Contribution of arbuscular mycorrhizal fungi and soil amendments to remediation of a heavy metal-contaminated soil using sweet sorghum
2022-12-14FayuanWANGPengCHENGShuqiZHANGShuwuZHANGandYuhuanSUN
Fayuan WANG,Peng CHENG,Shuqi ZHANG,Shuwu ZHANGand Yuhuan SUN
College of Environment and SafetyEngineering,QingdaoUniversityof Science and Technology,Qingdao266042(China)
ABSTRACT Owing to their potential advantages such as waste reduction,recycling,and economic attributes,fast-growing bioenergy crops have the capacity to effectively phytoremediate heavy metal-contaminated soils.However,little is known about the role of microbial and chemical amendments in phytoremediation using bioenergy crops.Here,we studied the contributions of inoculation with the arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ and three soil amendments,i.e.,hydroxyapatite(HAP),manure,and biochar,at doses of 0.1%and 1%(weight:weight)to heavy metal phytoremediation using sweet sorghum grown on an abandoned agricultural soil,with environmentally realistic contamination(2.6 mg kg−1 Cd,1 796 mg kg−1 Pb,and 1 603 mg kg−1 Zn),in a plant growth chamber.Mycorrhizal colonization,plant biomass and metal accumulation,metal availability,and soil pH were determined in harvested seedlings 12 weeks after sowing.The results showed that root colonization by indigenous AMFdecreased by 28%–46%with HAP,but increased after manure and biochar applications as compared to the no amendment control(CK).The AMFinoculation increased root colonization rates by 16%–128%and in particular,alleviated the inhibition of HAP.The remediation effects were highly dependent on the amendment type and dose.Among the three soil amendments,HAP was the most effective in promoting plant growth and phytostabilization of Cd,Pb,and Zn and phytoextraction of Cd,particularly at a dose of 1%.Compared to CK,1%HAP decreased DTPA-extractable Cd,Pb,and Zn concentrations in soil by 31%–43%,30%–38%,and 22%–23%,respectively.Manure and biochar also exerted positive effects on heavy metal immobilization,as indicated by lower DTPA extractability,but only the 1%manure treatment showed plant growth-promoting effect.The AMFinoculation did not affect plant growth,but increased soil pH and induced synergistic interactions with amendments on the immobilization of Cd and Pb.In conclusion,soil amendments,particularly HAP,produced positive impacts and synergistic interactions with AMFon the phytostabilization of heavy metals using sweet sorghum.Accordingly,sweet sorghum combined with soil amendments and AMFmay be an effective strategy for heavy metal phytoremediation.
KeyWords:biochar,bioenergy crop,hydroxyapatite,mycorrhizal colonization,phytoremediation,phytostabilization
INTRODUCTION
Heavy metal contamination in soil is a global environmental dilemma with adverse consequences on food safety and human health.It has been estimated that more than 2×107ha of land is polluted with heavy metal(loid)s globally(Liu L Wet al.,2018).According to an official survey in China,heavy metals account for the majority of agricultural soils classified as contaminated(Zhaoet al.,2014).Consequently,the accumulation of these contaminants in agricultural soil and growing crops poses potential health risks to consumers(Wei and Yang,2010;Yanget al.,2018).For these reasons,the development of soil remediation technologies,including variousin situandexsituremediation techniques,has been become a focus in recent decades(Khalidet al.,2017;Liu L Wet al.,2018).
Phytoremediation is a plant-based technique involving the growing of green plants in contaminated soils to remove heavy metals(phytoextraction)or immobilize them as harmless forms(phytostabilization)(Shah and Daverey,2020).Owing to its advantages such as environmental friendliness and low cost,phytoremediation has attracted increasing attention(Shah and Daverey,2020).However,this technique also has some disadvantages.For example,hyperaccumulators are usually metal-selective,and have low biomass and slow growth rates,whereas fast-growing or high-biomass plants generally lack sufficient tolerances to toxic metals,thereby leading to low remediation efficiencies(Shah and Daverey,2020;Khalidet al.,2021).Currently,phytoremediation is still in its growing stage and is far from mature.The selection of high-efficiency plants and effective measures to enhance phytoremediation should always be considered.
