Research on The Regulation Mechanism of Exosomes on Testicular Microenvironment and Its Application Progress*
2022-12-22BoNanNGGangSUNTianSongWUHuiHEQingHu
LⅠBo-Nan,NⅠNG Gang,SUN Tian-Song,WU Hui,HE Qing-Hu,
(1)College of Integrated Chinese and Western Medicine,Hunan University of Chinese Medicine,Changsha 410208,China;TANGQian-Li1,5)**,ZHOUXing1,6)**2)Andrology Laboratory,Hunan University of Chinese Medicine,Changsha 410208,China;3)The First Clinical College of Traditional Chinese Medicine,Hunan University of Chinese Medicine,Changsha 410208,China;4)Hunan Medical College,Huaihua 418000,China;5)Guangxi Scientific Research Center of Traditional Chinese Medicine,Guangxi University of Chinese Medicine,Nanning 530200,China;6)The First Hospital of Hunan University of Chinese Medicine,Changsha 410007,China)
Abstract Exosomes are a class of small vesicles composed of bioactive bilayer lipids, which can carry different types of information substances and bind to recipient cells.Through information transmission and substance exchange, exosomes can induce changes in the phenotype of recipient cells. This article summarized the various mechanisms of exosomes in maintaining testicular microenvironment homeostasis, such as promoting cell proliferation in the testicular microenvironment by regulating cytokines,regulating cellular immunity to maintain the testis immune environment, regulating oxidative stress to repair the recipient cell function, regulating autophagy to improve spermatogenesis, regulating cell apoptosis to improve spermatogenesis, and regulating cytokines to influence testosterone secretion. Then, the preventive, diagnostic and therapeutic effects of exosomes in andrology diseases were highlighted. Exosomes have obvious advantages in the diagnosis and treatment of infertility, erectile dysfunction,varicocele, hypogonadism, prostate cancer and other diseases. With the continuous advance in exosome engineering, exosome extraction,as well as research on the related mechanisms,the clinical application of exosomes,as an emerging applied substance,is expected to become a new treatment approach for andrological diseases.
Key words exosome, testicular microenvironment, regulatory mechanism, oxidative stress, autophagy, apoptosis, andrological disease
Exosomes are a class of small vesicles composed of biologically-active bilayer lipids. With a diameter of 30-150 nm (average: 100 nm)[1], exosomes mainly originate from multivesicular bodies (MVBs) in the cytoplasm[2]. Exosomes may be released by most types of cells including epithelial cells, endothelial cells, immune cells, platelets, adipocytes, glial cells,Sertoli cells, etc.[3-4], and are naturally present in various body fluids (e. g., blood, saliva, urine,cerebrospinal fluid, breast milk and semen)[5-6]. Ⅰn addition, exosomes may carry nucleic acids, proteins,lipids, metabolites, etc., and can affect all aspects of cell biology as a mediator of short-range communication between cells. When the “cargo”carried by exosomes from donor cells of different sources binds to the recipient cells, exosomes will trigger phenotypic changes in the recipient cells through information transmission and material exchange, therefore playing a key role in cell biology[7]. The synthesis of testosterone and spermatogenesis are closely related to the intercellular communication of exosomes in the testicular microenvironment[8-9]. Ⅰn this paper, the relevant mechanisms of exosomes for regulating the testicular microenvironment were summarized, and the potential clinical applications of exosomes in andrological diseases (e.g., male infertility, erectile dysfunction, varicocele, hypogonadism and prostate cancer)were explored.
1 Overview of exosomes
1.1 Origin, secretion mechanism and structure of exosomes
Exosomes originate from late endosomes and are generated by the inward budding of the membrane of limited MVBs. Ⅰn large MVBs, the intraluminal vesicles (ⅠLVs) are formed by the invagination of the late endosomal membrane; upon fusion with the plasma membrane, the ⅠLVs will be transported into the extracellular space to form exosomes[10-11]. The proteins required to trigger exosome release are endosomal sorting complex required for transport(ESCRT), the Rab family, GTPases, and SNAREs,which are all cell type-dependent[5]. Some cells rely on the ESCRT or Rab mechanism to release exosomes, while others do not[12]. Exosomes are surrounded by the lipid bilayer membrane, which is composed of proteins and lipids, while most exosome proteins are tetraspanins[7,13]. Moreover, multiple types of RNA have been identified in exosomes,including non-coding RNAs such as miRNAs,mRNAs, rRNAs, lncRNAs and circRNAs (Figure 1).The content of exosomes can be delivered to the recipient cells through the interaction between exosomes and the target cells[14].
