 9. The coefficient of determination (R2= 0.859) of WS in red ginseng versus PIC50is obviously higher than that (R2= 0.689 2) of WS in white ginseng versus PIC50. Taking PIC50as the abscissa and WS as the ordinate,establish a linear regression equation, the slope was thekvalue, and the constant value wasb. Finally, the equation for predicting the PIC50and ginsenosides interaction was generated as equation (7) and (8).

By equation (7) and (8), the WS of ginsenosides for white or red ginseng which have no actual activity data (IC50) could be calculated. It means that one could use this equation to predict the IC50of any potent saponins for white or red ginseng before doing the bioassay experiments.
3.5 Anti-α-amylase activity and docking studies of eight bioactive ginsenosides
The anti-α-amylase activity of eight bioactive ginsenosides,two triterpenoid saponins and acarbose were tested. The results were shown in Table 12. All the chemicals inhibitedα-amylase in a concentration dependent manner with IC50ranging from (0.033 ±0.002) to (0.502 ± 0.001) mg/mL, and the binding energy was ranging from –8.2 to –3.6 kcal/mol. The inhibitory effects of(20S)-protopanaxadiol and (20S)-protopanaxatriol were the lowest with the values of IC50was (0.421 ± 0.002) and (0.502 ± 0.001) mg/mL,respectively. (20S)-Protopanaxatriol had the highest binding energy of–3.6 kcal/mol followed by (20S)-protopanaxadiol (–4.2 kcal/mol).It was clear that (20S)-protopanaxadiol and (20S)-protopanaxatriol(final product after processing) exhibited the highest value of IC50,and the highest binding energy that interaction onα-amylase , and may have weaker inhibitory activity, which was in agreement with the conclusion in section 3.4 that the decreased inhibitory activity of red ginseng after processing.

Table 12 Anti-α-amylase activity and docking studies of eight bioactive ginsenosides.
Ginsenoside Rb1 ((0.034 ± 0.001) mg/mL) showed the most potent inhibitory activity againstα-amylase, which was closer to the positive control acarbose ((0.033 ± 0.002) mg/mL) than other ginsenosides. Molecular docking also revealed that the positive control acarbose (–8.2 kcal/mol) exhibited the lowest binding energy withα-amylase, compared with the eight ginsenosides and two aglycones. Ginsenoside Rb1 (–8.0 kcal/mol), which had a lower binding energy than other ginsenosides and two aglycones, might be the most possibleα-amylase inhibitor in ginseng. Therefore,ginsenoside Rb1 was selected for further docking studies. The interaction between ginsenoside Rb1 andα-amylase was shown in Fig. 10 and Fig. 11. Ginsenoside Rb1 formed six hydrogen bonds with amino acid residues such as Arg 10, Trp 280, Ser 289, Gly 334,Asp 402 and Phe 406 inα-amylase.
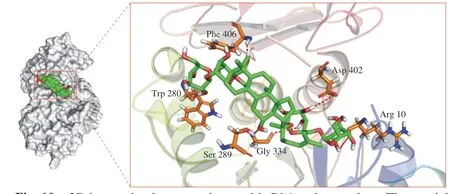
Fig. 10 3D interaction between ginsenoside Rb1 and α-amylase: The crucial residues of α-amylase and the compound structures were represented using stick structures. The red dashed lines represent hydrogen bonding interactions.The plots were generated by Pymol.
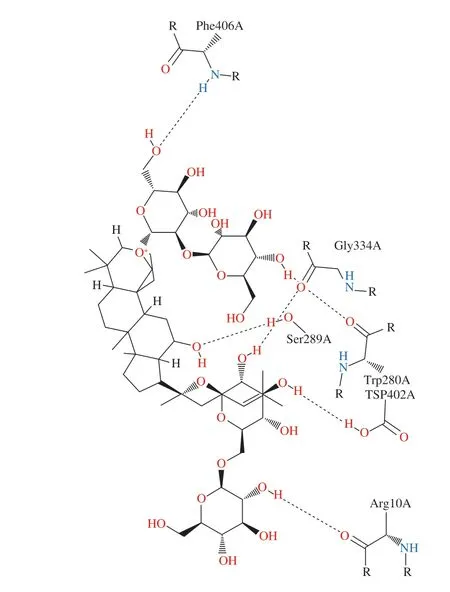
Fig. 11 2D interaction between ginsenoside Rb1 and α-amylase: Interaction analysis generated through Poseview. The interaction pattern is composed of hydrogen bonds, visualized as black dashed lines; and hydrophobic contacts,which are represented by the residue labels and spline segments along the contacting hydrophobic ligand parts.
Although noncovalent binding is often nonspecific and weak, a combination of many noncovalent bonds may alter the conformation and function of the protein. It was reported that ferulic acid interacted with the essential amino acid residues located in the noncatalytic site ofα-amylase [38]. We speculate that ginsenoside Rb1 interacted with the essential amino acid residues located in the noncatalytic site ofα-amylase to probably alter the conformation and function of the protein, in turn altering the enzyme activity.
4. Conclusions
In this work, we established a novel approach to assess the difference of physicochemical properties, ginsenosides content andα-amylase inhibitory effects between white and red ginseng. The results of physicochemical properties showed that processing had a significant impact on the color parameters. Especially, the color valueL* decreased significantly. Additionally, high processing temperature resulted in formation of a gel layer on the surface of red ginseng. The sun-drying strategy contributed to formation of a porous and loosely packed structure which went against keeping internal structure and quality of white ginseng stable. The established QAMS method by HPLC-PDA indicated that the white ginseng samples had higher bioactive ginsenosides than red ginseng. The anti-α-amylase activity of sample extracts decreased after processing. Molecular docking studies suggested that ginsenoside Rb1 might be the most possibleα-amylase inhibitor. The present study may deepen the understanding of the correlation between the chemical components and bioactive activity of white ginseng and red ginseng, which will facilitate its quality control. Moreover, this research provides detailed information could be useful to explaining the differences between white and red ginseng induced by processing.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgments
This study was supported by Tianjin Key R&D Plan-Key Projects Supported by Science and Technology (19YFZCSN00010).
杂志排行
食品科学与人类健康(英文)的其它文章
- Evaluation and selection of yeasts as potential aroma enhancers for the production of dry-cured ham
- Preserved egg white alleviates DSS-induced colitis in mice through the reduction of oxidative stress, modulation of inf lammatory cytokines,NF-κB, MAPK and gut microbiota composition
- Energy status regulated umami compound metabolism in harvested shiitake mushrooms (Lentinus edodes) with spores triggered to release
- Edible mushrooms as a potent therapeutics of subclinical thyroid dysfunction among adults, especially in obese individuals: a prospective cohort study
- Screening hepatoprotective effective components of Lonicerae japonica Flos based on the spectrum-effect relationship and its mechanism exploring
- Effects of the degree of oral processing on the properties of saliva-participating emulsions: using stewed pork with brown sauce as the model
