Purification conditions of extra-cellularmannanase produced by Lactobacillus casei 3MP-5-3
2023-01-12CHENXiYANGRuoXiJIHaiRuiZHANGHuiWenZHAOLiZHAODan
CHEN Xi, YANG Ruo-Xi, JI Hai-Rui, ZHANG Hui-Wen, ZHAO Li, ZHAO Dan,*
(Heilongjiang University,a.Key Laboratory of Microbiology, Life Science College, Harbin 150080, China;b.Engineering Research Center of Agricultural Microbiology Technology, Ministry of Education, Heilongjiang University, Harbin 150500, China)
Abstract:Lactobacillus casei 3MP-5-3 isolated from the fermentation liquid of pickled Chinese cabbage was employed as the starting strain. The extracellular β-mannanase was fermented in de Man-Rogosa-Sharpe (MRS) medium with konjac powder as the only carbon source. It was purified by using the supernatant of the fermentation solution as a crude enzyme solution. The results showed that the optimal volume ratio of the crude enzyme fluid to acetone was 1∶1.4. When an anion exchange chromatography system was used to purify the target protein, the optimal pH value of the elution system was 6.5, and the anion of CH3COO- exhibited the strongest elution capacity.
Key words:Lactobacillus casei;mannanase; acetone-precipitated protein; anion-exchange chromatography
0 Introcluction
β-1,4-mannanase (β-1,4-mannanase, EC 3.2.1.78, hereinafter referred to as mannanase) belongs to hemicellulose endonuclease hydrolase[1], which is able to hydrolyzeβ-1,4-glycosidic bonds of glucomannan, galactomannan and mannan[2-4]. Widely detected in animals, plants and microorganisms, it is increasingly used in paper industry, detergent and biomass energy, etc[5]. Microorganisms have the characteristics of short fermentation cycle, simple equipment operation, simple culture conditions, convenient product extraction, high efficiency in enzyme production. They are the main sources of mannanase producers and hotspots of current research[6].
The obtaining of extracellular mannanases produced by microorganisms usually including isolation and purification.The supernatant without microbial cells are considered as crude enzyme. The target protein can be separated and purified from the crude enzyme. Salting out, extraction and organic solvent are commonly used for separation. Purification methods usually include ultrafiltration, anion-exchange process, dialysis, gel filtration,isoelectric precipitation, affinity chromatography and density gradient centrifugation[7].Besides, different microbial species have different enzymatic properties, thereby the corresponding isolation and purification methods[8]. For example, Jana U K et al.[7],Seesom W et al.[8]separated and purified proteins by ammonium sulfate precipitation and anion column chromatography. Li Z P et al.[8]separated and purified the neutral protease fromVolvariellavolvaceaby ammonium sulfate precipitation and gel column filtration.
At present, the mannanase can be produced by bacteria (Pseudomonas,Bacillus[9]), fungi(Aspergillus[9],Penicillium[10]), and actinomyces (Streptomyces[11]). However, these microorganisms usually have low biological security and cannot meet the needs of food-grade applications. There are many kinds of Lactic acid bacteria(LAB), which are generally regarded as safe (GRAS) microorganisms[12]. The purification process of mannanase from LAB is relatively simple, and the culture can be directly used in the field of food grade. Therefore, mannanase produced by LAB is a research hotspot in recent years[13]. There are few reports that LAB are directly served as enzyme-producing strain. At present, there are only Zhang et al.[14]who separated and purified mannanase fromLactobacilluscaseiHDS-01, and Nadaroglu et al.[15]and Adiguzel et al.[16]who separated and purified mannanase fromPediococcusacidilactici,WeissellavirideseensandLactobacillusplantarum. In this study,L.casei3MP-5-3 was isolated from the fermentation liquid of pickled Chinese cabbage and used as the starting strain.The isolation and purification conditions of extracellular mannanase from 3MP-5-3 were optimized. The research results lay a certain foundation for the study of the nature and structure of this enzyme, and also provide basis for the large-scale industrial production of mannanase produced by LAB.