Increasing efforts have been made to improve phytoremediation,particularly the use of soil amendments and plantassociated microbes(Marqueset al.,2009;Rajkumaret al.,2012;Wiszniewskaet al.,2016).Commonly used amendments include natural organic materials,such as compost,sewage sludge,biochar,humic substances,plant extracts,and exudates,inorganic materials such as lime and phosphate,and chemical chelators such as ethylenediaminetetraacetic acid(EDTA),diethylenetriaminepentaacetic acid(DTPA),ethylenediaminedisuccinic acid(EDDS),malic acid,and citric acid(Marqueset al.,2009;Wiszniewskaet al.,2016).Many amendments can immobilize heavy metals through precipitation,complexation,ion exchange,and adsorption,and each has its own specific characteristics and dominant remediation mechanisms(Parket al.,2011;Khanet al.,2017;Lahoriet al.,2017;Zenget al.,2017;Heet al.,2019;Wang Y Fet al.,2019;Sunet al.,2020;Wanget al.,2020).Plantassociated bacteria and mycorrhizal fungi that benefit plant growth and health are mainly used to assist phytoremediation(Rajkumaret al.,2012;Ullahet al.,2015;Khalidet al.,2021).In particular,arbuscular mycorrhizal fungi(AMF)exhibit great potential for the phytoremediation of heavy metals by decreasing contaminant toxicity and improving soil properties and plant tolerances to contaminants(Cabralet al.,2015;Wang,2017a,b).Successful phytoremediation depends not only on plant performance and contaminants,but also on influencing factors such as soil properties and climate characteristics.Effective measures should be developed to enhance the remediation efficiencies of specific plants.Nevertheless,due to their different remediation mechanisms,the combined effects of AMFand soil amendments require further exploration.
Bioenergy crops are often recommended for phytoremediation because they have additional advantages,such as waste reduction,recycling,and economic potential(Van Ginnekenet al.,2007;Gomes,2012).In addition to being used as a food and feed crop,sweet sorghum is widely known as a biofuel crop used for ethanol production(Zhaoet al.,2009;Ratnavathiet al.,2011;Daret al.,2018),and can be grown in soils with disadvantage conditions,such as heavy metal stress(Zhuanget al.,2011).Previous studies have reported the advantages of sweet sorghum for phytoremediation,such as high accumulation of metals,high growth rate,and high biomass(Zhuanget al.,2009;Sathyaet al.,2016;Yuanet al.,2019).An endophyte(Bacillussp.SLS18)has shown potential for sweet sorghum biomass production and metal uptake(Luoet al.,2012).Our previous studies have found that AMFinoculation can enhance salinity and molybdenum resistances in sweet sorghum,implying a potential for biomass production in salted and heavy metal-contaminated soils(Wang FYet al.,2019;Shiet al.,2020).However,nothing is known about the roles of AMFand soil amendments in assisting heavy metal phytoremediation using sweet sorghum.
Therefore,we hypothesized that soil amendments and AMFcan jointly contribute to the heavy metal phytoremediation(i.e.,phytostabilization and phytoextraction)using sweet sorghum.Three widely used amendments with different remediation mechanisms,hydroxyapatite(HAP),manure,and biochar,were selected.Phosphate can form insoluble precipitates with some heavy metals such as Pb(Zenget al.,2017),and biochar can act on metals mainlyviacomplexation(Heet al.,2019).Manure can not only reduce heavy metal toxicity through complexation and adsorption,but can also provide nutrients for plants and soil microbes(Huanget al.,2016).The majority of previous studies on AMF-assisted phytoremediation have been conducted using artificially heavy metal(loid)-polluted and sterilized soils.The present soil culture experiment was performed to determine whether AMF and soil amendments can be used to enhance the phytoremediation efficiency of unsterilized soil with indigenous AMFand environmentally realistic contamination by combined heavy metals.Our objective was to identify appropriate soil amendments to assist the phytoremediation of a heavy metal-contaminated soil using sweet sorghum with and without AMFinoculation.
MATERIALS AND METHODS
Soil
The surface soil(0–20 cm depth)was taken from an abandoned rice farmland(25◦10′35.15′′N,113◦64′85.97′′S)in the vicinity of a Pb and Zn smeltery located in Dongtang Town,Renhua County,Shaoguan City,Guangdong Province,China.The soil was air-dried and passed through a 2-mm sieve to remove animal and plant residues and small stones.The basic physicochemical characteristics of the soil samples were determined using the methods described by Lu(2000)(Table I).To simulate realistic field conditions,the soil was left unsterilized.The concentrations of Pb and Cd in the sampled soil exceeded the risk intervention values listed in the China’s guideline of Soil Environmental Quality–RiskControl Standard for Soil Contamination of Agricultural Land(GB 15618–2018),and can be classified as pollution levels(Table I).The Zn concentration was eight times the screening value,and was also considered a pollution level(Table I).