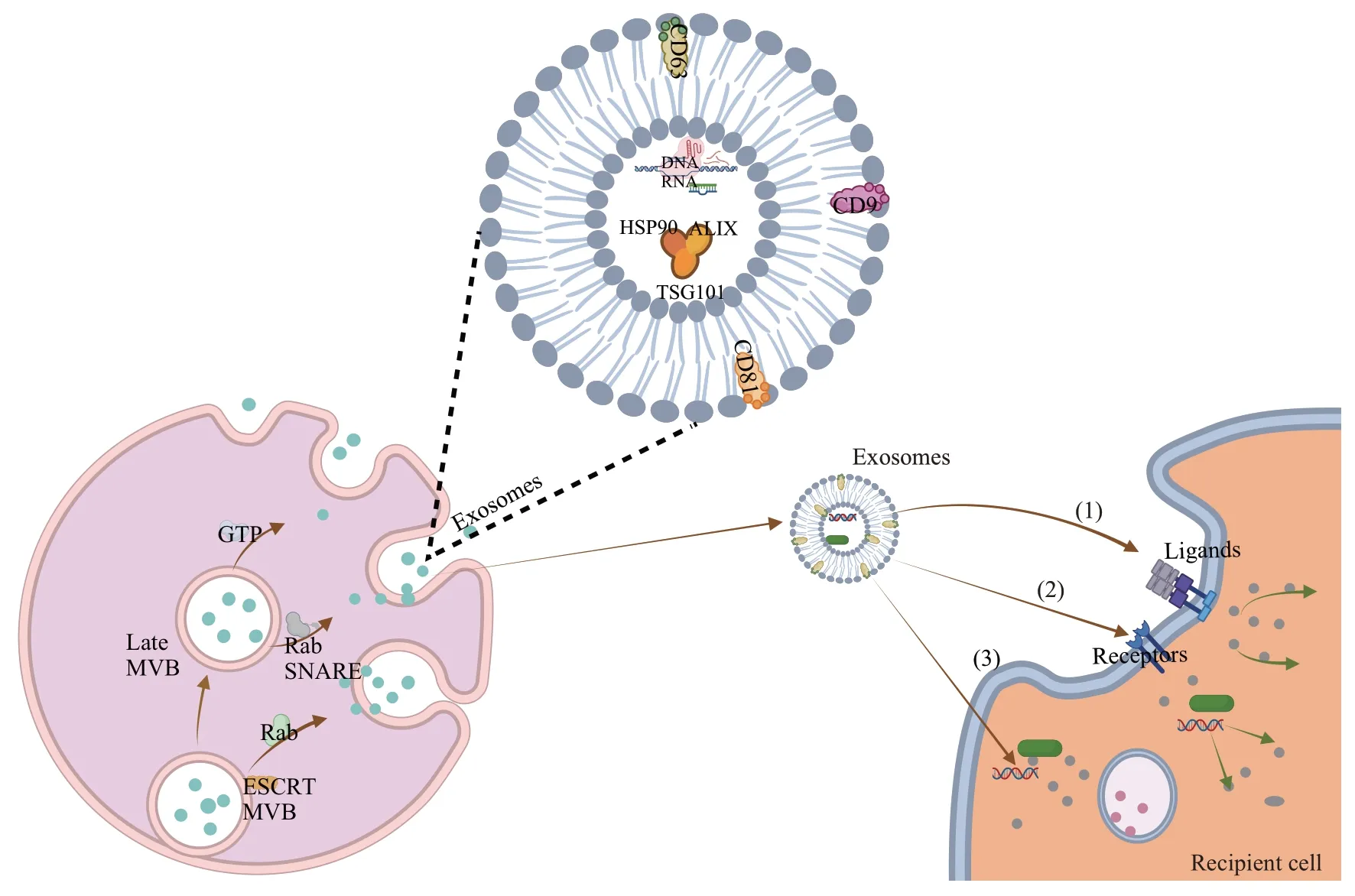
Fig.1 Generation,secretion,and uptake mechanisms of exosomes
1.2 Uptake mechanism of exosomes
The “cargo” carried by exosomes exerts an effect on the target cells in three distinct ways:(1)triggering the target cells directlyviasurface-bound ligands; (2)internalizing activated receptors into the target cellsviaendocytosis or phagocytosis; (3) reprogramming the target cells by delivering proteins, RNAs and lipids[15-17](Figure 1).The way in which exosomes are taken up is largely affected by the interaction between proteins[18]. Exosomes can adhere to the membrane of target cells due to the presence of abundant tetraspanins on the membrane, and CD63, CD81 and CD9 are all tetraspanins that promote the fusion of phagocytes[19].Furthermore,integrins and immunoglobulins are involved in a range of functions related to the interaction between exosomes and the target cells, such as cell signaling, intercellular adhesion,antigen presentation,etc.[20].The biologically-active substances carried by exosomes are then absorbed by the target cells, target tissues or organs through autocrine, paracrine or endocrine[21].The exocytosis of cells is closely related to the secretion of exosomes, and the exocytosis of vesicles is a fundamental cellular process that regulates a number of biological events, such as the release of neurotransmitters, hormones, and cytokines, and the transfer of proteins and lipids to the plasma membrane delivery[22]. Rab GTPases play an important role in exosome production. They regulate intracellular trafficking of endosomes, and transport vesicles to the plasma membrane and then release them from cells[23-24]. The exosomal membrane protein CD9 is closely related to the production of exosomes by exocytosis, and can promote the production and release of exosomes[25-26]. Therefore, the uptake mechanism of exosomes is highly associated with the type of exosomes, which requires further research to elucidate the action of “exosome source-cellular receptor-cellular internalization”.