1 Materials and methods
1.1 Strain
L.casei3MP-5-3 isolated from the fermentation liquid of pickled Chinese cabbage and stored in the Key Laboratory of Microbiology, Heilongjiang University.
1.2 Media
De Man-Rogosa-Sharpe medium (applied to activate test strain): glucose 20 g; beef extract 10 g; peptone 10 g; magnesium sulfate 0.2 g; yeast extract 5 g;ammonium citrate 2 g;dipotassium hydrogen phosphate 2 g;sodium acetate anhydrous 5 g;sodium sulfite 0.1 g; tween 80 1 mL;distilled water 1 L;pH 5.5; 121 ℃ for 15 min.
Konjac powder medium (applied to the fermentation and enzyme production of the test strain): konjac powder 10 g; sodium nitrate 5 g; dipotassium hydrogen phosphate 5 g; magnesium sulfate 0.2 g; yeast extract 5 g;distilled water 1 L;pH 6.5; 121 ℃ for 15 min.
1.3 Preparation of crude enzyme solution
The single colony ofL.casei3MP-5-3 was inoculated at 5% (v/v) in MRS medium at 37 ℃, 160 r·min-1for 18~24 h, and then the cell concentration was adjusted to 1×108cells·mL-1, which was seed liquid. The seed liquid was inoculated at 5% (v/v) in konjac powder medium at 5%, 37 ℃at 160 r·min-1for 36 h, followed by centrifugation at 6 000 r·min-1for 10 min. Then, the supernatant was obtained, which was crude enzyme solution.
1.4 Research on purification conditions
1) Precipitate protein with acetone. Acetone, which had been pre-cooled at -20 ℃ in advance, was added toL.casei3MP-5-3 fermented crude enzyme solution under the condition of ice bath. The precipitated protein was placed at 4 ℃ for 40 min, and then centrifuged at 8 000 r·min-1for 20 min. The supernatant was taken as crude enzyme solution. Protein content and enzymatic activity were determined to confirm the optimal volume ratio of crude enzyme solution and acetone.
2) Determine the pH of ion-exchange chromatography. Centrifuge tubes (10 mL) were taken and numbered 1~12. 2 mL of DEAE-Sepharose Fast Flow washed with citric acid-sodium citrate buffer solution of 20 mmol·L-1, pH 3.5~9 was added into each tube. 0.5 mL of the crude enzyme solution was added and fully mixed. After an hour of rest at 4 ℃, mannanase activity was measured and the binding between extracellular mannanase produced byL.casei3MP-5-3 and the gel was detected under different pH conditions[17].
3) Determine the anion with the strongest elution capacity. Firstly, the equilibrium of mannanase binding gel was selected under the optimum pH condition. Then, NaCl, Na2SO4, Na2SO3, NaNO3, CH3COONa, Na2HPO4, NaH2PO4, NaHCO3and Na2CO3were prepared at the concentration of 1 mol·L-1. The gel was washed and added into 10 mL centrifuge tube. A certain amount of crude enzyme liquid was added and fully mixed, and the gel was left at rest for an hour. At last, mannanase activity in supernatant was determined. The anion with the highest activity in supernatant was the anion with the strongest elution capacity that we’d like to confirm[18].
1.5 Measure mannanase activity with DNS
1)The standard curve of mannanase. Standard mannose solutions of 2 mg·mL-1with different volumes were added into 25 mL colorimetric tubes with plug numbered 1~6 and replenish with deionized water to 2.0 mL. Then,DNS reagent (3.0 mL) was added into tubes. After being boiled in boiling water for color rendering for 10 min and cooled to room temperature, water was added to 25 mL. With full mixture, the OD value at 540 nm was measured by spectrophotometer. The colorimetric tube numbered 1 was used as the blank control. Three repetitions were performed. The standard curve was drawn with mannose concentration (mg·mL-1) as x-coordinate and optional density (OD540 nm) as y-coordinate.
