Combining PD1 inhibitor,PARP inhibitor and antiangiogenic medication for lung squamous cell carcinoma with liver metastasis:a case report
2022-12-31RuoQiWangShanQiGuoJunChenWenYanFang
Ruo-Qi Wang,Shan-Qi Guo,Jun Chen*,Wen-Yan Fang*
1Graduate School,Tianjin University of Traditional Chinese Medicine,Tianjin 301617,China.2Department of Oncology,The First Teaching Hospital of Tianjin University of Traditional Chinese Medicine,National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion,Tianjin 300381,China.
Abstract
Keywords:immunotherapy;Programmed cell death protein 1;squamous cell carcinoma;Poly ADP-ribose polymerase inhibitors
Introduction
Programmed cell death protein 1(PD1)inhibitors have developed into a powerful therapeutic strategy and trend in driver gene-negative advanced non-small cell lung cancer(NSCLC)in recent years,which improved patients’clinical outcomes.A humanized IgG4 monoclonal antibody called Sintilimab targets PD1 and can disrupt the PD1/PDL1 pathway and the anticancer activity of reactivated lymphocytes to provide an anti-tumor impact.Due to primary and acquired drug resistance,solo immunotherapy has a limitation.Therefore,combined therapy with chemotherapy,immunotherapy,radiotherapy,or targeted therapy was applied to improve the therapeutic effect for patients.Poly ADP-ribose polymerase(PARP)inhibitors mediate DNA single-strand damage repair.Due to a failure in the homologous recombination repair function in tumor cells carrying the breast cancer susceptibility gene 1/2(BRCA1/2)mutation,DNA double-strand breaks cannot be repaired.The interplay of the two results in a deadly synergistic impact.This article presents the example of a patient with a big liver mass in addition to advanced squamous cell lung carcinoma and a BRCA1 mutation.Following treatment with Sintilimab,Anlotinib and Olaparib,the disease was locally managed,and the lung lesions dramatically decreased.
Case Report
A 68-year-old man attended the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine in March 2022.Because of frequent and rough cough for 2 weeks and left anterior chest pain,accompanied by suffocation within 24 hours.He had a history of alcoholism for 20 years and no history of hypertension,chronic obstructive pulmonary disease,and heart disease.The computed tomography showed the lung mass with pleural effusion(Figure 1A).Beyond that,an 11×12×14 cm well-defined hepatic mass was obvious(Figure 1C).In order to clarify the property of the tumor,we performed a needle aspiration biopsy from his hepatic mass for pathological examination.The pathological report showed the patient met the diagnosis of metastatic poorly differentiated squamous cell carcinoma,and the original tissue sample immunohistochemistry findings were P40(+),CK7(–),CK20(–),ARG1(–),TTF-1(–),NapsinA(–)(Figure 2).The positive rate of Ki-67 nuclear antigen cancer cells was 50%.Then the patient took genetic testing at Zhen He Science and Technology Center,which had a quality certificate issued by the government clinical laboratory center.The patients had mutations in BRCA1,FGFR1,RICTOR,TP53,ATR,DDR1,FAT1,FBXW7,KEAP1,NOTCH3,PAK7,PIK3C2G,RB1,and SLX4 in addition to the results of genetic testing.BRCA1,TP53,and NOTCH3 were positively correlated with immunotherapy response(Figure 3).Mutations in DNA damage repair genes include ATR,BRCA1(germline mutation),SLX4,and TP53.In comparison to patients with low tumor mutation burden,this patient’s tumor had 8.76 mutations per Mb and responded better to immune checkpoint inhibitors(Figure 4).According to the genetic test report,we choose Anlotinib,Olaparib,and Sintilimab as therapeutic strategies.Considering metastatic liver cancer,a two-cycle of epirubicin-based transarterial chemoembolization(TACE)was executed.Imaging revealed a considerable reduction in lung masses following a round of immunotherapy combined with targeted therapy and no disease progression in the liver(Figure 1B and 1D).We informed the patient and his families and got their permission before writing this manuscript.We are devoted to completely safeguarding the patient’s privacy.

Figure 1 The changes of lung cancer and hepatic metastases observed by computed tomography before(A,C)and after treatment(B,D).

Figure 2 Immunohistochemistry.(A)1:10 HE staning;(B)1:10 P40 staining;(C)1:10 Ki-67.