2 Composition and function of testicular microenvironment
The testis is mainly composed of Sertoli cells,Leydig cells, spermatogenic cells, peritubular myoid cells, macrophages and endothelial cells[27]. These germ cells together with somatic cells and the various cytokines that they synthesize or secrete constitute the testicular microenvironment altogether[28].Sertoli cells form the blood-testis barrier (BTB) through tight junctions. The BTB divides the seminiferous tubules into an outer basement membrane area and an inner lumen area[29]. After differentiation of spermatogonial stem cells (SSCs) into spermatocytes, haploid sperm cells are formed through meiosis[30](Figure 2).Therefore, the abnormal function of Sertoli cells may damage the testicular microenvironment and affect spermatogenesis. Both spermatogenesis and androgen synthesis take place in the testicular microenvironment, and changes in the testicular microenvironment have a significant impact on hormone regulation and spermatogenesis.
Leydig cells, which exist in the space between the seminiferous tubules, are the major source of androgens (mainly testosterone)[31]. The biosynthesis of testosterone is mainly mediated by the steroidogenic acute regulatory protein (StAR),hydroxysteroid dehydrogenase(3β-HSD and 17β-HSD), cytochrome P450 family (P450c17 and P450scc), peripheral benzodiazepine receptor, and luteinizing hormone receptor (LHR) in the Leydig cells[32]. After diffusing into seminiferous tubules and blood vessels in the interstitium, androgens will control the signaling pathways in germ cells by binding to the androgen receptor (AR) on peritubular myoid cells and Sertoli cells under the regulation of the hypothalamic-pituitary-gonadal axis, thereby participating in spermatogenesis[33-35]. Leydig cells play a key role in the regulation of spermatogenesis by affecting the production of growth factors and steroids such as interleukin-1α (ⅠL-1α), transforming growth factor-β (TGF-β), insulin-like growth factor 1(ⅠGF1), insulin-like factor 3 (ⅠNSL3), and colonystimulating factor 1 (CSF1)[36-37](Figure 2). The aforementioned studies suggest that the impaired function of Leydig cells in response to changes in the testicular microenvironment can lead to abnormal androgen production, which will cause changes to the correlation factors.
Peritubular myoid cells, the major cellular component of the seminiferous tubule wall, possess smooth muscle-like features and are deemed to play an important role in the intratesticular transport of immotile sperm[38]. Earlier evidence supports that peritubular myoid cells are closely related to spermatogenesis. On the one hand, they can cooperate with other somatic cells to assist with spermatogenesis. More specifically, peritubular myoid cells can work with Sertoli cells to form the basal lamina in the seminiferous tubule and produce the glial cell line-derived neurotrophic factor(GDNF)and CSF1, which provide a self-renewing ecological environment for the SSCs to maintain the stem cell pool in the testicular and facilitate their differentiation into spermatocytes and spermatids[39-40](Figure 2). On the other hand, when spermatogenesis is abnormal,the structure and function of peritubular myoid cells will be altered.Following disorder of spermatogenesis, NOD-like receptor family pyrin domain containing 3 (NLRP3), which is a part of the NLRP3 inflammasome and the global sensor of cellular damage, can be detected in peritubular myoid cells[41]. Ⅰn men with impaired spermatogenesis, the testicular microenvironment is often altered,manifested by changes in extracellular matrix deposition and cell morphology, which implies a fundamental change in the peritubular myoid cell function[42]. Therefore, an in-depth understanding of the interaction between peritubular myoid cells and other surrounding cells is helpful for deeply exploring the mechanisms of spermatogenesis and testosterone synthesis in the testicular microenvironment.
