Electroluminescence devices from carbazole-containing dibenzothiophene sulfone derivatives hybrid fluorophores
2023-01-12CHENShuoXUHui
CHEN Shuo, XU Hui
(School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, China)
Abstract:Three novel yellow emitters TD-xBPF (x=2, 3, 4) were synthesized by incorporating a dibenzothiophene sulfone acceptor core and arylamine (N-([1,1′-biphenyl]-4-yl)-9,9-dimethyl-N-phenyl-9H-fluoren-2-amine) donor moieties via D-A architecture. Three compounds feature maximum emission of 500~520 nm with the Commission International de L′ Eclairage (CIE) coordinates of (0.23, 0.56), (0.27, 0.60) and (0.19, 0.48) as well as high photoluminescence quantum yields of 0.53 in thin film. The doped devices fabricated by employing TD-xBPF as emitters obtained an external quantum efficiencies (EQE) of 3.3%, a maximum current efficiency (CE) of 10.28 cd·A-1 and a maximum power efficiency (PE) of 11.3 lm·W-1, respectively.
Key words:electroluminescence devices; quantum efficiencies;yellow emitters
0 Introduction
Organic light emitting diodes (OLEDs) have attracted much attention in recent years due to their potential application in full-color flat-panel displays and solid-state lighting[1-3]. During the last two decades, numerous efforts have been employed to enrich the chemistry of full color devices by designing blue, green and red luminescent complexes[4-6].In order to obtain excellent efficiency, Ir, Au and Pt complexes based phosphorescent OLEDs (PhOLEDs) are more popular as they can utilize nonradiative triplet energy for emission[7-9],which are rather expensive and dependent on limited global resources. Thus, organic molecule with free heavy-metal containing seems meet to the long-term mass production[10-12].Therein, the D-A type π-conjugated emitters have been extensively investigated for their cost advantage and versatility in tuning of emission color. Simultaneously, such D-A materials are also beneficial for the injection, transportation and recombination of carriers because of the separated D-A functions, which resulting the improvement of the device performance[13-14].
In this work, three D-A type yellow compounds TD-xBPF (x= 2, 3, 4) containing dibenzothiophene sulfone andN-([1,1′-biphenyl]-4-yl)-9,9-dimethyl-N-phenyl-9H-fluoren-2-amine-based with different linker site were designed and prepared (Scheme 1), in which arylamine serves as the electron donor and dibenzothiophene sulfone as the electron acceptor. Rigid π-conjugated electron-withdrawing cores with deep LUMOs are desired. planar rigid architecture dibenzothiophene sulfone is selected as the acceptor skeleton on the basis of their high electron affinity; optical, thermal, and chemical stabilities; promising electron-transporting properties, and tunable fluorescent properties. Furthermore, dibenzothiophene sulfone derivatives have proven to be promising because of their potential modification site as yellow chromophore. Arylamine can be functionalized at the dibenzothiophene sulfone nucleus with different substituents site, and this allows the photophysical and electronic properties of the targets to be fine-tuned. For TD-xBPF (x= 2, 3, 4), we demonstrate that the introduction of the arylamine has a different effect on the linker site of fluorescent nature. The HOMO and LUMO of TD-xBPF were almost complete separated, which is beneficial to give a good ability to transport charges. Employing TD-xBPF as a emitting layers, an yellow OLEDs with a CIE coordinates of (0.215 9, 0.579 9) and maximum EQE of 3.3% has been achieved. These results have motivated us to explore the utilization of dibenzothiophene sulfone derivatives for manufacturing and applications.

