Intraocular pressure fluctuation range and correction after small incision lenticule extraction
2022-12-27TaoLiangShengNanLiu2MeiGuangLiuZhongTaiJiangShuangSongAiPingZhang
Tao Liang, Sheng-Nan Liu2, Mei-Guang Liu, Zhong-Tai Jiang, Shuang Song, Ai-Ping Zhang
Abstract
KEYWORDS:small incision lenticule extraction; myopia; intraocular pressure; diurnal IOP fluctuation; correction
INTRODUCTION
In recent years, with the growing number of people with myopia, the incidence of the primary open angle glaucoma (POAG) is increasing, and as the diopter step of myopia continues to rise, the probability of developing into POAG is higher[1-3]. Intraocular pressure (IOP) is currently the best indicator for evaluating the risk of glaucoma, and it is also the most effectively monitored indicator[4-5]. Corneal refractive surgeries have made great progress. Among them, small incision lenticule extraction (SMILE) has become a emerging mainstream surgical treatment for myopia. Due to its advantages of small incision, high safety and good biomechanical stability, SMILE has become more and more myopic patients’ choices[6-8]. However, SMILE removes a complete stromal lens, which still causes a certain impact on the cornea, causing the tonometer’s measurement value to change[9-10]. We need to accurately assess the 24-hour IOP changes of myopia patients with different diopter steps after SMILE. This has an important clinical application value for the timely diagnosis and treatment of potential POAG patients after SMILE. This study will observe the changes in IOP fluctuations of myopia patients with various refractive levels after SMILE, and respectively correct the IOP values after reduction, which will provide a reference for postoperative follow-up of myopia patients.
SUBJECTSANDMETHODS
EthicalApprovalThe institutional review board at the Affiliated Hospital of Qingdao University approved this study protocol, which adhered to the tenets of the Declaration of Helsinki. All patients provided informed consent for surgery.
ObjectivesIn this prospective case series study, 79 patients (158 eyes) who underwent SMILE surgery for correcting myopia and/or astigmatism at the Department of Ophthalmology, Affiliated Hospital of Qingdao University, from March 2018 to September 2019 were involved. Among them, there were 44 males and 35 females, aged between 18 and 35 (mean 22.3±4.3) years.
The inclusion criteria were as follows: 1) age: 18-35 years old; 2) refractive status: spherical equivalent (SE) ≤ -10.00 D, cylinder degree ≤ -5.00 D, refractive dioptre progression ≤ 0.5 D within 1y, and best corrected visual acuity (BCVA) ≥1.0. Patients were off soft contact lenses for at least 2wk, off rigid contact lenses for at least 4wk and off orthokeratology for at least 3mo; 3) ocular conditions: corneal transparency, normal corneal topography, no keratoconus tendency, calculated residual stromal bed after treatment ≥280 μm, and no dry eye and other ocular surface diseases; 4) IOP requirements: IOPpre≤21 mmHg.
The exclusion criteria were as follows:1) ocular diseases: glaucoma, suspected glaucoma, visual field damage, cup-to-disk ratio >0.3, active inflammatory reaction or infection of the eye, history of previous eye surgery, history of trauma, cataract affecting vision, fundus disease,etc.; 2) systemic diseases: diabetes, hyperthyroidism, systemic connective tissue disease, mental disorders,etc. The grouping criteria were as follows (Table 1).

