Preliminary evidence in treatment of eosinophilic gastroenteritis in children: A case series
2022-12-19YingChenMeiSun
Ying Chen,Mei Sun
Abstract
Key Words: Eosinophil gastroenteritis; Children; Budesonide; Methylprednisolone; Montelukast; Absolute eosinophil count
lNTRODUCTlON
Eosinophilic gastritis/gastroenteritis is a rare inflammatory disorder in adult and children, characterized by diffuse or patchy eosinophilic infiltration of the stomach, intestine, and colon[1-4]. In recent years, the incidence and prevalence of eosinophilic gastroenteritis have gradually increased, especially in Western countries. The prevalence of eosinophilic gastritis in the United States is estimated to be 6.3 per 100000 cases, with the highest prevalence in children under 5 years old[5]. Although epidemiologic studies regarding eosinophilic gastroenteritis in Asia are very limited, the clinical, endoscopic, and histopathological characteristics of patients with eosinophilic gastroenteritis in Asia are mostly similar to those reported in Western countries[6-9].
Eosinophilic gastroenteritis in adult and children may present different gastrointestinal symptoms, depending on the location of the affected gastrointestinal tract and the extension of eosinophilic inflammation. Generally, the most common symptoms of eosinophilic gastroenteritis include abdominal pain, vomiting, diarrhea, nausea, bloating, burping, and intestinal obstruction[6,9-11]. Some patients may also experience loss of appetite, general weakness, foreign body sensation in the pharynx, dysphagia, focal mass, and massive ascites[12,13]. Moreover, eosinophilic gastroenteritis in children and adolescents may severely cause growth retardation, delayed puberty, and amenorrhea. The diagnosis of eosinophilic gastroenteritis generally includes the appearance of abnormal gastrointestinal symptoms, the presence of ≥ 20 eosinophils per high-power field (HPF), and exclusion of other secondary causes such as parasite or tuberculosis infection[14]. However, there is still no validated guideline for the clinical management of patients with eosinophilic gastroenteritis, let alone standard guideline for children. Some evidence in the case report/series suggests that dietary restrictions (or elemental diet therapy) and the use of corticosteroids and steroid-sparing agents such as prednisone and montelukast are effective as first-line treatments[2,15]. Considering the rarity of this disease in China and the limited understanding of its diagnosis and treatment, the aim of this study was to report our experience with the diagnosis and treatment outcome of 22 children with eosinophilic gastroenteritis in China.
MATERlALS AND METHODS
From January 2017 to December 2019, 22 children with histologically confirmed eosinophilic gastroenteritis were enrolled in the study. Inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease were excluded by biopsies on colonoscopy and fecal calprotectin examination. Clinical data of the children including demographics, allergic histories, and laboratory and endoscopic examination were retrospectively reviewed and analyzed. The diagnosis of eosinophilic gastroenteritis was based on Talley’s diagnostic criteria[16]: (1) The presence of gastrointestinal symptoms; (2) Histological evidence of eosinophil infiltration in one or more areas of the gastrointestinal tract; and (3) No parasites or extraintestinal disease. The study was approved by the Institutional Review Board (IRB) of our hospital, and the requirement for written informed consent was waived by the IRB due to the respective nature of this study.
The data collected for analysis in this study includes demographic characteristics (age, gender, and weight), laboratory parameters [white blood cell (WBC) count, absolute eosinophil count (AEC), hemoglobin, C-reactive protein (CRP), albumin, and immunoglobulin E (IgE)], history of atopic disease (asthma, eczema, urticaria,etc.), allergy history, physical examination results, clinical symptoms, medications, and endoscopic and imaging results. For statistical analysis, continuous variables are presented as the mean ± SD, and categorical variables are presented as numbers and percentages.