Scheme 1 Synthetic pathways toward y ellow emtiters TD-xBPF
1 Experimental sections
1.1 Materials and instruments
Unless otherwise noted, commercially available reagents empolying for the synthesis were purchased from Aldrich, J&K and TCI companies and used as received.1H magnetic resonance (NMR) spectra were recorded using a Bruker AVANCE III 400-MHz spectrometer, using CDCl3or DMSO[d6] as the solvent and tetramethylsilane (TMS) as the internal standard. High resolution mass spectra were recorded on AB SCIEX 5 800 matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF). Elemental analysis for C, H and N were performed on an Elementar Analysensysteme GmbH. All manipulations involving air-sensitive reagents were performed in an atmosphere of dry Ar. Absorption spectra of the target compounds were measured using a Perkin Elmer Lambda-750 UV-Vis-NIR spectrophotometer. Steady-state and transient-state emission spectra in both solution and solid were recorded with LS 55 fluorescence spectrometer. For the solid samples, the quantum yields for the compounds were determined at room temperature through an absolute method using an Edinburgh Instruments′ integrating sphere coupled to a modular Edinburgh FLS 920 fluorescence spectrophotometer. The absolute quantum yield was calculated using the following equation:
In equation,Lemissionis the emission spectrum of the sample, collected using the sphere,Esampleis the spectrum of the incident light used to excite the sample, collected using the sphere, andEreferenceis the spectrum of the light used for excitation with only the reference in the sphere. The method is accurate to within 10%. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed on Perkin Elmer TGA 4 000 and DSC 8 000 thermal analyzers under nitrogen atmosphere at a heating rate of 10 ℃·min-1. Cyclic voltammetric (CV) measurements were carried out in a conventional three electrode cell using a Pt button working electrode of 2 mm in diameter, a platinum wire counter electrode, and a saturated calomel electrode (SCE) reference electrode on a computer-controlled CHI660d electrochemical workstation at room temperature. Reduction CV of all compounds was performed in CH2Cl2containing teterabutylammonium hexafluorophosphate (Bu4NPF6, 0.1 M) as the supporting electrolyte. Ferrocene was used as an external standard. Electrochemistry was done at a scan rate of 100 mV·s-1.
1.2 Computational method
The theoretical investigation of geometry optimization was performed with theGaussian09program package. Density functional theory (DFT) was calculated at Beck′s three-parameter hybrid exchange functional and Lee, and Yang and Parr correlation functional B3LYP/6-31G (d). The spin density distributions were visualized usingGaussview5.0.8.
1.3 Device fabrication and measurement
PEDOT:PSS, TmPyPB and LiF were purchased from Lumtec Corp. (Taiwan, China). Prior to the device fabrication, the patterned ITO-coated glass substrates were scrubbed and sonicated consecutively with detergent water, deionized water, and acetone, dried in drying cabinet, and then exposed to a UV-ozone environment for 30 min. After these processes, the substrates were transferred into a vacuum chamber for sequential deposition of all the organic layers by thermally evaporation with a base pressure (~4.0×10-4Pa) at a rate of 0.1~0.2 nm·s-1monitored in situ with the quartz oscillator. LiF covered by Al is used as cathode without breaking the vacuum. All the samples were measured directly after fabrication without encapsulation at room temperature under ambient atmosphere. The electroluminescent spectra were recorded using an Ocean Optics spectrometer. The current-voltage-luminance characteristics were measured using a PR655 Spectrascan spectrometer and a Keithley 2400 programmable voltage-current source. The external quantum efficiency (EQE) and luminous efficiency (LE) were calculated assuming Lambertian distribution, and then calibrated to the efficiencies obtained at 1 000 cd·m-2in the integrating sphere (Jm-3200).
1.4 Synthesis
General Procedure of Oxidation for Sulphone. A sample of x-Bromodibenzothiophene (x=2, 3 or 4) (1 973.6 mg, 7.5 mmol) was dissolved into 20 mL of dichloromethane at room temperature, and then 75% 3-chlorobenzoperoxoic acid (6 471.4, 37.5 mmol) was added to the mixture and stirred for 6 h.
