Effect of intravitreal anti-VEGF injection on choroidal thickness in patients with diabetic macula edema
2022-12-27JiaCherngChong1HanizasuranaHashim2TajunisahIqbal
Jia Cherng Chong1,2,3, Hanizasurana Hashim2, Tajunisah Iqbal
Abstract
KEYWORDS:anti-VEGF; Aflibercept; choroidal thickness; diabetic retinopathy; diabetic macula edema; Ranibizumab; optical coherence tomography; enhanced depth image-optical coherence tomography
INTRODUCTION
Diabetic retinopathy(DR) and diabetic macula edema (DME) is one of the leading cause of blindness[1]. Diabetes mellitus (DM) is an extremely common chronic metabolic disease which occurs across all populations. It is a state of persistent hyperglycemia of the body, due to either insulin insufficiency (Type 1 DM) or insulin resistance (Type 2 DM). Uncontrolled DM is a leading cause of blindness, renal failure, heart disease, stroke and limb amputation[2]. WHO data showed global increment of diabetic prevalence from 4.7% in 1980 to 8.5% in 2014[2]. Our Malaysian National Health and Morbidity Survey 2015 showed a higher than average prevalence of DM among Malaysians at 17.5%[3]. Malaysian Diabetic Eye Registry in 2007 showed a diabetic retinopathy prevalence of 36.8%, with 11% having diabetic maculopathy[4]. This was again higher than global average where a 35-studies global Meta-analysis has given a prevalence of 7.48% for DME[1]. DME is the third commonest cause of preventable blindness, just trailing behind refractive errors and cataract[1].
Chronic hyperglycemia leads to deposition of advanced glycated products on vessels which then causes inflammation, oxidative stress and hypoxia in the retina and vessels. These events are the backbone pathology of DR, and eventually trigger the up-regulation of vascular endothelial growth factor (VEGF) by the Müller-glial cells and retinal pigmented epithelium cells. DME was caused by VEGF which breaks down blood-retinal-barrier, followed by a cascade of events including leakage, exudation and neovascularization[5]. DME refers to the condition where fluid accumulation occurs over the macula area, which could be intra-retinal and/or sub-retinal. DME causes significant visual loss and prompt treatment is important to improve and maintain vision, as well as to prevent permanent visual impairment.
VascularEndothelialGrowthFactorandAnti-VEGFVEGF is the protein responsible for the vessels regulation and formation of new blood vessels in an attempt to re-perfuse the area with retinal ischaemia. With the understanding of pathogenesis in DME, anti-VEGF medications were developed as a treatment modality. Anti-VEGFs are medications that bind to VEGF-molecules or their receptor binding sites in order to stop their signalling pathway and thus impairing their effect.

In Malaysia, the two approved anti-VEGFs for the treatment of DME are Ranibizumab (ACCENTRIX, Novartis, Switzerland) and Aflibercept (EYLEA, Regeneron-Bayer HealthCare, Germany). Ranibizumab works by binding to the VEGF-A (a subtype of VEGF) receptor-binding site, which then prevents the binding of all VEGF-A isoforms to their endothelial cell surface receptors and impair the action ofFigure1Cross section of choroid[11].
VEGF. Aflibercept works as a high affinity decoy receptor for VEGF-A, VEGF-B and placental growth factor, which again inhibits the action of all these growth factors. For the case of Malaysia, Ranibizumab was approved in 2011 and Aflibercept was approved in 2017. Following the introduction of anti-VEGF, its proven efficacy and safety profile by multiple large sample, multi-centre randomized control trial studies have made them the first line treatment for DME worldwide[6-10].
ChoroidChoroid is the posterior part of the ocular uveal tissue which lies between the outer scleral shell and the inner retinal pigmented epithelium. Choroid is highly pigmented and it extends anteriorly from the optic disc margin to ora serrata. Choroid is made up of multiple layers microscopically but generally it is a highly vascularized structure which serves multiple functions (Figure 1). Being the most vascularized tissue in the eye. The principal function of choroid is to nourish the outer retina and prelaminar portion of the optic nerve. Other functions include ocular thermoregulation, acting as a conduit for vessels travelling to other parts of the eye, control of light scattering within the eye ball and probably a small influence on intraocular pressure. Choroidal thickness varies at different sites, with the thickness at posterior pole measure approximately 220-250 μm and near the ora serrata it measure approximately 100 μm. The choroidal thickness and choriocapillaris are continuously regulated by VEGF in response to the requirement.
OpticalCoherenceTomographyOptical coherence tomography (OCT) was invented as early as 1990. OCT has given a great boost in the field of ophthalmology in terms of disease diagnosis, disease monitoring and treatment monitoring. It is a non-contact and non-invasive imaging modality which provides objective measurements and images of the anterior segment of the eyes and/or retina layers. OCT uses the theory of low coherence interferometry, splitting a light source (laser beam) into reference and sample arm which then was reflected upon reaching different surfaces and depth. The reflected laser beam then was captured by the machine and reconstructed into cross-sectional images or even 3-dimentional imaging.