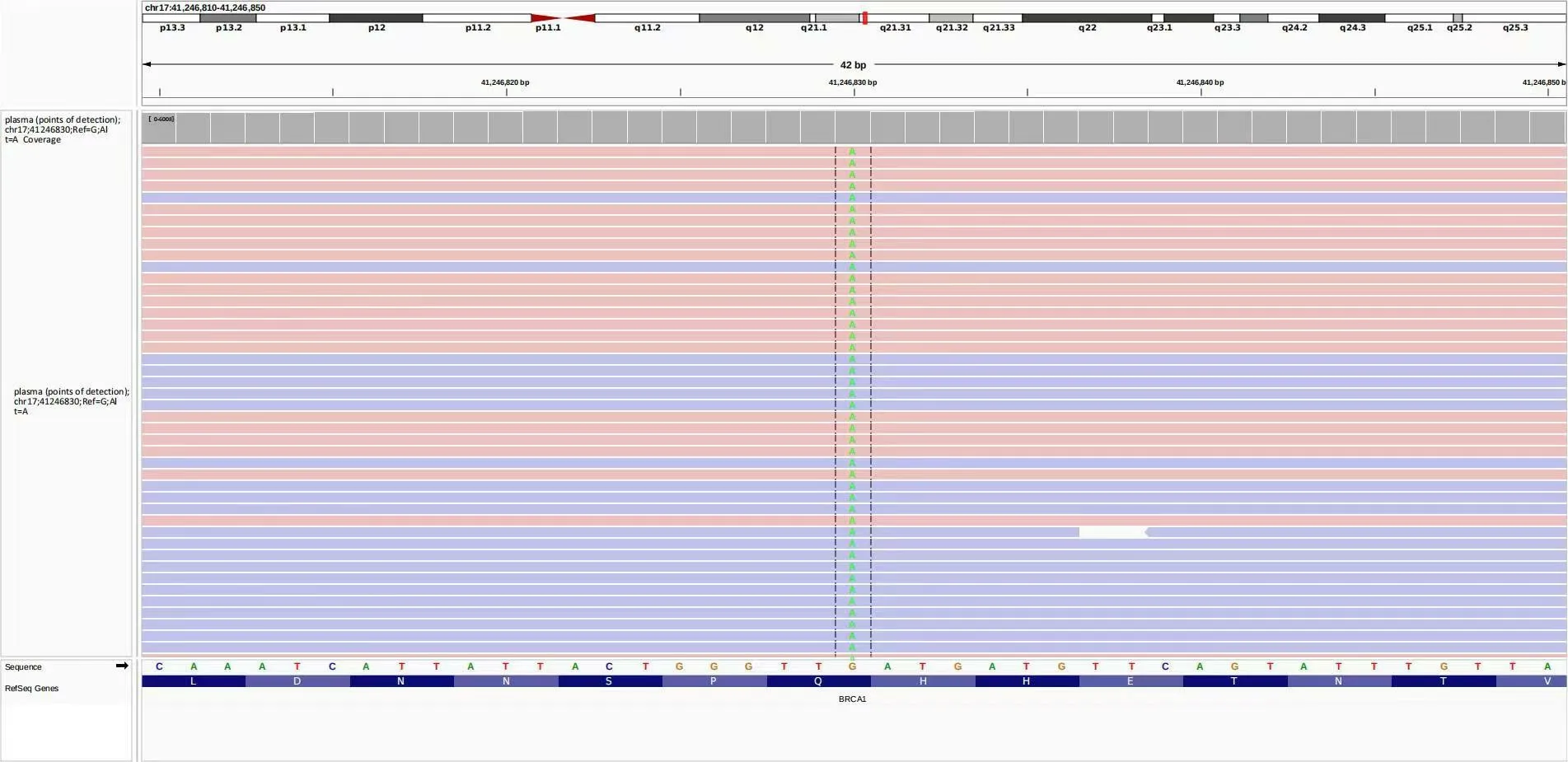
Figure 3 The image information of gene detection.