For 2-Bromodibenzothiophene Sulfone (2TDSO):the crude was filtered under reduced pressure and washed using EtOH (3×10 mL). The product was used without further purification for next reaction.1H NMR (500 MHz, CDCl3, δ) 7.94 (s, 1H), 7.83~7.85 (d,J= 7.5, 1H), 7.77~7.78 (t,J=7.5 Hz, 1H), 7.66~7.70 (dd,J= 8.0 Hz, 3H), 7.56~7.59 (t,J= 7.5 Hz, 1H). EI-MS (m/z): calcd for C12H7BrO2S 293.94; found, 295.3 [M+].
For 3-Bromodibenzothiophene Sulfone (3TDSO):the crude was filtered under reduced pressure and washed using EtOH (3×10 mL). The product was used without further purification for next reaction.
For 4-Bromodibenzothiophene Sulfone (4TDSO): The precipitate was removed invacuoto afford the mother liqued. Then, the solution was stirred with sodium thiosulfate for 1 h and extracted with CH2Cl2/NaHCO3. The organic layer was combined and dried with anhydrous sodium sulfate. The solvent was removed invacuo, and then the residue was further purified by column chromatography with CH2Cl2as eluent to afford the white product.1H NMR (500 MHz, CDCl3, δ) 7.84~7.86 (d,J= 7.5 Hz, 1H), 7.78~7.80 (d,J= 7.5, 1H), 7.65~7.68 (t,J= 5.5 Hz, 1H), 7.60~7.63 (dd,J= 4.0 Hz, 1H), 7.56~7.58 (t,J= 7.5 Hz, 1H), 7.45~7.49 (t,J= 4.5 Hz, 1H). EI-MS (m/z): calcd for C12H7BrO2S 293.94; found, 295.3 [M+]
Synthesis of BPFB:
PdCl2(dppf)2(1,1′-Bis(diphenylphosphino)ferrocene-palladium(II)dichloride) (109.8 mg, 0.15 mmol) was added to a suspension ofN-([1,1′-Biphenyl]-4-yl)-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine (1 549.4 mg, 3.0 mmol), bis(pinacolato)diboron (990.4 mg, 3.9 mmol), potassium acetate (588.8 mg, 6.0 mmol) in dioxane (20 mL) at room temperature. After stirred at room temperature for 20 min, the mixture was heated to 80 ℃ and stirred for 24 hours. The reaction mixture was extracted with DCM and further purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 20:1, v/v) to attain a white solid (1 183.4 mg, 70%).1H NMR (500 MHz, CDCl3, δ) 7.76~7.80 (dd,J= 5.5 Hz, 2H), 7.66~7.67 (t,J= 7.0, 4H), 7.58~7.59 (d,J= 8.5 Hz, 2H), 7.51~7.53 (d,J= 7.0 Hz, 1H), 7.43~7.46 (t,J= 7.5, 2H), 7.27~7.35 (m, 4H), 7.15~7.16 (d,J= 8.5 Hz, 2H), 7.00~7.06 (m, 3H), 1.39 (s, 6H), 1.28 (s, 12H). EI-MS (m/z): calcd for C39H38BNO2563.299 011; found, 563.299 440 [M+].
Synthesis of TD-2BPF:
A mixture of 2TDSO (590.4 mg, 2 mmol), BPFB (1 127.2 mg, 2 mmol), tetrakis(triphenylphosphine)palladium (231.0 mg, 0.2 mmol), tetrabutylammonium bromide (66.4 mg, 0.2 mmol), and an aqueous solution of sodium hydroxide (2 mol·L-1, 12 mmol) in THF (25 mL) was stirred under argon at 80oC for 48 h. After quenching with an aqueous NH4Cl solution, the mixture was extracted with CH2Cl2. The combined organic extracts were washed with brine and dried over anhydrous MgSO4. After removing the solvent, the residue was purified using column chromatography on silica gel with petroleum ether/CH2Cl2= 1∶2, v/v as the eluent to give a white power. Yield: 58%. EI-MS (m/z): calcd for C45H33NO2S 651.222652; found, 651.224457 [M+].