2) Measure mannanase activity. The konjac powder was soaked in 75% alcohol for several days and dried so as to remove the reducing sugar and prevent it from interfering with the experiment. Locust bean gum solution (0.5 mL)with a concentration of 0.5% and 2 mL of crude enzyme solution were added into a 25 mL colorimetric tube with plug, and put in 55 ℃ water bath and kept warm for 30 min. Then 3 mL of DNS reagent was added into the tube. After being boiled it in boiling water bath for color rendering for 10 min and cooled to room temperature, water was added to 25 mL.With full mixture, the OD value at 540 nm was measured by spectrophotometer. The locust bean gum solution with a concentration of 0.5% was used as the blank control. And then, the mannanase activity was able to be calculated according to the standard curve we get[19]:
The activity of mannanase(U·mL-1) =
1.6 Protein content analysis
Protein content was assayed by Bradford[20].
1)The standard curve of Bovine Serum Albumin. 1% BSA standard solution under different volumes was successively added into six tubes numbered 1 to 6, followed by adding pure water to 1 mL. Coomassie brilliant blue G-250 reagent (5 mL) was added into each tube and fully mixed, After 5 min, the OD value at 595 nm was measured and the standard curve was drawn with OD595nmas x-coordinate and the density of BSA (mg·mL-1) as y-coordinate.
2) Determination of protein content. Coomassie brilliant blue G-250 reagent (5 mL) was mixed with 100 μL crude emzyme solution fully, and the mixture was left for a rest for 2 min. Deionized water (0.1 mL) was added into 5 mL coomassie brilliant blue G-250 reagent and mixed fully as the blank control. The OD value at 595 nm was measured by spectrophotometer, and the protein content was calculated according to the standard curve we get.