RESULTS
Case summary
A total of 22 children (17 males and 5 females) were diagnosed with eosinophilic gastroenteritis, with a mean age of 9.3 ± 3.2 years and a mean weight of 32.0 ± 13.8 kg (Table 1). In all the 22 pediatric patients, the results of tuberculosis testing and parasite stool testing (larvae, cyst, and ova) were negative. The mean WBC count was (11.7 ± 8.9) × 109cells/L. The mean AEC was 1692.9 ± 3845.6 cells/μL, and the converted eosinophil percentage was 9.5% ± 14.5%. Except for patient #19, the hemoglobin levels of the remaining 21 patients were within the standard range. The mean hemoglobin level of the 22 patients was 126.2 ± 15.2 g/L. Except for patients #7, #19, and #21, the albumin levels of the remaining 19 patients were within the normal range. The mean albumin level was 39.9 ± 8.4 g/L. Among the 22 patients, 15 (68.2%) had abnormal CRP levels, with a mean value of 11.5 ± 12.1 mg/dL. The serum IgE levels of nine patients (40.9%) exceeded the normal IgE level of children for the corresponding age, with a mean value of 520.3 ± 351.2 kU/L. All the 22 patients had a history of allergies or atopy disorders. Among them, 11 patients (50.0%) were allergic to food (wheat, egg, milk,etc.), and 6 (27.3%) were allergic to environmental allergies (house dust, dust mite, mold,etc.). In addition, three (13.6%), two (9.1%), and one (4.5%) patient had a history of asthma, eczema, and urticaria, respectively. The most common symptoms on admission included abdominal pain in 17 children (77.3%), vomiting in 9 (40.1%), diarrhea in 3 (13.6%), and nausea in 2 (9.1%).
The symptoms of eosinophilic gastroenteritis are heterogeneous, mainly depending on the region and layer of the intestinal wall affected by the eosinophilic infiltration. According to the location of eosinophil infiltration in the gastrointestinal tract[17], eosinophilic gastroenteritis can be further classified into mucosal/sub-mucosal pattern, muscle layer pattern, and serosal/sub-serosal pattern. In this study, most children (21/22, 95.5%) were diagnosed as having mucosal pattern. Only one child (4.5%) was diagnosed as having serosal pattern with unusual presentation of eosinophilic ascites.
Gastrointestinal endoscopy depicted that 20 children (95.2%) had erythematous exudative gastritis and a rough gastric antrum, accompanied by scattered erosion of the duodenal mucosa and hyperemia. Histological examinations showed that all the 22 children (100%) had eosinophilic gastroenteritis infiltration in the duodenum (mean number of eosinophils/HPF = 53.1 ± 81.5), while 20 children (90.9%) had eosinophilic gastroenteritis infiltration in the stomach (mean number of eosinophils/HPF = 36.8 ± 50.5). Only two children (9.1%) had eosinophilic gastroenteritis infiltration in the terminal ileum (mean number of eosinophils/HPF = 49.0 ± 24.0). The molecular examination ofFIP1L1-PDGFRAfusion gene in the peripheral blood cells of all the 22 patients was negative.
Treatment and response
Table 2 shows the clinical characteristics and treatments of 22 children with eosinophilic colitis. All the 22 children (100%) received dietary restrictions. Except for patient #4, the remaining 21 children (95.5%) received initial drug treatment, including methylprednisolone, montelukast (Singulair), budesonide, and lansoprazole. Three children relapsed after initial treatment, including patient #4 who did not receive drug treatment and two patients (2/21, 9.5%) who received initial drug treatment. It is worth noting that 17 children (17/17, 100%) with low eosinophil percentage (< 14%) responded very well to the above-mentioned medications without relapse. Two of the four children (#20 and #21) with high eosinophil infiltration (> 14%) and CRP levels (> 1 mg/dL) relapsed after treatment with methylprednisolone and montelukast. Moreover, for children with high eosinophil infiltration and CRP levels (#19, #21, and #22), budesonide as a first-line and relapse treatment relieved the clinical symptoms and endoscopic appearance of eosinophils.
DlSCUSSlON
Due to the rare prevalence of eosinophilic gastroenteritis in Asia, the diagnosis and treatment of the disease can only be understood through a few case reports. In addition, there is currently no standard guideline for the treatment of eosinophilic gastroenteritis in children due to the lack of prospective study[4]. The treatment of eosinophil gastroenteritis is still empirical. Current treatments for eosinophilic gastroenteritis include restricted diet/elemental diet therapy, corticosteroids, and steroid-sparing agents[2,15,18,19]. Although dietary therapy was reported to be effective in relieving allergic eosinophilic gastroenteritis[20,21], low patient compliance limits its usefulness, especially in adolescents and adults. The only patient in our study who received restrictive diet therapy relapsed (case 4). The patient was advised to avoid exposure to allergens and not to receive steroid treatment because of low eosinophil count (0.06 × 109cells/μL, AEC = 60 cells/μL), low eosinophilic infiltration (25/HPF), low eosinophil percentage (1.02%), and normal hemoglobin, albumin, CRP, and total IgE levels. However, the child relapsed with increased eosinophilic infiltration in the ileum (40/HPF), stomach (20/HPF), and duodenum (55/HPF). Following montelukast (5 mg/d) treatment for 1 mo, his abdominal pain and vomiting were relieved. After discussing with the child’s parents, the relapse may be the actual difficulty of restricting diet at home. It may also be because it is difficult to accurately identify diseasecausing foods through allergen testing. In clinical practice, the effect of eliminating diets based on allergen testing may be different. Therefore, corticosteroids are still the best treatment if dietary restrictions are not feasible or symptoms cannot be relieved. Based on the results of our study, methylprednisolone/montelukast is an effective first-line treatment, especially for children with lower eosinophil percentage (< 14%).