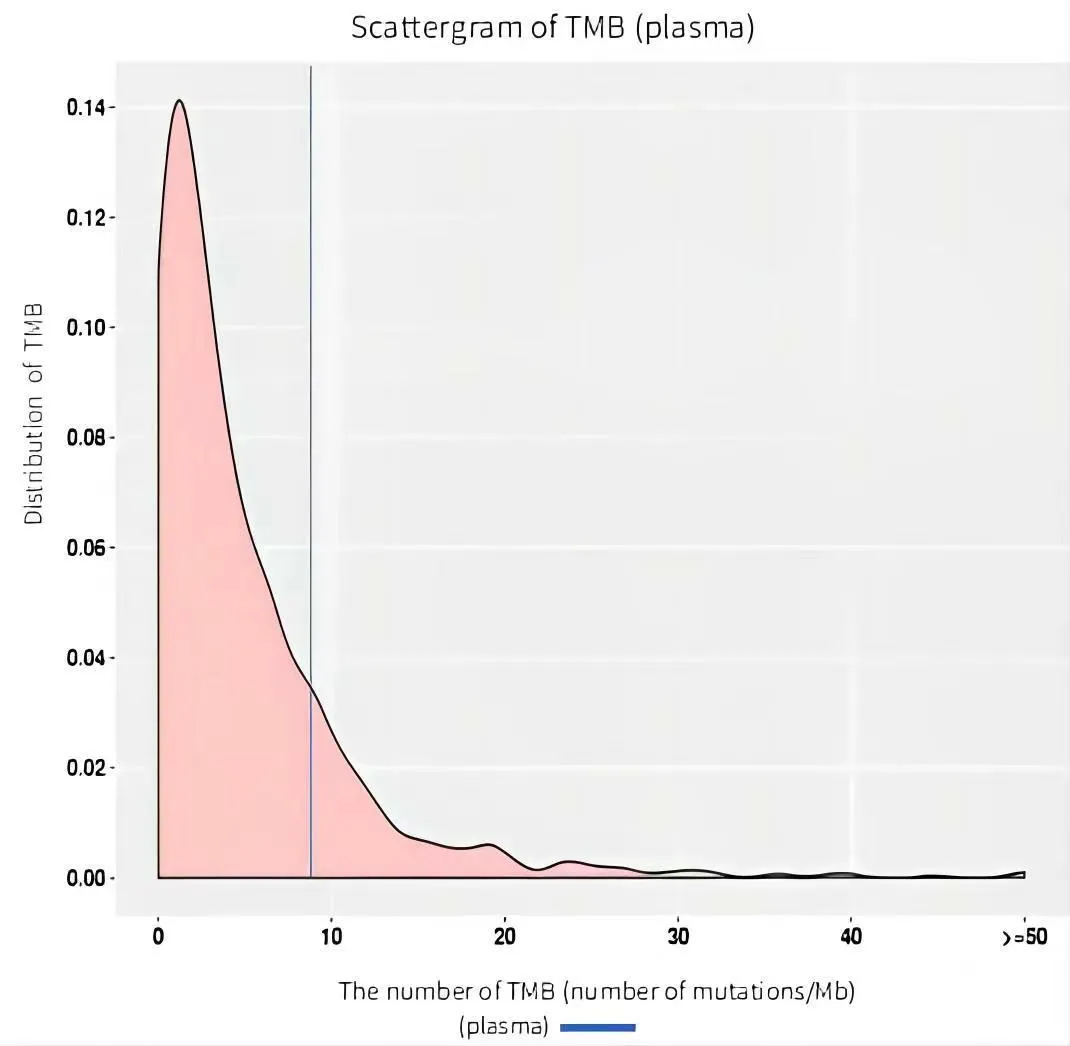
Figure 4 Tumor mutation burden test result.TMB,tumor mutation burden.
Discussion
Due to variations in molecular genetics,different patients respond differently to the same anticancer therapy or medicine in terms of sensitivity and resistance,as well as efficacy and side effects.Pharmacogenomics influences individual variances in efficacy.Therefore,choosing a customized treatment through genetic testing is crucial to increase clinical efficacy for cancer patients.Targeted therapy is not usually beneficial for patients with lung squamous cell carcinoma since driver mutations are uncommon in this cancer.Since they have been used in clinical practice,PD1 inhibitors have stood apart from other anti-tumor medicines in recent years.This has helped alleviate the problem that standard chemotherapy-resistant tumor patients face.Immune therapy has become the focus of high-profile and emerging oncology field treatment strategies.As a result,there is a positive trend for future development and a wide range of opportunities.
This study is a case of immunotherapy compared to anti-angiogenesis,and PARP inhibitors obtained temporary success.It is essential to investigate the use of immune checkpoint inhibitors in treating lung squamous cell carcinoma without driving genes.
According to studies,Sintilimab can also treat other types of cancer,such as liver cancer,esophageal cancer,and non-squamous non-small cell carcinoma.Sintilimab monotherapy was added as a Grade II recommendation for second-line therapy in stage IV squamous cell carcinoma lacking driver genes in the 2021 CSCO Guidelines for NSCLC.According to multiple latest clinical trials,Sintilimab has significantly improved tumor patients’chances of survival.According to a Randomized,Double-Blind,Phase 3 Study(Oncology pRogram by InnovENT anti-PD-1-11),patients with locally progressed or metastatic non-squamous NSCLC who had not previously received treatment.The Sintilimab,Pemetrexed,and platinum combination significantly increased progression-free survival compared to the placebo-alone group.Between the Sintilimab and placebo groups,the verified objective response rate was 51.9% versus 29.8%.The frequency of adverse responses of grade 3 and higher was 61.7%versus 58.8%[1,2].The treatment of patients with advanced resectable esophageal squamous cell carcinoma with Sintilimab in combination with paclitaxel and carboplatin was confirmed in a prospective,single-arm,phase 2 trial;the complete pathological response rate was 22.2%,and the incidence of neutrophils and leukopenia was 12.8% and 17%,respectively[3].With a median progression-free survival(PFS)of 5.5 and 4.9 months,respectively,compared to first-line chemotherapy regimens,ORIENT-12 confirmed that Sintilimab coupled with gemcitabine and platinum significantly extended PFS in patients with advanced non-small cell lung squamous cell carcinoma.In the treatment with citicoridumab and placebo groups,the incidence of grade 3 and above acceptable adverse events was 83.1% and 86.6%,respectively[4].