1.7 Data processing
The values were expressed as mean ± standard deviation and independent triplicates were set. Excel 2019 (Microsoft, U.S.A.) and Origin 2020(OriginLab Institute Inc, U.S.A.) were used for drawing in this study. JMP 10.0 (LISHISAS) was used for multiple comparison with test data. Duncan’s new multiple range method was used for analyzing whether the differences between different data were significant andP<0.05 indicates significant difference.
2 Results
2.1 Standard curve
The standard curve of mannose was shown in Fig.1, the regression equation wasy=0.732 5x+0.031 6,R2= 0.994 8, which was used for calculating mannanase activity. The standard curve of protein was shown in Fig.2, and the regression equation wasy= 1.000 3x+ 0.139 8,R2= 0.998 2, which was used for calculating protein content.

Fig.1 Standard curve of mannose

Fig.2 Standard curve of protein
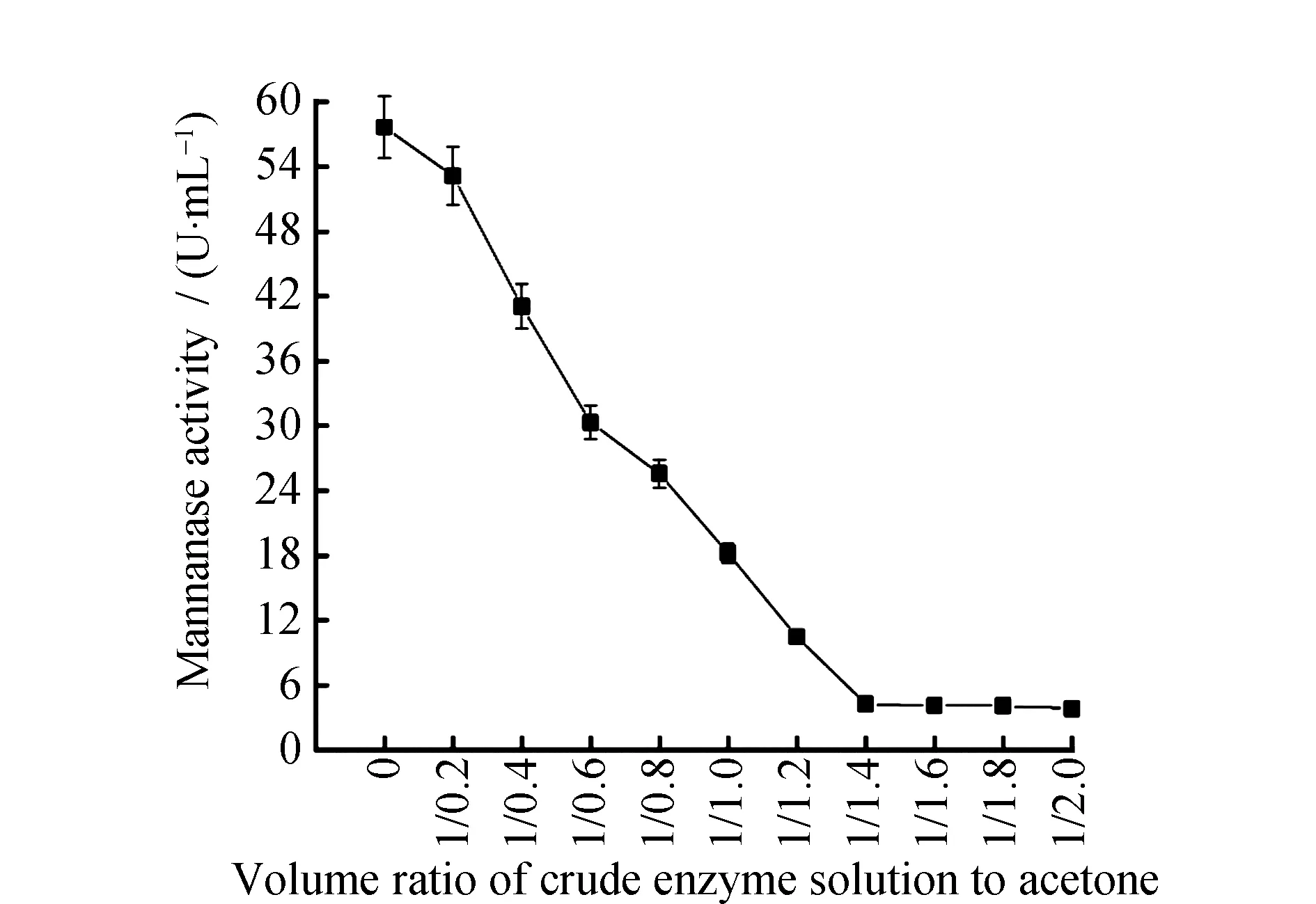
Fig.3 Enzymatic activity under different volume ratio of crude enzyme solution and acetone
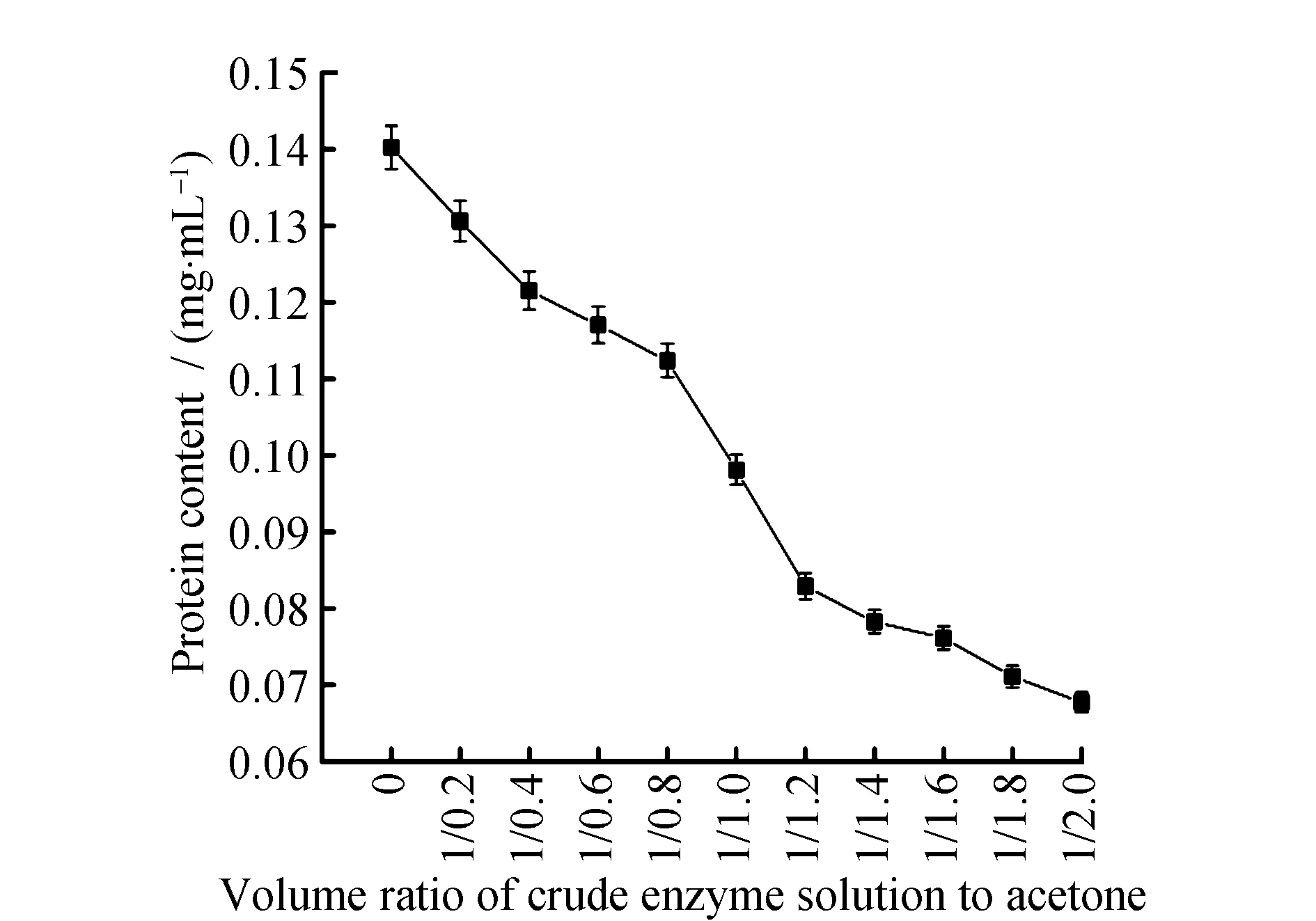
Fig.4 Protein content under different volume ratio of crude enzyme solution and acetone
2.2 Precipitate mannanase with acetone
As was shown in Fig.3 and Fig.4, when the volume ratio of crude enzyme solution and acetone reached 1∶1.4, the protein content in supernatant was 0.078 ± 0.01 mg·mL-1, and the enzymatic activity was 4.26 ± 0.09 U·mL-1. When the volume ratio of the crude enzyme solution and acetone was greater than 1∶1.4, the target protein in the crude enzyme solution was almost precipitated. And with the increasement of acetone, the