Table 1 Baseline characteristics of the study subjects
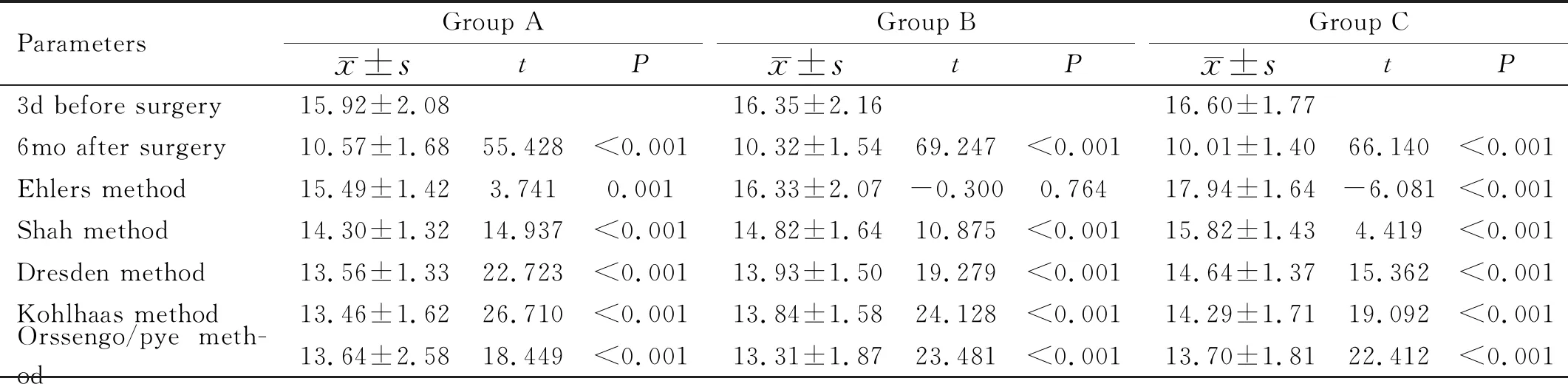
Table 2 Comparison of IOPpre, IOPpost and Pentacam-corrected IOP in each group mmHg
InspectionMethodsAll patients underwent a conventional SMILE preoperative examination, including assessments for uncorrected distance visual acuities (UDVA) and corrected distance visual acuities (CDVA), general optometry, dilated optometry, 24-hour IOP (24-hour IOP measured at 2:00, 6:00, 10:00, 14:00, 18:00, and 22:00), slit-lamp microscopy, corneal topography and a fundus examination. The follow-up period was 6mo. The UDVA, CDVA, general optometry, 24-hour IOP, and corneal topography were reviewed at 1wk, 1, 3mo and 6mo after SMILE surgery. This study used the 6mo postoperative data for statistics.
1) Vision: UDVA and CDVA before and after surgery were measured using a standard logarithmic visual acuity chart. A five-point recording method was used; 2) Optometry: A NIDEK AR-1 computer refractometer was used to check the patient’s dioptre, which was combined with manifest refraction results and converted to an spherical equivalent (SE); 3) IOP value: Perform the non-contact tonometer (NCT, NT-510, Canon, Japan) inspection first, followed by the goldmann applanation tonometer (GAT, Haag-Streit, Bern, Switzerland) inspection, with an interval of 15min. During the NCT measurement, patients were required to relax, avoid blinking and holding their breath, and gaze at the indicator light. For each eye, the arithmetic average of the 3 measurement values was taken, and the difference between the measured values was less than 3 mmHg. Before GAT measurement, the subject’s eyes were drunk with 0.5% tetracaine for surface anesthesia. After 0.25% sodium fluorescein solution was dripped, three measurements were made. The difference between the measured values is required to be less than 1 mmHg, and the average value is taken; 4) Corneal topography: The Oculus Pentacam three-dimensional anterior segment analyser was used to observe the front and back corneal surface morphology. The corneal volume (CV) before and after surgery, the central 3 mm-diameter CV (CCV3mm), horizontal corneal diameter (WTW), and corneal curvature (Kf, Ks, Km) were recorded. Eyes were maintained, in a good state of tear film in a dark room before the examination, and the average of the 3 measurement values was taken for the study. The above checks were performed by an experienced technician.
SurgicalMethodsA VisuMax femtosecond laser system (Carl Zeiss Meditec AG, Jena, Germany) was used for surgical refractive corrections in all patients. The thickness of the corneal cap was set to 120 μm, the diameter was 7.8 mm, the lens diameter was 6.7 mm, the thinnest edge was 15 μm, and the astigmatism transition zone was 0.1 mm. The patient was placed in supine position, and the eye was anaesthetized. According to the surgical parameters, the posterior surface of the lens was first scanned and cut laterally. Then, the front surface of the lens was scanned, and the 2 mm edge of the corneal cap was scanned. After the end of the scan, the substrate lens was bluntly separated and completely removed. All procedures were performed by the same surgeon.
PostoperativeMedicationPostoperatively, all the patients received 0.5% levofloxacin eye drops for 7d and 0.1% fluorometholone eyedrops for 2wk. Sodium hyaluronate eye drops were used for more than 4wk.

RESULTS
ComparisonofIOPpre,IOPpostandPentacam-correctedIOPA pairedt-test was used in Table 2. The IOPpostcorrected by Ehlers was higher than the IOPprein patients with high myopia, and the IOPpostcorrected by Ehlers was lower than the IOPprein patients with low myopia (P<0.01). There was no significant difference between the IOPpreand IOPpostcorrected by Ehlers in patients with moderate myopia (t= -0.300,P=0.764). The NCTpostand Pentacam-corrected IOPs (Dresden, Orssengo, Shah, and Kohlhaas) were significantly decreased in each group, and the difference was statistically significant (P<0.01).