Following technological advancement, OCT has also improved over time. Scanning speed and resolution has improved significantly from the initial time-domain OCT (TD-OCT), to spectral-domain OCT (SD-OCT) and the latest swept-source OCT (SS-OCT) which does not require a spectrometer. Enhanced depth image (EDI) mode was introduced around the year of 2004[12]. It was first applied onFigure2Sample picture of EDI-OCT (In Black/White mode).

Figure 3 Sample picture of EDI-OCT (In White/Black mode).
the SD-OCT, where the positioning of the OCT was closer to the eye which gives a good resolution choroidal images. With high-speed scanning, eye tracking and image averaging, high quality choroidal image can be obtainedviaEDI-OCT (Figures 2 and 3). With the application of EDI on SS-OCT, images are expected to be of even higher resolution and clarity.

Figure 4 Scanning protocol.
The OCT system used in our study was the Spectralis OCT System (Heidelberg Engineering, Heidelberg, Germany). It is an enhanced depth imaging mode-enabled spectral domain OCT. The EDI images of SD-OCT are highly reproducible, even among different model of OCT machines[13-15]. By obtaining an EDI-OCT choroidal image of our study subjects, measurement of choroidal thickness could be done using the built-in calliper function, where the measurements were then analysed for any significant changes.
RationaleofStudyThe purpose of this study is to look for any change in choroidal thickness following intravitreal anti-VEGF treatment for DME patients, especially in Malaysian sub-population. While previous studies may have shown a choroidal thinning after injections, there were only little comparison analysis between different anti-VEGFs used. Results were previously analysed as a whole regardless of the type of anti-VEGF used, namely Pegaptanib, Bevacizumab and Ranibizumab. Previous studies were also mainly conducted in the Western countries, where no significant data on Malaysian population was published.
In our prospective study, we had the same time frame for all study subjects, and we separated the data for analysis for subjects receiving Ranibizumab or Aflibercept. By doing so, we were able to monitor the effect of a specific anti-VEGF on the choroidal thickness after treatment. This study also included subjects who were receiving Aflibercept for DME. These data analysis and results were not previously available nor published.
Another advantage of our study was that all subjects received the same number of injections and same dose of medication. In comparison to previous studies, they were done retrospectively with variable number of injections among their subjects. Once our study results were available, it could serve as a platform for future studies in relation to choroidal thickness.
SUBJECTSANDMETHODS
This was a prospective observational cohort study strictly adhering to the guidelines in Declaration of Helsinki. All patients who were planned for intravitreal anti-VEGF treatment for DME and fulfilled the study criteria were invited for the study. Sampling was doneviaconsecutive sampling method. Sampling and data collection were carried out from 1stJune 2019 to 30thSeptember 2019 in Eye Clinic, Hospital Selayang. Study proposal was submitted to Clinical Research Centre (CRC), Hospital Selayang for evaluation and approval prior to submission for ethical approval. Ethics approval was then obtained from the Medical Research & Ethics Committee (MREC), Ministry of Health Malaysia prior to the commencement of this study. Research was also approved by the Director of Hospital Selayang and Head of Ophthalmology Department, Hospital Selayang. MREC study approval number was NMRR-18-2893-43909.
InclusionCriteria1) Patients with central involving DME with central retinal thickness >250 μm, with vision worse than 6/9 on Snellen; 2) Patients planned for intravitreal anti-VEGF (Ranibizumab/Aflibercept) injection for the treatment of central involving DME by treating consultant; 3) Patients who has no contraindication for intravitreal anti-VEGF injection; 4) Treatment naive patients with no previous history of intravitreal anti-VEGF injection in the target eye.
ExclusionCriteria1) Patients with pre-existing ocular co-morbid specifically uncontrolled glaucoma, chorioretinal pathologies and inflammatory ocular diseases; 2) High axial myopia > -6.0 D; 3) Active smokers; Patients on phosphodiesterase type 5 inhibitor treatment (e.g.sildenafil) of any indication; 4) Severe hypertension patients on 3 or more anti-hypertensives.
MethodologyThis study was a prospective observational cohort study. We recruited patients with central involving DME who required and were agreeable for intravitreal anti-VEGF (Ranibizumab or Aflibercept) injections. These patients were recruited from Ophthalmology Clinic, Hospital Selayang. We employed a consecutive sampling method to recruit all eligible patients as subject in order to obtain more and sufficient samples from a limited number of eligible subjects for better statistical power.
The recruited subjects were intravitreal anti-VEGF injection-naive patients. There were all planned for 3 doses of monthly (every 4 weekly) intravitreal Ranibizumab 0.5 mg/0.05 mL or intravitreal Aflibercept 2 mg/0.05 mL injections. Baseline EDI-OCT were taken prior to the each injection and at approximately 12wk since the first injection during clinic review. Standard aseptic precautions in intravitreal injections such as sterile environment, sterile precautions, normal intraocular pressure and infective free status of the subjects were applied. It is worth reminding that being an observational study, we have no control over the choice of medication used on each subject, where the decision was made by the treating consultant based on multiple factors including medication availability.
The OCT facility used in this study was the Spectralis OCT System (Heidelberg Engineering, Heidelberg, Germany).A custom scanning protocol was made to cater to our study (Figure 4). The protocol obtains a 20° × 20° EDI-OCT high resolution macula scan divided into 25 horizontal cuts, with a gap of 240 μm between each cut and consists of 512 A-scan per at per horizontal cut. The automatic retinal tracking (ART) mode was on and was set to 60 frames per retinal location for adequate image quality acquisition within a reasonable time frame without burdening the subject.