Table 1 Demographic and clinical characteristics of 22 children with eosinophilic colitis (n = 22)

AEC: Absolute eosinophil count; HB: Hemoglobin; CRP: C-reactive protein; IgE: Immunoglobulin E; HPF: High-power field.
Glucocorticoids (methylprednisolone) are considered to be effective drugs for the treatment of eosinophil gastroenteritis, and about 90% of adult patients respond to glucocorticoids[22]. A recent single-center study in China reported that increased eosinophil infiltration count is the predictive factor for glucocorticoid therapy in children with eosinophil gastroenteritis[23]. However, long-term use of methylprednisolone/prednisolone has been shown to cause Cushing syndrome, weight gain, growth retardation, and hypertension, as well as increased susceptibility to infection[24]. Although budesonide is also a topical glucocorticoid, the metabolized budesonide has less than 1% of its original activity. Thus, systemic exposure can be minimized. In addition to effectively alleviating Crohn’s disease, autoimmune hepatitis, and ulcerative colitis in adults[25-27], a recent small-scale retrospective study showed that budesonide is effective in children with eosinophilic gastroenteritis[28]. In our clinical practice of the 22 children, methylprednisolone/montelukast and budesonide did be effective in the clinical and pathological remission of eosinophil gastroenteritis in children.
However, methylprednisolone/montelukast appears to be ineffective for children with high eosinophil infiltration and CRP levels. In this study, two patients with high eosinophil infiltration and CRP levels relapsed after receiving initial methylprednisolone/montelukast treatment. Case #21 is a 5-year-old boy with a history of asthma and food allergies, presenting with periorbital edema and swelling of his limbs. His hemoglobin level (116 g/L) was normal, and the results of tuberculosis and parasite stool examination were negative. Moreover, the patient showed elevated CRP level (13.7 mg/dL), increased serum IgE level (649 IU/mL), and low albumin level (21.7 g/L). Gastrointestinal endoscopy depicted erythematous, exudative and erosive gastritis, and congestion (Figure 1A). In addition, obvious hyperemia and edema were observed in the anterior wall of the duodenal mucosa. Histopathological examination (Figure 1B) depicted a high degree of eosinophilic infiltration. High eosinophil counts were observed in the duodenum (400/HPF) and stomach (70/HPF). The eosinophil percentage was as high as 41.63%. The patient was prescribed a dose of 2 mg/kg methylprednisolone and 5 mg/kg of montelukast for 1 wk, followed by a maintenance dose of 0.5 mg/kg of montelukast for 1 wk. Although a rapid response to methylprednisolone/montelukast was observed, the patient still relapsed four times. Finally, the treatment regimen was changed to methylprednisolone (2 mg/kg) and then maintained on budesonide (3 mg/d). The symptoms were completely relived. No symptoms of eosinophilic gastroenteritis were further observed at the revisit 2 mo later.
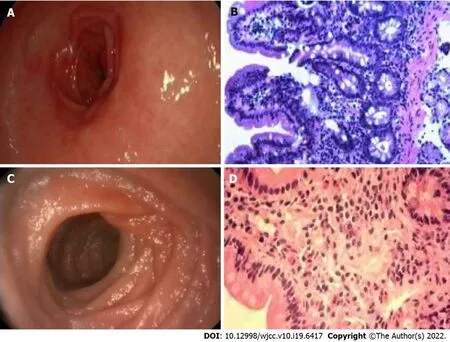
Figure 1 Gastrointestinal endoscopy and histopathological examination. A: Gastrointestinal endoscopy depicted erythematous, exudative and erosive gastritis, and congestion in case #21; B: Histopathological examination depicted a high degree of eosinophilic infiltration in case #21 (magnification, 40 ×); C: The gastric mucosa was normal under gastrointestinal endoscopy in case #19; D: Mucosal biopsy revealed a high degree of eosinophilic infiltration in case #19 (magnification, 40 ×).