Table 3 Comparison of the 24-hour IOP values for the right eye and left eye in myopic patients after SMILE (n=79, mmHg)

Table 4 Changes in the range of IOP values before and after surgery in each group mmHg
Comparisonofthe24-hourIOPoftheLeftandRightEyesThe difference between the two eyes preoperatively was 0-5 (mean 2.16±1.48) mmHg, and the difference between the two eyes postoperatively was 0-4 (mean 1.70±1.24) mmHg, indicating that the difference between the two eyes postoperatively was smaller than that before surgery.
The IOPpreof the right eye was 10-20.3 mmHg, with an average of 16.19±2.09 mmHg; the IOPpreof the left eye was 11-21 mmHg, with an average of 16.30±2.03 mmHg, and the difference was not statistically significant (t= -0.895,P=0.371). Repeated measures analysis of variance showed a statistically significant difference in intraocular pressure fluctuations in both eyes (F=24.903,P<0.001), and there was no interaction between the right and left eyes at any time point (F=0.888,P=0.477), indicating that time had no influence on the eyes.
According to the repeated measures variance analysis (Table 3), the multivariate test results showed that the IOP of the right eye (F=23.101,P<0.001), the IOP of the left eye (F=17.027,P<0.001), and the IOP at different time points after SMILE were significantly different, indicating that there was diurnal fluctuation in intraocular pressure. There was no interaction between the time points and the two eyes (F=1.474,P=0.202), indicating that time did not affect the eye. An analysis of variance of the IOP in the right eye and left eye for 24h showed thatF=38.495 andP<0.001, indicating that there was a significant difference between the right eye and left eye 24-hour IOP in myopia patients after surgery. The IOP of the right eye and left eye were analysed with pairedt-tests at each time point, indicating that there was no significant difference in the IOP of the right and left eyes at any time point in patients with myopia after SMILE (P>0.05).
Comparisonof24-hourIOPpreand24-hourIOPpostThe differences in IOP measured by NCT and GAT were not statistically significant (P>0.05), but the differences in IOP of the three groups were statistically significant (P<0.05). The normal IOP range of 10-21 mmHg was not applicable to the IOP value after SMILE. The IOPpostrange by NCT were 8-17 mmHg in group A, 7-16.3 mmHg in group B, and 7.7-14.3 mmHg in group C. The IOPpostrange by GAT were 8-17 mmHg (Table 4). The IOP fluctuation before surgery was ≤8 mmHg, and the IOP fluctuation after surgery was ≤6 mmHg (Table 5).
Repeated measures analysis of variance was performed on the IOP values from before and after SMILE in patients with low myopia, which was listed in Table 6. The following IOP measured by NCT. The multivariate test results showed that the IOPpre(F=14.814,P<0.001), IOPpost(F=4.432,P<0.001), and IOPs at different time points were significantly different and that there was no interaction between the preoperative and postoperative measurements at any time point (F=2.587,P=0.111), indicating that the effect of time does not vary for the preoperative and postoperative measurements. The analysis of variance before and after surgery showed thatF=17.615 andP<0.001, indicating a significant difference between the IOPpreand IOPpostmeasurements. A pairedt-test was performed on the IOPs at each time point before and after surgery and showed thatP<0.001, indicating that there was a significant difference between the IOP at each time point before and after surgery. Similarly, there was a significant difference between the preoperative and postoperative IOP measurements in patients with moderate and high myopia (group B:F=31.211,P<0.001; group C:F=80.121,P<0.001) (Table 7, Table 8).
CorrelationsBetweenIOPpostMeasurementsandVariousInfluencingFactorsThe IOPpostmeasurements of each group were positively correlated with IOPpre, CCTpost, 3 mm/WTW,CVpost, CCVpost3mm, and CCVpost3mm/ CVpost(P<0.05; Table 9), and negatively correlated with WTW (P<0.05; Table 9). There was no significant correlation with corneal curvaturepost, ΔCV, and ΔIOP (P>0.05).