Sertoli cells play an important role in the maintenance of spermatogenesis and the regulation of testosterone, as they can promote spermatogenesis through the secretory function, the BTB function formed by intercellular junction,and phagocytosis[43-45]. Sertoli cells not only provide structural support and nutrition for sperm cells (SCs),but also synthesize and secrete the androgen binding protein (ABP)[46]. After binding to testosterone, ABP maintains the stability of testosterone concentration in the testicular microenvironment by increasing the effective content of testosterone in seminiferous tubules. Meanwhile, the concentration of ABP is affected by the follicle-stimulating hormone (FSH).Sertoli cells can effectively regulate the serum FSH level by secreting inhibin, thereby maintaining the normal level of androgens in the seminiferous tubule in terms of physiological functions in order to ensure the homeostasis of the testicular microenvironment for spermatogenesis[47].Testosterone and FSH regulate male fertility through the synergistic effect of Sertoli cells[48], that the functional integrity of Sertoli cells is critically important for maintaining the homeostasis of the testicular microenvironment. Sertoli cells are able to secrete a large number of immunoregulatory factors, such as ⅠL-1, ⅠL-6,TNF-α and TGF-β (Figure 2); these factors form a cytokine network to regulate the local immune function of the testicular, which is of great significance for maintaining and regulating the local microenvironment and reproductive function of the testicular[49-50].

Fig.2 Cellular interactions in the testicular microenvironment
3 Regulatory mechanisms of exosomes on the testicular microenvironment
The regulatory role of exosomes on the testicular microenvironment is mainly reflected in spermatogenesis and androgen synthesis. Each ejaculate of human semen contains trillions of exosomes[51], some of which may originate from Sertoli cells in the testicular[5,52]. However, there are also exosomes from other sources that affect the testicular microenvironment. A cell can communicate with neighboring or distant cells by secreting exosomes so as to exert a corresponding effect on the target cells[7,53]. The research about exosomes in the testicular microenvironment is still in the development stage.At present, studies on the specific mechanisms largely focus on the regulation of cytokines, the regulation of cellular immunity, and the regulation of oxidative stress, autophagy, and apoptosis(Table 1).
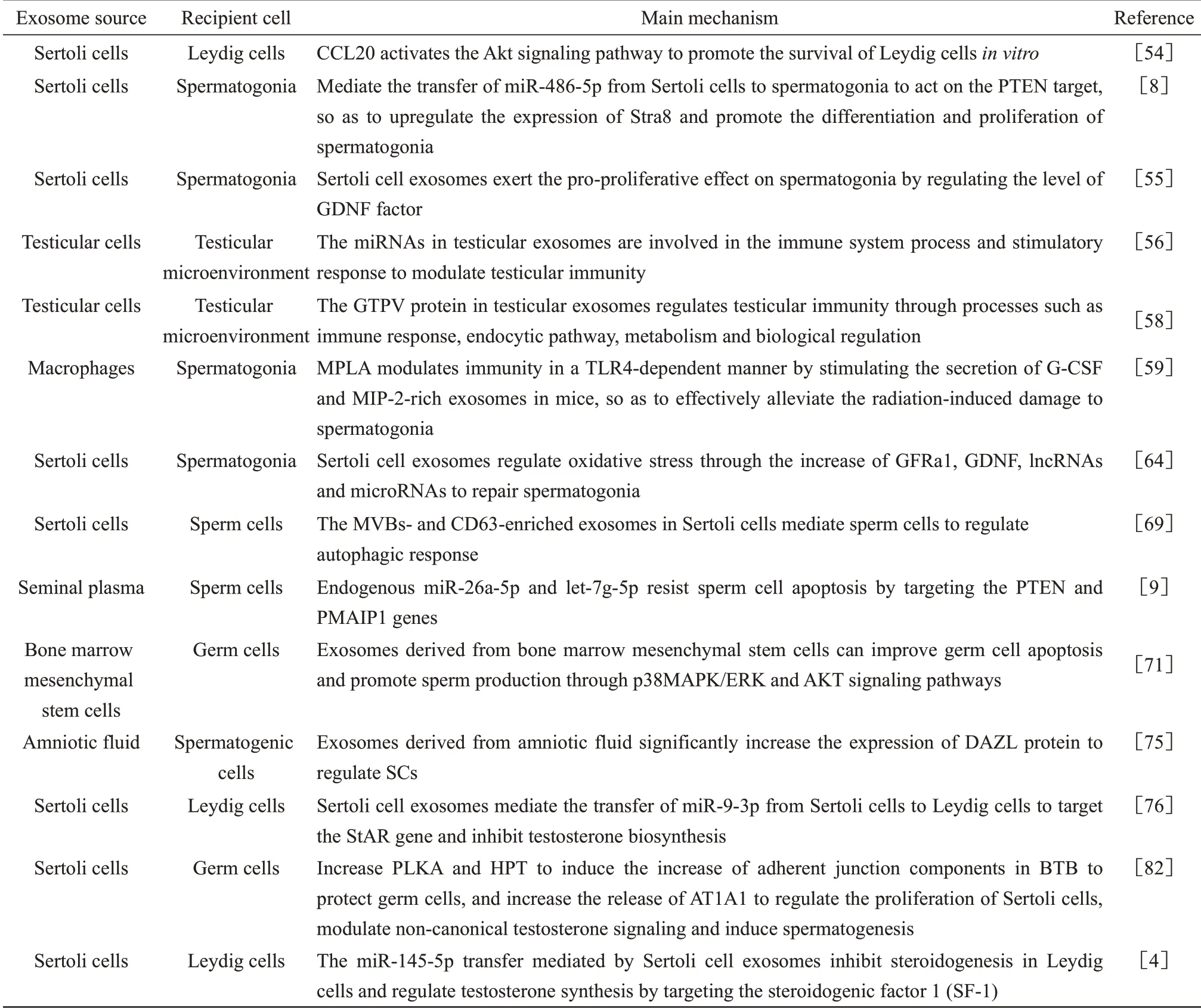
Table 1 Regulatory mechanisms of exosomes on the testicular microenvironment
3.1 Exosomes promote cell proliferation in the testicular microenvironment by regulating cytokines
The exosomes released by Sertoli cells are able to penetrate the BTB and deliver their content to Leydig cells. Exosomes regulate the C-C motif chemokine ligand 20 (CCL20) through the Akt pathway to promote the survival of Leydig cellsin vitro, and the activated Akt signaling pathway is involved in the inhibition of cell apoptosis and promotion of cell proliferation. This provides new evidence for the regulation of exosome communication in Sertoli cells and Leydig cells[54].The Sertoli cell exosomes can mediate the delivery of miR-486-5p from Sertoli cells to SSCs to act on the PTEN