Figure 5 Drop-out rate.
EDI-OCT was obtained after 12wk, 0 and 12wk sub-foveal choroidal thickness were measured by two researchers, where one was unmasked while the other was masked. Intra-class correlation coefficient was tested on the reproducibility of the results. All image qualities were at least with instrument quality score of 15 out of 20 (Medium Quality). Choroidal imaging were all done between 10 a.m. to 12 p.m. in order to reduce the effect of diurnal variation in choroidal thickness[16].
Choroidal thickness measurement is extremely challenging despite the introduction of EDI mode in OCT. The newer generation of swept-source OCTs give a picture of higher resolution compared to spectral-domain OCTs. However, Narendranetal[17]and Zafaretal[18]have shown that choroidal thickness measurement between swept-source OCTs and spectral-domain OCTs were comparable. All spectral-domain OCT are equipped with built-in calliper function for manual measurement of choroidal thickness following manual identification/delineation of choroidal scleral junction interface. Multiple algorithm/software had been developed in an attempt to delineate the choroidal-scleral-junction and gives automated measurement[19-21]. This software had been proven to have high repeatability, however their validity was undetermined. Multiple methods have been used to improve the visualization of the choroidal scleral junction interface, which include image editing with adaptive compensation, white-black colour reverse or heat colour mode[22-23].
In our study, we used white/black, black/white and heat colour mode to facilitate choroidal-scleral junction interface visualization. Image enhancements with contrast/brightness adjustments were also done to improve choroid-scleral-junction
visualization.The inner border of the choroidal thickness was taken at the outer border of RPE reflective line, and the outer border was taken at the choroid-scleral-junction, or the outer border of the choroid stroma[24].