Table 5 Changes in the 24h fluctuation range of IOP before and after surgery in each group mmHg

Table 6 Changes in IOP 24h before and after SMILE in patients with low myopia (n=53, mmHg)

Table 7 Changes in IOP values 24h before and after SMILE in patients with moderate myopia (n=65, mmHg)

Table 8 Changes in IOP values 24h before and after SMILE in patients with high myopia (n=40, mmHg)
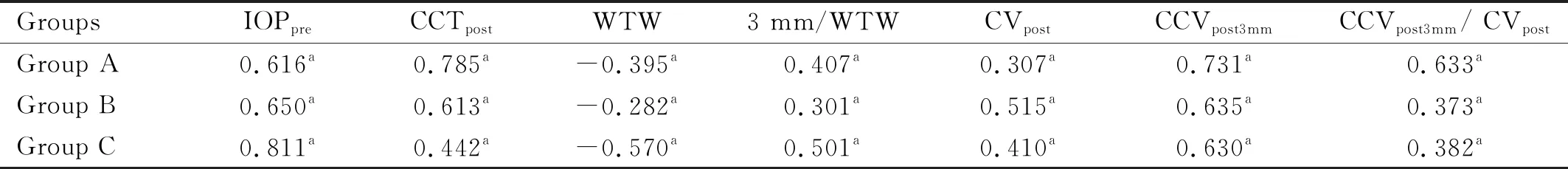
Table 9 Correlations between IOPpost measurements and corneal parameters and their differences in each group r
CorrectionFormulaforIOPpostMeasurementsThe IOP 6mo postoperatively was used as a dependent variable, and the SEpre, CCTpost, WTW, 3 mm/WTW, CVpost, CCVpost3mm, CCVpost3mm/CVpostwere used as independent variables. A staged multivariate regression model (stepwise method) was established for each group of IOPpostmeasurements.
NCTpost corrected (A)=0.349 × NCTpost+ 4.137 × CCVpre3mm- 1.533 (F=17.165,P<0.01), adjustedR2=0.383; NCTpost corrected (B)=0.477 × NCTpost+ 3.643 × CCVpost3mm-1.125 (F=41.819,P<0.01), adjustedR2=0.561; NCTpost corrected (C)= 0.638 × NCTpost+ 3.426 × CCVpost3mm-0.716 (F=69.858,P<0.01), adjustedR2= 0.779.
DISCUSSION
At present, the safety, effectiveness, stability, and predictability of SMILE correction for myopia and/or astigmatism have been widely recognized[11-12]. The femtosecond laser is the shortest pulse laser that humans have built under laboratory conditions. It rapidly ionizes the corneal tissue to form a hot plasma, vaporizes the corneal tissue and generates expanded blisters and CO2bubbles. The corneal tissue is thus separated so that the complete stromal lens can be cut out of the corneal tissue[13].
Accurate evaluation of the IOP after SMILE is essential for the diagnosis and treatment of potential glaucoma patients[9]. The Goldmann applanation tonometer (GAT) is still considered the gold standard for tonometers[14]. However, the GAT is an applanation tonometer, and corneal thickness, corneal curvature, tear film stability, and corneal stroma elasticity may all influence the measurement of intraocular pressure[15]. At present, NCT is designed based on the Imberk-Fick principle. Its automatic microcomputer sensor calculates the intraocular pressure value according to the time required for the light to reflect from the corneal surface and flatten the 3.6 mm-diameter area of the central corneal area[16-17]. NCT does not require surface anaesthesia and does not touch the cornea[18]. It is suitable for large-scale screening of potential glaucoma patients, especially for patients who have undergone myopic surgery. In this study, we found that there was no statistical difference between the IOP measured by NCT and GAT (P>0.05). Animal experimental studies have shown that the eyeball structure and aqueous humour circulation are not affected by corneal refractive surgery and that the IOP will not change significantly[19]. However, the cornea is not an ideal plane. Because of changes in the central corneal volume, corneal curvature, corneal biomechanics,etc., after SMILE, the force required to flatten the cornea of the same area is reduced, and the postoperative IOP value is lower than the true value[20]. Thus, the IOP evaluation standard[21], in which 10-21 mmHg is the normal IOP range, the binocular difference is ≤5 mmHg and the diurnal IOP fluctuation is ≤8 mmHg, is no longer appropriate.
LASIK changes the curvature of the front surface of the cornea through the ablation of the corneal stroma with an excimer laser, thereby changing the refractive power of the cornea to achieve the purpose of correcting vision[22]. Iatrogenic corneal dilatation is a vision-threatening complication after LASIK and is associated with weakened corneal mechanical strength[22-23]. Previous studies have found that the Pentacam correction formula by Ehlers is more reliable in the correction of IOP after LASIK and EK. The Ehlers method uses 545 μm as the standard corneal thickness; for every 15 μm increase or decrease, the IOP increases or decreases by 1 mmHg, respectively[24]. Follow-up after LASIK found that corneal thickness was related to preoperative and postoperative IOP[25]. The greater the preoperative CCT and cutting depth, the greater the postoperative IOP reading changes were[26-27].