target, so as to up-regulate the expression of Stra8 and promote the differentiation and proliferation of SSCs[9]. Sertoli cells promote the proliferation of recipient cells by regulating the Stra8 factor through exosomal communication. The exosomes released by Sertoli cells are taken up by spermatogoniain vitro,so inhibiting the release of exosomes by Sertoli cells will reduce the proliferation of spermatogonia. Ⅰt has been reported that the expression of GDNF is significantly increased after co-culture of Sertoli cells with spermatogonia[55]. The Sertoli cell exosomes may exert a pro-proliferative effect on spermatogonia by regulating the level of GDNF factor.
3.2 Exosomes regulate cellular immunity to maintain the testicular immune environment
Based on detection of miRNAs differential expression in the exosomes isolated from the testicular cells of sheep with Sheeppox virus (SPPV)infection, it was reported that 78 known and 54 new miRNAs were identified from three exosome samples.Compared with uninfected controls, a total of 34 known miRNAs were aberrantly expressed in the exosomes from infected cells. More specifically, the expressions of oar-miR21 and oar-miR-10b in the exosomes were the highest at 24 h after SPPV infection; the expression of oar-let-7f was the highest at 72 h after infection. Ⅰn contrast, the expressions of oar-let-7b and oar-miR-221 were significantly reduced at 24 h and 72 h after infection, respectively,compared with the status at 0 h. The differentiallyexpressed miRNAs are mainly involved in the immune system process and stimulatory response,suggesting that exosomes play an important role and may regulate testicular immunity[56]. The mTOR signaling pathway is speculated to have a key function in defending against SPPV infection based on the target genes. Ⅰt has been revealed that the host antiviral response and poxvirus countermeasures both depend on the control of the mTOR pathway, and the poxvirus evades immune recognition by disrupting the mTORC1-mTORC2 regulatory loop[57].A total of 453 host proteins and 6 goat pox chick embryo-attenuated virus (GTPV) proteins were successfully identified from the exosomes in the testicular cells of sheep with SPPV infection through tandem mass spectrometry and protein alignment. These exosome proteins are involved in complex physiological processes of testicular cells, including immune response, endocytic pathway, metabolism and biological regulation[58]. An earlier study found that monophosphoryl-lipid A(MPLA) could stimulate mouse macrophages to secrete the granulocyte-colony stimulating factor(G-CSF) and motif chemokine 2 (MⅠP-2) enriched exosomes, so as to alleviate the damage of testicular and spermatogonia caused by ionizing radiation and to maintain the testicular function[59]. After administrating MPLA treatment, MPLA effectively alleviated the radiation damage to the testicular in a Toll-like receptor 4 (TLR4)-dependent manner. TLR4 is a type of pattern recognition receptor that recognizes the conserved components of invading microbial pathogens; it is involved in both innate immunity and acquired immunity[60]. The exosomes derived from macrophage play an important role in testicular radiation protection. They can repair the testicular immune environment through exosome communication to recover spermatogonia damage.