Pre-injection choroidal thickness and post-injection choroidal thickness for both Ranibizumab and Aflibercept arms were analysed using Wilconxon-Signed-Ranked test as our results were not normally distributed. A 95% confidence interval with 5% level of significant was use to retain or reject the hypotheses.P-value of less than 0.05 indicates significant change in choroidal thickness between pre-injection and post-injection was present and null hypothesis is rejected. The pre-injection values and post-injection values used for analysis were the mean value of two readings done by two different researchers. Two-way random intraclass correlation coefficient test was performed to ensure the reliability and reproducibility of the choroidal thickness measurements done by the two researchers.
Independent Mann-Whitney U test was used to analyse for any difference between subjects who received Ranibizumab or Aflibercept. AP-value of less than 0.05 indicates the presence of statistically significant difference between two groups and the null hypothesis is to be rejected.
Bivariate Spearman’s correlations were performed to look for significance between pre-treatment choroidal thickness with vision improvement. Correlations between choroidal thickness changes with age, vision improvement, as well as central retinal thickness changes were also performed.
RESULTS
Demographic,VisionandCentralRetinalThicknessWe managed to collect a total of 17 subjects who were able to complete our study. Drop-outs are shown in the flow-chart below (Figure 5).We have total of 15 drop-outs (46.88%) throughout our study follow-up for various reasons including sepsis, ocular injections, infected wound as well as subjects who failed to turn up half-way along the study. The summary of our subjects’age, gender and ethnicity as well as intraocular pressure are summarized in Table 1. From Table 1,

Table 1 Subject demographic details and pre-treatment intraocular pressure
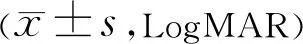
Table 2 Pre and post-treatment vision acuity
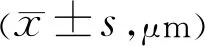
Table 3 Pre and post-treatment central retinal thickness
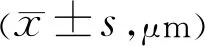
Table 4 Pre and post-treatment sub-foveal choroidal thickness
we could clearly see that our subjects between Ranibizumab and Aflibercept group were matched in terms of age, gender as well as ethnicity. Pre-treatment intraocular pressure was also similar in both groups. Pre and post-treatment visual acuity has been summarized in Table 2. Table 3 shows the changes in central retinal thickness before and after treatment. Both groups achieved significant visual improvement and reduction of central retinal thickness.
ChoroidalThicknessChangesTable 4 below summarizes the change in choroidal thickness following intravitreal anti-VEGF injection. We can see that there is significant sub-foveal choroidal thinning after injections however the amount of thinning among the two groups were insignificant. Figure 6 showed the boxplot graph interpretation of pre and post-injection choroidal thickness. Example of pre and post-treatment measurement of a single subject are shown below in Figures 7 and 8.

Figure 6 Box-plot representation of pre and post-treatment sub-foveal choroidal thickness.

Figure 7 Pre-treatment EDI-OCT.

Figure 8 Post treatment EDI-OCT.
Intraclass correlation coefficient (ICC) were done on pre and post-treatment measurement done by two researchers in order to ensure reliability of the readings and if they were in agreement between the two researchers. The ICC test result we had was 0.993 for average measurements, which indicated that the readings made by our two researchers were in agreement and reliable.
CorrelationsWith our data collected, we ran Spearman’s correlation test to look for relationship between certain variables. The result is summarized in Table 5.Our result showed that there is no significant correlation between pre-treatment choroidal thickness with the amount of vision improvement. There were also no significant correlation between choroidal thickness changes with vision change, age of subjects and central retinal thickness change.