On the other hand, two other patients with high eosinophil infiltration and high CRP levels were initially treated with budesonide and methylprednisolone, and clinical remission was achieved without recurrence. In this study, case #19 is a 3-year-old boy with a history of asthma and food allergies, presenting with periorbital edema and swelling of his limbs. Although the gastric mucosa was normal under gastrointestinal endoscopy (Figure 1C), mucosal biopsy revealed a high degree of eosinophilic infiltration (Figure 1D). There were inflammatory infiltrations with high eosinophil count in the duodenum (30/HPF) and stomach (200/HPF). The eosinophil percentage was as high as 14.66%. The child had no oral ulcer or perianal lesions and was negative for fecal calprotectin, ruling out the possibility of inflammatory bowel disease. In addition, endoscopic biopsy examination also excluded intestinal lymphangiectasia. Since the patient showed elevated CRP level (2.25 mg/dL), raised serum IgE level (337 IU/mL), and low albumin level (17 g/L), methylprednisolone was prescribed for 1 wk, and budesonide, azathioprine, and thalidomide were used as a maintenance therapy for 1 wk. The symptoms were relived and the eosinophil counts improved rapidly. A 3-mo follow-up showed no symptoms. Although there are not many cases of using budesonide to treat children with high eosinophil infiltration and high CRP levels in this study, our clinical experience suggests that budesonide can be considered as the first-line or relapse treatment for children with high eosinophil infiltration and CRP levels. In the future, large-scale prospective randomized controlled studies should be designed to confirm whether budesonide is better than methylprednisolone/montelukast regimen in the treatment of pediatric eosinophilic gastroenteritis with high eosinophil infiltration (> 14%) and high CRP levels.
Compared with adults, eosinophilic gastroenteritis may cause growth retardation, failure to thrive, delayed puberty, and amenorrhea in children[29]. The diagnosis of eosinophilic gastroenteritis mainly depends on the clinical manifestations and endoscopic and radiographic examinations. Endoscopic abnormalities include erythema, mucosal hyperemia, thickened folds, fragile, rough areas, whitish spots, erosions, superficial ulcers, and nodules[30]. Because patients with eosinophilic gastroenteritis do not always have the characteristics of peripheral eosinophilia, it is very important to confirm the infiltration of eosinophils by histological biopsy. Eosinophils usually exist in the lamina propria of the intestinal mucosa, gradually increasing from the duodenum to the cecum, and gradually decreasing from the right colon to the rectum. Although there is no consensus on the diagnostic threshold of eosinophil count in various parts of the gastrointestinal tract for eosinophilic gastroenteritis[31], most of the current case reports/series have suggested a threshold of > 20 eosinophils/HPF under microscopic examination[7]. In addition, degranulation of eosinophils, degeneration and regeneration of epithelial cells, and eosinophil cryptitis/abscess may also be observed. In this study, all the 22 patients showed eosinophil infiltration in the duodenum, stomach, and/or ileum, but only seven patients (31.8%) had abnormal peripheral eosinophilia counts. In addition, eosinophil infiltration does not always occur in sites where abnormalities are found by endoscopy or radiography. Instead, eosinophil infiltration is often found in normal mucosa due to patchy in distribution. Therefore, we recommended that multiple biopsies be examined to avoid misdiagnosis. It should be noted that endoscopic biopsy is mainly limited to the mucosa and submucosa. For patients with eosinophil infiltration in muscle layer or serosal pattern, mucosal biopsy may be negative. If eosinophilic gastroenteritis is highly suspected, a fullthickness surgical biopsy may be required but this is not feasible in pediatric patients.
The pathology of eosinophilic gastroenteritis is still unclear. In addition to the esophagus, eosinophils are often found in the lamina propria of various parts of the gastrointestinal tract. In addition, the number of eosinophils in the gastrointestinal tract varies, with the highest count in the cecum and appendix[31]. However, the number of eosinophils also tends to increase in the pathogenesis of various inflammatory processes, including parasitic infections and allergic diseases. Activated eosinophils can release a variety of inflammatory mediators with high biological activity. Meanwhile, the degranulation of mast cells and the release of cytokines, chemokines, and lipid mediators are not only cytotoxic to the epithelium of the gastrointestinal tract, but also trigger the Th2 immune responses and intestinal inflammation[32,33]. On the other hand, anatomical malformations and intestinal dysbiosis play a role in the pathophysiological mechanism of eosinophilic gastroenteritis[2]. In this study, only one pediatric patient (#20) showed abnormal superior mesenteric artery and intestinal malrotation on abdominal ultrasound examination.