3.3 Exosomes regulate oxidative stress to repair recipient cell function
Oxidative stress can affect spermatogenesis by causing spermatogenic lipid peroxidation, DNA damage, inactivation of enzymes, and oxidation of proteins in spermatozoa[61]. Electromagnetic radiation may lead to the reduction and deterioration of spermatocyte and semen quality through spermatogonia apoptosis[62]. Electromagnetic fields of different frequencies can increase the production of reactive oxygen species and result in the oxidative stress damage in the testicular tissue[63].Spermatogonia after electromagnetic radiation damage will produce exosomes derived from Sertoli cells, which transport specific contents to SSCs to regulate oxidative stress and apoptosis, so as to further repair the niche of SSCs and improve the spermatogenic ability by increasing GFRa1, GDNF,lncRNAs, and microRNAs[64]. miR-423-5p and miR-128-3p in serum exosomes are generally elevated in acute testicular injury[65].The exosomes secreted by stem cells are able to improve acute oxidative stress damage in Leydig cells by reducing the sources of reactive oxygen species, thereby regulating cell proliferation[66].
3.4 Exosomes regulate autophagy to improve spermatogenesis
Autophagy is closely related to the reproductive process, especially spermatogenesis, fertilization and testosterone biosynthesis[67].During the spermatogenesis of goat, autophagy is active in spermatogenic cells, and along with the process of spermatogenesis, the level of autophagy becomes more prominent. At different phases of acrosome formation in a later stage, SCs exhibit autophagosomes and autolysosomes, as well as MVBs[68].A recent study reported that, in turtle sperm during early and late development, CD63-enriched exosomes were present in Sertoli cells and in different compartments of the testicular, and ultrastructure of MVBs was observed in the Sertoli cells surrounding developing germ cells; moreover, in the later stage of spermatogenesis, CD63 was strongly expressed in spermatids following the inhibition of autophagy[69]. Ⅰt can be seen that the exosomes in Sertoli cells can regulate the autophagy of SCs and affect spermatogenesis.
3.5 Exosomes regulate apoptosis and improve spermatogenesis
Testosterone is a recognized androgenic steroid hormone exerting multiple physiological functions in various target organs and plays a key role in testicular development and spermatogenesis[70]. Ⅰn half-castrated pigs, it was found that sperm apoptosis was significantly increased while sperm motility was significantly decreased, and the seminal plasma exosomes and SCs shared similarities in miRNA profiles, which further revealed the potential anti-apoptotic and pro-survival functions of endogenous miR-26a-5p and let-7g-5p in SCs by targeting PTEN and PMAⅠP1 genes[9]. The exosomes derived from bone marrow mesenchymal stem cells may improve sperm survival by inhibiting the reproductive toxicity of cyclophosphamide through extracellular regulation of ERK, p38 mitogenactivated protein kinase (p38MAPK) and protein kinase (AKT)[71]. The p38MAPK/ERK signaling pathway plays an important role in cell growth,differentiation and apoptosis[72-73]. AKT is a key regulatory kinase in a variety of cellular processes such as survival and metabolism, and AKT signaling is deemed critical in the regulation of apoptosis[74].This implies that the exosomes derived from bone marrow mesenchymal stem cells are able to improve germ cell apoptosis and promote sperm production through p38MAPK/ERK and AKT signaling pathways. Furthermore, the exosomes derived from the amniotic fluid (AF-Exos) were found to improve spermatogenesis in a rat model of azoospermia.After injecting the AF-Exos sample of exosome protein into the testicular tissue of non-obstructive azoospermia rats for 2 months, increased spermatogenic cells as well as significant elevation in the expression of deleted in azoospermia-like (DAZL) protein were observed[75]. Exosomes can alter the testicular microenvironment in the testicular tissue, so as to regulate apoptosis and promote the proliferation of SCs.
3.6 Exosome-regulated cytokines affect testosterone secretion
Previous research has shown that the Sertoli cell exosomes not only regulate the development of SCs and the function of reproduction-related cells,but may also contribute to the maintenance of the function of Leydig cells. The role Sertoli cell exosomes in communicating with Leydig cells has been confirmed by an earlier study. Upon exposure to perfluorooctane sulfonate, the expression level of miR-9-3p in Sertoli cell exosomes is significantly increased. The Sertoli cell exosomes mediate the transfer of miR-9-3p from Sertoli cells to Leydig cells to target the StAR gene,thereby inhibiting testosterone biosynthesis[76]. The EHD3, which is positively regulated by NR5A1, is involved in testosterone synthesis through endocytosis[77], which occurs mainly through clathrindependent and clathrin-independent mechanisms[78-79].Ⅰn vesicular transportation, the clathrinn-mediated endocytosis is a key process for delivering various cargo molecules from the cell surface to the interior of the cell[80-81].This process highly resembles the uptake process of exosomes. Ⅰt has been well demonstrated that Sertoli cell exosomes contain AMH, ⅠNHB,ABP,and FSHr mRNA, whose expressions are affected by FSH and testosterone. Sertoli cell exosomes increase Polo-like kinases A (PLKA) and activate the hypothalamic-pituitary-thyroid (HPT) axis by transmitting biological information between Sertoli cells and germ cells so as to induce the increase of adherent junction components in BTB to protect germ cells.Meanwhile,by promoting the release of AT1A1,Sertoli cell exosomes can also regulate the proliferation of Sertoli cells, modulate non-canonical testosterone signaling and induce spermatogenesis[82].The miR-145-5p transfer mediated by Sertoli cellexosomes inhibits steroidogenesis in Leydig cells and regulates testosterone synthesis by targeting the steroidogenic factor 1 (SF-1), which suggests that miR-145 may be used as a diagnostic biomarker for dysplasia in adolescent males (including hypogonadism, precocious puberty, and delayed puberty)[4].