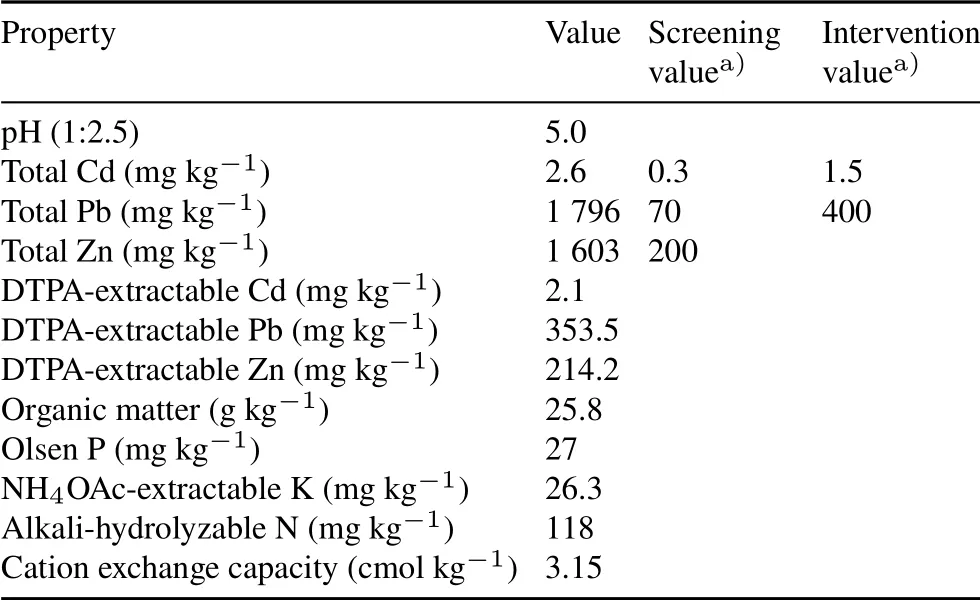
TABLE IPhysicochemical properties of the test soil taken from an abandoned rice farmland near a Pb and Zn smeltery
Inoculum and soil amendments
Acaulospora melleaZZ isolated from an abandoned coal mine was used as a target inoculant because our previous findings have demonstrated its excellent ability to colonize maize and sweet sorghum,and to enhance plant growth and tolerance in the Cu-and Mo-contaminated soil(Wanget al.,2007a;Shiet al.,2020)and saline soil(Wang FYet al.,2019).The AMFinoculant was propagated using maize grown in sterilized sand,consisting of a mixture of spores,mycelia,sand,and root fragments(Wanget al.,2007a;Wang FYet al.,2019).The inoculum contained more than 50 AMF spores g−1.
Hydroxyapatite was purchased from Hualan Chemical Technology Co.,Shanghai,China,with Ca10(PO4)6(OH)2content≥99.5%and average particle size≤40µm.Cow dung-based manure was kindly provided by Wonong Agricultural Technology Co.,Luoyang,China,with pH 8.58,organic matter 600 g kg−1,water content 300 g kg−1,N 30 g kg−1,P2O510 g kg−1,and K2O 10 g kg−1.
Biochar was prepared from pine shoots at approximately 700◦C in an oxygen-free environment for 2 h,with the following properties:pH 9.64,average specific surface area 139.4 m2g−1,C 861.5 g kg−1,H 31.7 g kg−1,N 2.9 g kg−1,S 5.1 g kg−1,O 73.6 g kg−1,P 3.0 g kg−1,K 2.8 g kg−1,zeta potential−26 mV,and average particle size 9.5 µm.Scanning electron microscopy images and Fourier transform infrared spectra of the biochar are shown in Fig.S1(see Supplementary Material for Fig.S1).