protein content and enzymatic activity in supernatant precipitated by acetone did not decrease but remain stable. Protein in supernatant was almost precipitated and the enzymatic activity was zero, the best volume ratio of crude enzyme solution and acetone to precipitate the protein was 1∶1.4.
The reason why acetone is able to precipitate protein is that, on the one hand, the acetone added to water can lower volumetric permittivity and increase the appeal of the opposite electric charge. On the other hand, as acetone belongs to strongly hyrophilic reagent, it’ll compete for the hydration water that is on the surface of protein and damage the protein gel hydration film on the surface of molecule so as to precipitate it. Precipitation effects were various under different volume ratio of crude enzyme solution and acetone. Therefore, different volumes of acetone could be used for precipitating different kinds of proteins. When Wang J et al.[21]precipitated the protein in the plasma through acetone, the optimal volume ratio of crude enzyme solution and acetone was 1∶4, which was significantly different from the optimum volume ratio of crude enzyme solution and acetone inL.casei3MP-5-3 mannanase in this study. It displayed that precipitation effects were various under different volume ratio of crude enzyme solution and acetone. When Yang W D et al.[22]precipitated theβ-mannanase produced byAspergillusnigerthrough acetone,the optimum volume ratio of crude enzyme solution and acetone was between 1∶0 and 1∶1.6, which was consistent with the results in this study. It displayed that acetone might have the same effect on the precipitation of proteins produced by different species of microorganisms.