Table 5 Spearman’s correlation test(n=17)
DISCUSSION
Our results showed a significant sub-foveal choroidal thinning following 3 loading doses of monthly anti-VEGF injection among the Malaysian sub-population of Malay, Chinese and Indian. The reduction was in agreement with data published earlier by authors in the western countries[25-28]. The choroidal thinning effect of Aflibercept in DME subjects, to the best of our knowledge, was never published before. It was only in age-related macular degeneration(ARMD) subjects where their choroidal thinning effect was established[29-32].
Choroidal thickness became an area of interest following the invention of EDI-OCT. The detection of choroidal thinning effect following anti-VEGF injection has raised the concern on possible functional impairment of choroid, particularly its function of blood supply to the outer retina layers. Cennamoetal[30]has conducted a prospective study on choroidal vasculature and choroidal thickness following three monthly loading doses of Bevacizumab in ARMD patients. They have found that there were no reduction of superficial, deep choroidal vasculature and choriocapillaris density on OCT-Angiography at baseline and 12wk after first injection. Another study conducted on diabetic mice by Lechneretal[33]following five anti-VEGF antibody injection within four months, again did not show any reduction in their choroidal vasculature. This led to the postulation that choroidal thinning following injections was due to reduction of extravascular fluid collection within the choroidal tissue, rather than a loss of choroidal vessels or perfusion[30]. Furthermore, choroidal thickness in healthy person among various population and ethnicity varies as shown by multiple studies mentioned above[34-38]. The choroidal thickness also reduces with age without compromising visual acuity among the elderly[34-39]. Our pre-treatment mean sub-foveal choroidal thickness measure 312.8 μm, which is slightly thicker than normal Malaysian population aged 40-60, measuring 302.8 μm.
Following injections, our subjects has reduced sub-foveal thickness to 292.1 μm, accompanied by significant vision and retinal anatomical improvement. To date, there is no publication on any established relationship between choroidal thickness and its ability to function. The choroidal thinning effect of anti-VEGF may not be as detrimental as expected, at least for the short term. At this point of time, choroidal thickness monitoring is probably more useful in monitoring disease activity or treatment response like in the case of ARMD, retinitis pigmentosa or chorioretinal and macula degenerations[40].
Thicker pre-treatment choroid did not guarantee better vision improvement in our subjects, which indirectly supports the postulation that choroidal thickness may not affect visual function. The amount of reduction in choroidal thickness was also not related to the age of our subject, degree of vision improvement and central retinal thickness reduction. The disproportionate thickness reduction between retina and choroid may be explained by the disproportionate amount of fluid collection, evidenced by more thickening in pre-treatment central retinal thickness than sub-foveal choroidal thickening. Intravitreal anti-VEGF injection has been established as the first line treatment following its proven safety profile, effective anatomical recovery and visual gain[6-10,41]. Ranibizumab and Aflibercept are two anti-VEGFs approved for treatment of DME in Malaysia and their usage is expected to increase with the increasing population of DM patients. Treatment with anti-VEGF in indicated subjects should not be delayed in concern to its choroidal thinning effect, not until its long term side effects has been clinically proven.
There are certain limitations in our study. First of all, as our study was not regarded as interventional study, the treatment planned for our subjects were not randomized. We employed consecutive sampling on patients who had been planned for anti-VEGF (Ranibizumab / Aflibercept) treatment as our subjects. Consecutive sampling was endorsed in order to obtain the maximum number of subjects for maximum study power. The choice of treatment was decided by the treating medical retina consultant and thus no alteration was allowed. The biggest flaw in non-randomized sampling was that the subjects recruited for both arms could be skewed in term of their baseline disease severity or disease duration, which may or may not affect the statistical analysis.
However, our subjects in both group were age matched (P=0.270). Our pre-treatment vision between two groups were statistically insignificant as well (P=0.743). Same goes to our pre-treatment central retinal thickness, statistically insignificant withPof 0.481. Most importantly, our pre-treatment choroidal thickness was also statistically insignificant (P=0.888). The other limitation in our study was the small sample size as a result of high drop-out rate throughout our follow-up period. The loss of 15 study eyes (46.88%) throughout the follow-up was significant. However, the reasons of drop-out were hardly preventable as the subjects developed unexpected systemic infections, ocular infections or even defaulted treatment, which disrupted the treatment duration and protocol. Subjects drop-out from study is common and the chance is higher with a longer duration of follow-up. We were unable to replace the dropped-out subjects due to the time limitations of our study, as most of the drop-out subjects who were unable to continue, were already in the late phase of the follow-up, or were recruited near the end of recruitment period. Our strict inclusion criteria, especially recruiting only subjects who were anti-VEGF naive, also contributed to our limited sample size. Despite the drop in our number of subjects, we were still able to recruit more than sufficient sample size required to maintain a study power of 0.8. These limitations could be addressed in future studies by planning a randomized control trial with longer study period.