Experimental setup and procedure
To explore the single and combined effects of AMF and soil amendments,a trifactorial experiment was designed,including 1)two inoculation treatments,that is,non-inoculation and inoculation withA.melleaZZ,2)three types of soil amendments,that is,HAP,organic manure,and biochar,and 3)two doses of soil amendments,that is,0.1%and 1%(weight:weight).A blank with no soil amendment was used as a control(CK).Each treatment included four replicates.An appropriate amount of each amendment was mixed into the soil to obtain the target concentrations.Thereafter,1.2 kg of soil mixture was placed into each pot(1.4 L volume,15.5 cm top diameter,10.1 cm bottom diameter).For AMFinoculation treatments,50 g of AMFinoculum was mixed into each pot.Non-inoculation treatments received an equal amount of AMFinoculum sterilized by autoclaving at 121◦C for 2 h per pot.To provide similar non-AMF microbial communities,all pots were added with 10 mL of the AMFinoculum control filtrate,which was passed through a 37-µm nylon mesh(Wang FYet al.,2016).
Sweet sorghum(Sorghum bicolorL.var.Dalishi),which is widely grown in China,was selected as the test plant.Twenty surface-sterilized seeds were grown in each pot on October 3,2018,and after germination,the seedlings were thinned to 12 per pot on October 26,2018.The pots were randomly placed in a plant-growth chamber at approximately 20–25◦C,with a photoperiod of 16 h of light(light intensity of 10 000 lux)and 8 h of darkness and relative humidity of 50%–60%.During the plant growth period,weeds were manually removed at their emergence.The pots were weighed every three days and irrigated with deionized water when necessary to maintain soil water content of approximately 70%.
Sample analysis
Seedlings were harvested on December 25,2018,12 weeks after sowing.Roots and shoots were separately sampled,washed with tap water and deionized water before measurement of fresh weight.Fresh subsamples of roots were sampled for root colonization rate assessment and microscopic observation.The remaining fresh materials were oven-dried at 70◦C for 48 h,and their dry weights were recorded.The soil from each pot was mixed evenly and sampled for the analyses of soil pH and metal availability.
The root colonization rate was determined based on the method proposed by Giovannetti and Mosse(1980)after ink staining(Vierheiliget al.,1998).Briefly,fresh root samples were cleared in boiling KOH solution(10%,weight:volume)for 3 min,acidized in vinegar for 5 min,and then stained for 3 min in a boiling ink(5%,volume:volume)-vinegar solution.Fifty root segments(approximately 1 cm in length)were scanned under a microscope to determine the frequency of mycorrhizal colonization in the root system.The dried plant materials were ground and digested in HNO3using a graphite digester(SH220 N,Shandong Hanon Instruments Co.Ltd.,China)to extract the total metal content.The concentrations of metals and P were determined using inductively-coupled plasma mass spectrometry(ICP-MS)(Agilent 7700,USA)(Chaveset al.,2010).For quality assurance and quality control of the data,reagent blanks,standard solutions,and standard plant reference material(GBW07603,GSV-2)were included during the sample analysis.
Available forms of metals in soil were extracted using DTPA solution(0.005 mol L−1DTPA,0.1 mol L−1triethanolamine,and 0.01 mol L−1CaCl2,pH 7.3)at a soil/solution ratio of 1:2(weight:volume),and estimated using ICP-MS.Soil solution(soil:water=1:2.5,weight:volume)was used to assay soil pH using a pH meter.
Data analysis
The total uptake of each element by plant tissues was calculated as the product of plant dry weight and element concentration in plant tissues.The translocation factor(TF)was calculated to measure the degree of translocation of metals from underground tissues to aerial parts.

whereCshootis the metal concentration in shoots andCrootis the metal concentration in roots.
Data were analyzed for statistical significance in SPSS 22.0,using analysis of variance(ANOVA).The results are presented as mean±standard deviation(SD).The Shapiro-Wilks test was used to test the normality of the distribution of variables,and Levene’s test was used to test the homogeneity of variance.A three-way ANOVA was conducted to calculate the main and interaction effects between three independent factors(amendment type,amendment dose,and AMFinoculation),while a two-way ANOVA was performed to analyze the interactions between AMFinoculation and each amendment.One-way ANOVA followed by Duncan’s multiple range test(P<0.05)was used to compare the effect of a specific factor within each group.Pearson’s correlation coefficients were calculated to analyze the correlations between the different parameters.