There are some limitations in this study. Since this was a retrospective study of 22 patients, the accurate incidence of eosinophilic gastroenteritis in children remains unclear. In addition, there may be a diagnostic bias on patients because there is still no consensus on the diagnostic threshold of eosinophilic gastroenteritis in children. Despite the promising preliminary evidence of budesonide in the relapse treatment of children with high eosinophil infiltration, multicenter prospective or retrospective studies with a large sample size should be conducted to further validate the correlation between eosinophil percentage and budesonide in the treatment of eosinophilic gastroenteritis in children.
CONCLUSlON
For children with recurrent or persistent gastrointestinal symptoms and increased peripheral eosinophils, gastrointestinal endoscopy and endoscopic biopsy examinations should be performed multiple times to confirm the diagnosis of eosinophilic gastroenteritis. Although there are currently no standard treatment guidelines for pediatric eosinophilic gastroenteritis, we recommend corticosteroids as the first-line treatment, especially when dietary restriction is not feasible or ineffective. Budesonide can be considered as the first-line or relapse treatment for children with high eosinophil infiltration and CRP levels. In the future, large-scale prospective studies are needed to explore the efficacy of budesonide and other corticosteroids in the treatment of eosinophilic gastroenteritis in children with high eosinophil infiltration and CRP levels.
ARTlCLE HlGHLlGHTS

Research methods
A total of 22 children with histologically confirmed eosinophilic gastroenteritis were enrolled in the study. The diagnosis of eosinophilic gastroenteritis was based on Talley’s diagnostic criteria. Clinical data of the children including demographics, allergic histories, and laboratory and endoscopic examination were retrospectively reviewed and analyzed.
1.文稿应具有科学性、真实性,重点突出,文字简练,数据可靠。论著与临床报道类稿件一般不超过8000字(包括摘要、图、表、参考文献等),病例报道不超过4000字。
Research results
All children received dietary restrictions. First-line drug treatment included methylprednisolone, montelukast, budesonide, and lansoprazole. All children with low eosinophil percentage (< 14%) responded very well to first-line drug treatment without relapse. Half of children with high eosinophil infiltration (> 14%) and C-reactive protein (CRP) levels (> 1 mg/dL) relapsed after treatment with methylprednisolone and montelukast. However, budesonide is an effective first-line and relapse treatment for children with high eosinophil infiltration (> 14%) and CRP levels.
Research conclusions
Based on our clinical practice, we recommend corticosteroids as the first-line treatment for low eosinophil infiltration (< 14%). Budesonide is recommended as the first-line or relapse treatment for children with high eosinophil infiltration and CRP levels.
Research perspectives
Although our clinical practice showed the promising preliminary evidence of budesonide in the relapse treatment of children with high eosinophil infiltration, multicenter prospective or retrospective studies with a large sample size should be conducted to further validate the findings.
FOOTNOTES
Author contributions:Chen Y and Sun M were gastroenterologists, conceptualized the study, reviewed the literature of eosinophilic gastroenteritis, and drafted the manuscript; Chen Y searched the computerized database of our hospital, collected the patients’ data, and analyzed the data; and all authors read and approved the final manuscript.
lnstitutional review board statement:This study was approved by the Institutional Review Board of ShengJing Hospital of China Medical University (No. 2020PS806K).
lnformed consent statement:The requirement for written informed consent was waived by the Institutional Review Board due to the respective nature of this study. All procedures performed in the present study were in accordance with Declaration of Helsinki.
Conflict-of-interest statement:The authors declare that there is no conflict of interest to disclose.
Data sharing statement:The data used and analyzed in this study are available from the corresponding author upon reasonable request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Ying Chen 0000-0003-1378-9621; Mei Sun 0000-0002-9839-4372.
S-Editor:Wang JJ
L-Editor:Wang TQ
P-Editor:Wang JJ
猜你喜欢
杂志排行
World Journal of Clinical Cases的其它文章
- Hem-o-lok clip migration to the common bile duct after laparoscopic common bile duct exploration: A case report
- Identification of risk factors for surgical site infection after type II and type III tibial pilon fracture surgery
- Sustained dialysis with misplaced peritoneal dialysis catheter outside peritoneum: A case report
- Delayed-onset endophthalmitis associated with Achromobacter species developed in acute form several months after cataract surgery: Three case reports
- Diagnostic accuracy of ≥ 16-slice spiral computed tomography for local staging of colon cancer: A systematic review and meta-analysis
- Family relationship of nurses in COVID-19 pandemic: A qualitative study