Synthesis of TD-3BPF and TD-4BPF:
The procedure for TD-3BPF and TD-4BPF was similar to the preparation of TD-2BPF starting from 3TDSO and 4TDSO (310.2 mg, 1 mmol) instead of 2TDSO. Yield: 59%,65%.
2 Results and discussion
2.1 Synthesis and characterization
The synthesis route of TD-xBPF (x=2, 3, 4) was outlined in Fig.1. By treatment with bromine substituted dibenzothiophene withm-CPBA under refluxing for 2 h, the initial compoundsxTDSO (x= 2, 3, 4) was obtained over 80% yield. Then, the target compounds TD-xBPF were prepared with coupling reaction in 70% yield. Herein, this synthesis method could conveniently construct dibenzothiophene sulfone derivatives with various structures. The target products is afforded through the typical Suzuki reaction between the bromide intermediate and boric acid ester derivatives.1H NMR and high-resolution mass (MALDI-TOF MS) characterization reveal the intermediates and the final products have the right structure and high purity. Both of the molecules have good solubility in common organic solvents such as THF, dichloromethane, chloroform and toluene. The detailed synthetic procedure and analysis were depicted in the experimental section.

Fig.1 Synthesis route TADF emitters TD-xBPF (x = 2, 3, 4)
2.2 Photo-physical properties
The absorption spectra of theTD-xBPF in different solvents are presented in Fig.2. TD-2BPF and TD-4BPF have maximum absorption bands both at 350 nm, which could be assigned to the intramolecular charge transfer (CT) transition from the donor units to the acceptor moiety. In addition, the absorption thresholds of TD2-BPF and TD4-BPF reached 445 nm and 419 nm, respectively. The corresponding optical band gaps were estimated to be 2.79 eV and 2.96 eV on the basis of absorption edge, respectively.
The photoluminescent emissions of TD-2BPF, TD-3BPF and TD-4BPF were observed at 570 nm and 515 nm in DCM, respectively in Fig.3. The huge bathochromic-shift of 145 nm is primarily due to the CT effect. High photoluminescence quantum yield (PLQY) is vital to evaluate the device performance of nondoped OLEDs. We measured the PLQYs of TD-xBPFviathe vacuum evaporated film (100 nm), giving the value of 0.53. High PLQY suggested that three emitters would be a promising candidate as high performance yellow OLEDs.

Fig.2 Absorption spectra of TD-2BPF and TD-4BPF in different solvents at room temperature
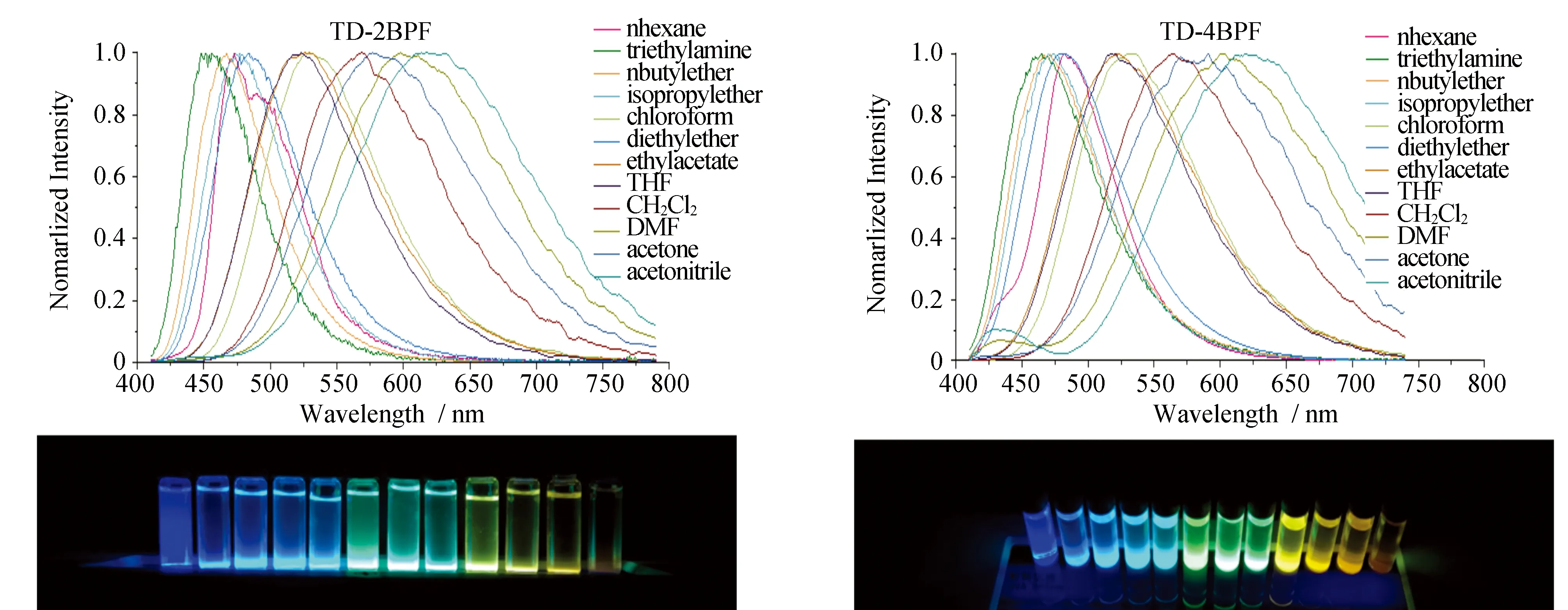
Fig.3 Photoluminescence spectra of TD-2BPF and TD-4BPF in different solvents at room temperature. Insert: photographs of TD-2BPF and TD-4BPF in different solvents under 365 nm hand-lamp irradiation
2.3 Thermal properties