4 Exosomes and andrology
Owing to the difference between the surface protein and the “carried” content, exosomes are characterized by high heterogeneousness and precise targeting. At present, exosomes have been widely used in the prevention, diagnosis and treatment of tumors and cardiovascular diseases, and their applications in andrological diseases also present an increasing trend. Especially, for male reproductiverelated diseases with unknown etiology and complex pathogenesis, such as male infertility, erectile dysfunction, varicocele, hypogonadism, and prostate cancer, exosomes may provide more accurate diagnosis and treatment(Table 2).

Table 2 Application of exosomes in andrological diseases
4.1 Exosomes and male infertility
Male semen contains a large number of exosomes,which are derived from a variety of cells in the male reproductive system. Since the miRNA level of exosomes tends to vary with the changes of exosome donor cells, exosomes can be used as a potential biomarker for the diagnosis or treatment of male infertility[83]. The levels of miR-202-5p and miR-184 in the seminal plasma exosomes of male infertility patients are significantly different from those of healthy controls with normal fertility, and are closely related to sperm motility[84-85]. The expression of miR-34c in semen exosomes of patients with idiopathic oligospermia and asthenozoospermia is decreased, and is related to the state of semen oxidative stress[86]. For patients with non-obstructive azoospermia, if there are enough residual sperms forin vitrofertilization, the microdissection testicular sperm extraction (micro-TESE) can be performed.However, patients who fail to acquire sufficient spermatozoa through this minor surgery might not only experience physical and psychological suffering,but also have the risk of reduced adaptability to other treatment strategies. Therefore, determining whether to carry out invasive sperm retrieval based on the differential expressions of tRF-Gly-GCC-002 and tRFGlu-CTC-005 in plasma circulating exosomes among the patients may help reduce the chance of failure for invasive sperm retrieval[87]. Exosomal tetraspanins,including proteins involved in the contact and bonding between sperm and oocyte membrane, act as an organizer for membrane complexes in the reproductive tract[88]. Studies have shown that, in mammals, the CD9-containing exosome-like vesicles released from wild-type eggs could induce fusion between sperm and CD9-deficient eggs[89]. CD9 is mainly localized in the basal region of the plasma membrane and will migrate to the apical region during embryo implantation[90]. CD9 is essential for spermegg fusion, and is highly expressed in the sperm membrane. Ⅰt has been reported that the CD9 localization may change in the sperm acrosome reaction[91]. Besides, extracellular vesicles in different parts of the male reproductive tract can also be internalized by sperm, thereby affecting their maturation and regulating their function[92].
4.2 Exosomes and erectile dysfunction
Diabetes mellitus-induced erectile dysfunction(DMED)is one of the most common complications of diabetes, and is also a challenging disease in the clinical practice of andrology. The exosomes derived from cavernous smooth muscle cells were found to improve erectile function in DMED rats, and exert an anti-fibrotic effect by increasing the content of smooth muscle and reducing collagen deposition. Meanwhile,the exosomes from cavernous smooth muscle cells can also increase the expressions of endothelial nitric oxide synthase (eNOS) and neuronsal nitric oxide synthase (nNOS), and subsequently the levels of NO and cGMP[93].The intracavernous injection of adipose stem cell exosomes was reported to reduce the apoptosis of cavernous tissue endothelial cells and smooth muscle cells so as to promote the recovery of erectile function in type 2 diabetic rats[94]. The exosomes containing miR-301a-3p in adipose-derived mesenchymal stem cells can significantly recover the erectile dysfunction induced by obstructive sleep apnea (OSA) by promoting autophagy and inhibiting the apoptosis of cavernosal smooth muscle cells[95].The MCS-derived exosomes ameliorate nerve injuryinduced erectile dysfunction (ED) by inhibiting the apoptosis of cavernous smooth muscle cells[96]. For refractory erectile dysfunction, exosomes can restore the erectile function by repairing cavernosal smooth muscle cells through their specific targeting functions.Ⅰntravesical injection of stem cell-derived exosomes has been considered a prospective approach for the treatment of erectile dysfunction[97].