2.3 Ion-exchange chromatography elution equilibrium system
As was shown in Fig.5, the activity ofL.casei3MP-5-3 mannanase decreased first and then increased in citric acid-sodium citrate buffer solutions under different pH conditions, and when the pH was 6.5, mannanase activity could hardly be detected in supernatant. As was shown in Fig.6, protein reached the minimum when the pH was 6.5, which was consistent with the histogram significance analysis results. The results indicated that the target protein bound the gel best when the pH was 6.5.Different kinds of enzymes have different charge polarities and quantities on their surfaces, which leads to different optimal pH. Hu S Q et al.[23]found that the optimal pH of the target protein was 7.5 when studied the properties of oxidoreductin in wheat endoplasmic reticulum by electrophoresis. Optimal pH ofL.casei3MP-5-3 mannanase elution was 6.5, indicating that the electronegative activity of the latter was weaker.

Fig.5 Protein content in sodium citrate at different pH valuesNote: Different letters indicate significant differences among protein content (P<0.05).

Fig.6 Mannanase activity in sodium citrate at different pH valuesNote: Different letters indicate significant differences among mannanase activity (P<0.05).
Different anions have different elution effects onL.casei3MP-5-3 mannanase. As shown in Fig.7, the best choice for the elution was CH3COO-. After elution by CH3COO-buffer, the protein content in supernatant reached the maximum of 0.131 ± 0.01 mg·mL-1. Meanwhile, as shown in Fig.8, mannanase activity reached the maximum of 20.09 ± 0.28 U·mL-1.
L.caseiHDS-01 isolated from the fermentation liquid of pickled Chinese cabbage was used as the starting strain by Zhang X et al.[18]and the anion with the strongest capacity to elute target proteins in anion-exchange chromatography system was determined to be CH3COO-that was consistent with the results in this study. It indicated that the mannanase produced by different strains of the same genus from the same source had the same adsorption effect on anions.L.plantarumM24 isolated from the fermented sausage was used as the starting strain by Nadaroglu H et al.[20]and the anion with the strongest capacity to elute target proteins in anion-exchange chromatography system was determined to be Cl-that was different from the results in this study. It indicated that the mannanase produced by different strains of the same genus from the different sources had different adsorption effects on anions.

Fig.7 Protein content in elution buffer at different anionsNote: Different letters indicate significant differences among protein content (P<0.05).
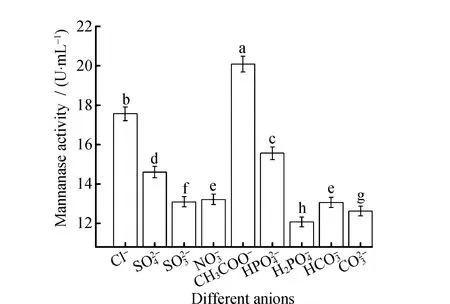
Fig.8 Mannanase activity in elution buffer at different anions Note:Different letters indicate significant differences among mannanase activity (P<0.05).
3 Conclusions
Conditions on acetone-precipitated protein and anion-exchange chromatography were studied in order to improve the purification efficiency of extracellular mannanase produced byL.casei3MP-5-3. The experimental results manifest that acetone was able to precipitate mannanase effectively and when the volume ratio of crude enzyme solution and acetone reached 1∶1.4, the effiency of precipitation reached 56.8. The target protein is negatively charged acidic protein, so it was isolated and purified with a cationic resin in the buffer of pH 6.5, and eluted with different kinds of anions. Comprehensively considered the influence of pH and the effect of anion elution on the enzymatic purification process, CH3COONa solution with pH 6.5 and a concentration of 1.0 mol·L-1was determined as the eluent. The research results lay a certain foundation for the applications of extracelluar mannanase produced byL.casei3MP-5-3 in various areas.