The thermal properties of TD-xBPF were evaluated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), respectively. As shown in Fig.4. TD-xBPF shows good thermal stability, which is indicated by high decomposition temperature (Td, corresponding to 5% weight loss) of 260~450 ℃. But the thermal stability of TD-3BPF is poor. TGA test suggests that the TD-4BPF exhibit thermal stabilities with decomposition temperatures of 450 ℃, which indicating that TD-4BPF can retain amorphous morphology in a wide range of temperature and be expected to decrease the phase separation rate of the guest-host system.

Fig.4 TGA thermogram of TD-xBPF (at 10 ℃ min-1under notrogen atmosphere)
2.4 Theoretical calculations
Further insight into the molecular orbital distributions of both materials was gained by density functional theory (DFT) calculations, which was carried out at the level of Lee-Yang-Parr functional (B3LPY)/6-31(d) basis to investigate their ground states. Fig.5 shows the minimized energy structure of TD-xBPF in the gas phase. The distributions of frontier molecular orbital (FMO), electron exchange energy (Est) as well as the energy gap (Eg) between the HOMO and LUMO levels are depicted in Evidently, TD-xBPF exhibited a clear pattern of electron density-distribution separation between the HOMO and LUMO.
For three emitters, the LUMO is located predominantly on the central dibenzothiophene sulfone acceptor, while the HOMO was mainly distributed over the entire arylamine donor and extended to the phenyl part of dibenzothiophene sulfone. The multiple twist architecture could prevent the formation of excimers and exciplexes in the solid state. Meanwhile, the twist structure contributes significantly to achieve an effective separation between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Showing similar distribution pattern, the HOMOs of TD-xBPF were distributed on electron-rich arylamine moiety. While, the electron distributions of corresponding LUMOs were dominantly localized on the electron-deficient dibenzothiophene sulfone. Therefore, typically observable extensive HOMO-LUMO separation and little HOMO-LUMO overlap at the dibenzothiophene sulfone segment were apparent in the molecular orbital diagram. The almost complete spatial separation of HOMO and LUMO energy level suggests that the HOMO-LUMO excitation would shift the electron density distribution from donor to acceptor. It is essential that such separation of electron cloud of HOMO and LUMO could afford the transporting channels between hole and electron, which may endow the molecules with the ability of bipolar charge transport.