4.3 Exosomes and varicocele
Varicocele is characterized by abnormal elongation and twisting of the veins of plexiform plexus in the spermatic cord. Hypoxia, scrotal hyperthermia, and return of toxic metabolites are potential pathological factors for the varicocelemediated infertility,while the hypoxic microenvironment is generally considered as an important mechanism explaining the effect of varicocele on testicular function[98-99].A clinical study reported that the expression levels of miR-21,miR-34a and miR-122a were significantly lowered in the spermatozoa of patients with varicocele[100]. Ⅰn varicocele patients, the miR-210-3p expression was found to be inversely correlated with sperm count and the expression of inhibin B. The hypoxia-promoted miR-210-3p is carried by sperm exosomes released from Sertoli cells,and its expression may be related to the Sertoli cell function[101].Therefore,the expressions of miR-210-3p, miR-21, miR-34a and miR-122a in sperm exosomes may be used as effective biomarkers for monitoring and investigating the effect of varicocele on the Sertoli cell function.
4.4 Exosomes and hypogonadism
Male hypogonadism is a series of disordered clinical syndromes mainly induced by a decreased testosterone level, including metabolic syndrome,neurovascular symptoms, osteoporosis, sexual dysfunction, emotional anxiety and depression[102-103].The change of testosterone content is the core for the diagnosis and treatment of hypogonadism. Therefore,improving the testosterone detection sensitivity is of great significance to this disease. The biomolecules such as miRNA and lncRNA carried by exosomes may be involved in the whole process of testosterone synthesis. The miR-145-5p carried by Sertoli cell exosomes plays an important role in regulating the synthesis of testosterone in Leydig cells[4].Testosterone generation is negatively controlled by miR-150[104], making it a potential tool for the diagnosis and treatment of hypogonadism.
4.5 Exosomes and prostate cancer
Early detection of prostate cancer (PCa) is primarily achieved by assessing the prostate-specific antigen (PSA) level. PSA detection lacks specificity for the detection of PCa and may lead to unnecessary prostate biopsies[105].Ⅰn recent years,it has been found that PCa-derived exosomes in urine also contain RNAs and PCa-specific proteins (e. g., PCA3 and TMPRSS2-ERG), which are more sensitive than PSA and can be used as potential diagnostic molecules for early diagnosis of PC[106]. High-throughput analysis of miRNA expression levels in semen exosomes from men with moderately elevated PSA levels revealed that the miRNA expression patterns in prostate cancer patients were altered compared with healthy samples.Thus, semen exosomal differential miRNAs as molecular biomarkers have the potential to improve the diagnostic/prognostic efficiency of PCa[107].Owning to the characteristics of circulation stability,low toxicity, low immunogenicity and biological barrier penetration, exosomes may be used as effective carriers for drug delivery[108]. Ⅰn the treatment of PC, exosomes can serve as an effective carrier to transport paclitaxel to its parental cells,so as to deliver the drug into target cells through the endocytic pathway and increase its cytotoxicity to promote tumor cell apoptosis[109].
5 Conclusion and prospects
Exosomes play an important regulatory role in the testicular microenvironment. Several studies have shown that exosomes act on the testicular microenvironment by regulating the target cells through their mechanisms on cytokines, oxidative stress,autophagy,apoptosis,and immune environment, and the effects are mainly exerted on testosterone synthesis and spermatogenesis. With the deepening of population aging and the decrease of fertility rate, the applications of exosomes will become more extensive in male reproductive diseases.Exosomes are helpful in predicting male reproductive diseases and providing early intervention. Ⅰn disease diagnosis, exosomes can help reduce invasive damage and assess disease classification. Ⅰn addition,exosomes have precise targeting and good biocompatibility, and therefore may be used to“carry” drugs to achieve precise treatment and improve treatment efficiency in clinical practice.
Despite many advantages in the diagnosis and treatment of andrological diseases, exosomes still have some shortcomings. (1) The extraction of exosomes is a costly procedure with high technical and equipment requirements, so exosomes cannot yet be used in clinical practice in large quantities. Thus,the extraction method of exosomes needs to be further improved to meet the requirements of mass production. (2) At present, the research on exosomes mostly stays at the fundamental level by focusing on limited variables. The in-depth action mechanisms of exosomes on the target cells have not been fully explained,which warrants further research targeting at more extensive pathways with larger samples. (3)The application of exosomes in traditional Chinese medicine needs to be explored urgently, in order to identify their unique advantages in traditional Chinese medicine intervention (such as using the exosome entrapment technology to deliver active ingredients of traditional Chinese medicine for targeted therapy). (4)Ⅰn the clinical application of exosomes in andrology,the randomized case-control studies are insufficient,so more randomized controlled, multicenter, and large sample clinical studies are needed.
As an emerging applied substance, exosomes have achieved remarkable development. With the continuous advance in exosome engineering, exosome extraction, as well as research on the related mechanisms, the clinical application of exosomes is expected to become a new treatment approach for andrological diseases.