Based on the BRCA1/2 defective cells’sensitivity to PARP inhibitors,PARP inhibitors work synergistically with BRCA-mutated tumors to kill tumor cells.Olaparib provided a more significant prognostic benefit than non-platinum-based medications in platinum-sensitive ovarian cancer patients with BRCA mutations,according to a Randomized Phase III Trial(SOLO3)[5],with objective response rate of 84.6% and 61.5%,and PFS of 13.4 months and 9.2 months,respectively.After achieving a full or partial response to first-line platinum-containing chemotherapy,numerous additional clinical trials have shown that Olaparib is suitable for maintenance therapy in patients with advanced epithelial ovarian,fallopian tube,or primary peritoneal cancer who carry harmful or potentially harmful BRCA embryonic or somatic mutations.It is indicated for the treatment of patients with metastatic castration-resistant prostate cancer who carry BRCA germline or somatic mutations and have developed disease progression after prior use of novel hormone therapy;maintenance therapy for adult patients with recurrent epithelial ovarian,fallopian tube,or primary peritoneal cancer who have achieved complete or partial response to platinum-containing chemotherapy(NMPA approved).Based on this information,we chose Olaparib for this patient with a BRCA somatic mutation to evaluate Olaparib’s effectiveness in treating non-small cell lung squamous cell carcinoma.
In order to achieve synergistic results,we also chose the antiangiogenic medication Anlotinib in this case study.Anlotinib combined with Sintilimab is a safe and effective treatment for patients with advanced NSCLC,according to phase Ib research[6].The patients in the research had objective response rates of 72.7%,100%,and 100%,respectively,and disease control rates of 100%.The prevalence of grade 3 adverse events was 54.5%,with no grade 4 adverse reactions,and the 12-month progression-free survival rate was 71.4%.The outcomes described in this study are undoubtedly encouraging as a treatment without chemotherapy.Malignant pleural effusion and brain metastases in older NSCLC patients may also benefit from antiangiogenic therapy[7,8].
The patient underwent two epirubicin-based TACE in addition to immunotherapy.TACE can destroy tumors as a local interventional technique by injecting anticancer medications and embolic agents into tumor arteries in the liver to cut off the nourishment supply of tumors.The effectiveness of anti-tumor therapy can also be increased by using TACE in combination with targeted medications by partially inhibiting the expression of vascular endothelial growth factor and platelet-derived growth factor.
After a cycle of combined therapy,the patient’s computed tomography scan revealed that the left pleural effusion was partially absorbed in contrast to earlier.The space-occupying lesions of the left hilum and the left inferior lobe of the lung were smaller than before.The enormous masses of the right lobe of the liver showed no progress compared to before,which was accompanied by tumor tissue necrosis and absorption,and the multiple intrahepatic masses and nodular shadow had not progressed.Thus,this report demonstrates the safety and efficacy of a personalized therapy plan that combines TACE with PD1 inhibitors,PARP inhibitors,and antiangiogenic medicines.
Conclusion
The treatment of advanced lung cancer has increasingly shifted toward a precision approach with the advancement of diagnosis and treatment technology.Numerous forms of combination therapy have been developed,including targeted therapy,immunotherapy,antiangiogenic therapy,and others.For patients with driver-negative NSCLC,immunotherapy based on immune checkpoint drugs targeting PD1/PDL1 offers additional therapeutic options and survival prospects[9,10].This report presents a staged victory of immunotherapy that gives us direction and confidence as we pursue clinical trials of innovative anti-tumor medications to treat advanced NSCLC.Antiangiogenic therapy is also used to treat advanced NSCLC and exhibits a synergistic relationship with other anticancer treatments.It is helpful for patients with various disease types or specific patients at various treatment stages.The best method of administration and combination therapy strategy for patients with various pathological conditions or receiving treatment at various stages still needs further investigation.Additionally,finding new therapeutic and drug resistance targets and efficacy biomarkers are urgent research areas for precision therapy.
杂志排行
Precision Medicine Research的其它文章
- The therapeutic mechanism of Polygonatum sibiricum polysaccharide on T2DM rats based on the Nrf2 signaling pathway
- Regulatory effects of evodiamine on glucose metabolism-related factors in CT26 colorectal carcinoma-bearing mice
- Network pharmacology study of drug pair Tubeimu-Zhebeimu in the treatment of breast cancer