Fig.5 FMOs (HOMO and LUMO) of TD-xBPF calculated with DFT on a B3LYP/6-31G(d) level
2.5 Electrochemical properties
To study the electrochemical performance of TD-xBPF, cyclic voltammetry (CV) was carried to evaluated the optoelectronic applications. The ferrocene was used as a standard reference and the ferrocene/ferrocenium (Fc/Fc+) redox shows a half-wave potential (E1/2) at 0.47 V. The HOMO energy level can be calculated by the empirical equation HOMO = (Eox+ 4.40) eV, whereEoxis the onset potential of oxidation. The CV curves of TD-xBPF are shown in Fig.6. TheEoxare 0.65 eV, 0.63 eV and 0.64 eV for TD-2BPF, TD-3BPF and TD-4BPF, respectively. By calculation, the HOMO energies of TD-2BPF, TD-3BPF and TD-4BPF were 5.05 eV, 5.03 eV and 5.04 eV, respectively. The LUMO levels are calculated through subtraction of the optical energy gap (Eg= 2.79, 2.79 and 2.96 eV) from the HOMO energy levels. The Egis estimated by the wavelength of the ultraviolet absorption edge. By calculation, the LUMO levels of TD-2BPF, TD-3BPF and TD-4BPF were calculated to be 2.26 eV, 2.24 eV and 2.08 eV, respectively.

Fig.6 Cyclic voltammogram of TD-xBPF, in each case, anodic scan was performed in CH2Cl2 at a scan rate of 5 mV s-1. Working electrode: platinum wire; the auxiliary electrode: platinum wire with a porous ceramic wick; the reference electrode: calomel electrode
2.6 Electroluminescence properties
In order to investigate the applications in OLEDs, we fabricated using the following configuration: indiumtin oxide (ITO) | PEDOT:PSS (10 nm) | TD-xBPF (40 nm) | TPBi(40 nm) | LiF (1 nm) | Al (100 nm). In this device structure, PEDOT:PSS and LiF were used as the hole-injection layer (HIL) and the electron-injection layer (EIL), respectively. TPBi (1,3,5-tris(phenyl-2-benzimidazolyl)-benzene) served as the hole-transporting layer (HTL) and the electron-transporting layer (ETL). TD-xBPF were used as the electroluminescent layer (EML) in this device. As displayed in Fig.7, the undoped device showed a yellow emit with the maximum emission of 507, 499 and 514 nm, respectively. The CIE coordinates of TD-2BPF, TD-3BPF and TD-4BPF were (0.215 9,0.579 9), (0.198 9,0.513 1) and (0.240 8,0.604 9), respectively. As the result shown in Fig.8, the devices based TD-2BPF has the maximum forward-viewing current efficiencies of 10.28 cd·A-1, power efficiencies of 11.3 lm·W-1and EQE values of 3.30%(Table 1).

Fig.7 Electroluminescence spectra for devices based TD-xBPF

Table 1 EL performances of the fabricated OLEDs

Fig.8 (a) EL spectra of devices based TD-xBPF, inset: CIE coordinates;(b) Current density-voltage-luminance characteristics for devices;(c) Efficiency versus luminance curves of non-doped devices based on TD-xBPF
3 Conclusions
In summary, we have designed and synthesized three yellow emitters TD-xBPF, by incorporating arylamine donor and dibenzothiophene sulfone acceptor. OLED based on TD-2BPF exhibited a maximum EQE of 3.3% with emission at peak wavelength of 507 nm. More importantly, dibenzothiophene sulfone derivatives are demonstrated to be a quite promising yellow-green candidate to construct effective electroluminescent devices.