The incision in SMILE is only 2 mm, and a corneal cap is made instead of a corneal flap. The collagen fibre damage is significantly reduced, the integrity of Bowman’s membrane and the anterior stroma layer is better preserved, and the corneal elasticity is better maintained[9-10,28]. The SMILE matrix lens has a diameter of 6.5-7.0 mm and has no transition zone. The changes in the shape of the peripheral cornea are significantly less than those in LASIK. It greatly reduces the changes in corneal biomechanics[12,23]. Thus, it is possible to reduce the effect on IOP measurements. It can be seen that SMILE is different from previous procedures in terms of surgical design, corneal biomechanical changes,etc. Therefore, the previous LASIK IOP-corrected formula cannot be applied to the evaluation of IOP after SMILE.
This study found that the IOPpostcorrected by Ehlers was higher than the IOPprein patients with high myopia and that the IOPpostcorrected by Ehlers was lower than the IOPprein patients with low myopia. There was statistical significance (P<0.01). There was no significant difference between the IOPpreand IOPpostcorrected by Ehlers in patients with moderate myopia (t= -0.300,P=0.764). It was considered that the Ehlers correction for the assessment of IOP in patients with moderate myopia after SMILE is relatively reliable. The NCTpostand Pentacam-corrected IOPs (Dresden, Orssengo, Shah, and Kohlhaas) were significantly decreased in each group, and the difference was statistically significant (P<0.01). Therefore, it is believed that Pentacam’s IOP correction formula is insufficient to correct the IOP after SMILE. Lietal[29]obtained the following formula: IOP after SMILE = NCTpost+ 0.389 NCTpre-0.431 SE -4.61815. However, at present, the evaluation of IOP after SMILE remains to be further studied.
Pathologically elevated IOP is the main risk factor for glaucoma, and large IOP fluctuations are independent risk factors for progressive damage to the optic nerve. In this study, repeated measures analysis of variance showed that there was no statistically significant difference between the right and left eyes, both before and after surgery. There were significant differences in IOP at different time points during the day before and after surgery in patients with low myopia, moderate myopia, and high myopia. Studies have shown that the IOPpostrange by NCT are from 8-17 mmHg in group A, 7-16.3 mmHg in group B, and 7-14.3 mmHg in group C. The IOPpostdifference between the two eyes is 0-4 mmHg, and the diurnal IOP fluctuation after the operation is less than 6 mmHg; both are decreased compared with the preoperative values. With an increase in the preoperative refractive power, the postoperative IOP value decreases.
This study found that the higher the IOPpre, the larger the CV, the higher the SE, the thicker the base lens, and the smaller the corneal diameter, the greater the change in IOP measurements after surgery. In addition, the statistical results show that the greater the CV change rate before and after surgery, the lower the IOPpostmeasurement. The preoperative CV was not consistent among patients. When the surgical lens volume was the same, the postoperative corneal rigidity changes were different, and the IOPpostmeasurements varied. The patients were divided into three groups according to the SE level. The IOPpremeasurement value and the CCVpostcan be used to roughly predict the IOPpostmeasurement of each group, which is convenient to evaluate the actual IOP after surgery and guide postoperative hormone medication administration. The results of this study have important clinical significance for the diagnosis and treatment of patients with potential glaucoma after SMILE with different refractive levels before surgery.
There are some limitations in this study. There are differences between tonometers, and different designs in corneal cap thickness and optical area diameter in surgical parameters will affect the changes of corneal biomechanical factors after the operation, subsequently affecting the size of the IOP measurement[30-31]. The prediction of IOP after SMILE is still to be further studied by increasing the sample size.
In summary, factors such as the IOPpreand cutting volume should be considered when assessing the IOPpost. The IOPpostvalue is lower than the real value and may be evaluated relative to the normal IOP range of 7-17 mmHg, a binocular difference of ≤4 mmHg, and a diurnal IOP fluctuation ≤6 mmHg. The greater the IOPprevalue, the larger the CV, the smaller the corneal diameter, the higher the degree of myopia, the larger the surgical removal of the stromal lens, the greater the change in IOPpostmeasurements. For different patients, the greater the change in CV before and after surgery, the lower the IOPpostmeasurement. CCV3mmis an important indicator in the evaluation of IOP measurements after SMILE, and it is of great significance the administration of medication after SMILE. The correction of IOP after SMILE remains to be further explored.