RESULTS
Root colonization
All plants had significant mycorrhizal colonization of their roots(Fig.1).The uninoculated plants were also colonized,with root colonization rates ranging from 30%to 78%.Compared to non-inoculation,the AMFinoculation significantly increased root colonization rates in all amendment treatments,ranging from 73%to 90%.Compared to CK,only HAP among the three soil amendments significantly decrease root colonization in uninoculated plants,by 28%–46%,but not in inoculated plants.Comparatively,both manure and biochar exhibited stimulatory effects on root colonization,particularly in inoculated plants.Threeand two-way ANOVA results showed that AMFinoculation and soil amendments always had significant independent influences on root colonization,but significant interactions only occurred between AMFinoculation and HAP(Tables II and III).

Fig.1 Root mycorrhizal colonization rates of sweet sorghum plants uninoculated(−AMF)and inoculated(+AMF)with arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ under three soil amendment treatments,including hydroxyapatite(HAP),manure,and biochar,at doses of 0.1%and 1%(weight:weight).Vertical bars indicate standard deviations of the means(n=4,degree of freedom=55).Different letters above the bars indicate significant differences based on the Duncan’s test at P<0.05.Three-and two-way analysis of variance results are shown in Tables II and III,respectively.CK=control with no soil amendment.
Plant biomass
Overall,plant biomass was significantly influenced by amendment type and dose(three-way ANOVA,Table II),and by the three amendments separately(except root biomass by biochar)(Table III).Compared to CK,HAP showed significant plant growth promotion,particularly at a dose of 1%.Significant plant growth promotion by manure was observed only at a dose of 1%.However,manure at a dose of 0.1%had no significant effect(Fig.2).Although biochar did not significantly change shoot and root biomass(oneway ANOVA,Fig.2),the overall effects on root biomass were significant(two-way ANOVA,Table III).Compared to the non-inoculation treatments,AMF inoculation did not significantly change plant biomass,independent of soil amendments(Fig.2,Table II).
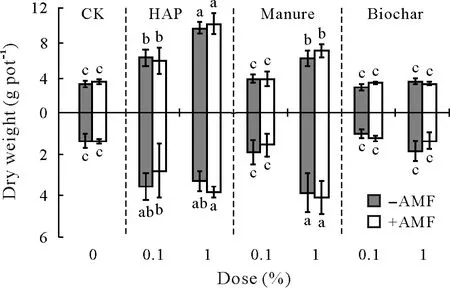
Fig.2 Shoot(above X-axis)and root(below X-axis)dry weights of sweet sorghum plants uninoculated(−AMF)and inoculated(+AMF)with arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ under three soil amendment treatments,including hydroxyapatite(HAP),manure,and biochar,at doses of 0.1%and 1%(weight:weight).Vertical bars indicate standard deviations of the means(n=4,degree of freedom=55).Different letters above or below the bars indicate significant differences based on the Duncan’s test at P<0.05.Three-and two-way analysis of variance results are shown in Tables II and III,respectively.CK=control with no soil amendment.
Concentrations and TFof heavymetals in plant tissues
Overall,Cd,Pb,and Zn concentrations in plant tissues were significantly influenced by amendment type,dose,and their interactions,but only shoot Pb concentration was changed by AMFinoculation(Table II).Compared to CK,HAP caused significant decreases in Cd,Pb,and Zn concentrations in both shoots and roots,and the higher dose(1%)produced more pronounced effects(Fig.3).Manure also exhibited decreasing trends in heavy metal concentrations in plant tissues,but in most cases,only a dose of 1%produced significant effects.Biochar generally decreased shoot Pb and Zn concentrations,but did not alter root Cd,Pb,and Zn concentrations,and even increased shoot Cd concentration.In general,1%HAP produced the most significant reduction in all three contaminants in plants.
Compared to the non-inoculation treatments,AMFinoculation decreased shoot Pb concentration in plants when applied independently or in combination with manure and biochar(Fig.3,Table III).The AMFinoculation decreased shoot Zn concentration in plants that received no amendments or biochar.Overall,AMFinoculation caused no alterations in shoot Cd concentration,except for an increase in plantsreceiving 1%biochar.In most cases,AMFinoculation did not significantly alter the concentrations of Cd,Pb,and Zn in the roots of plants treated with or without soil amendments(Tables II and III).

Fig.3 Shoot(above X-axis)and root(below X-axis)Cd,Pb and Zn concentrations in sweet sorghum plants uninoculated(−AMF)and inoculated(+AMF)with arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ under three soil amendment treatments,including hydroxyapatite(HAP),manure,and biochar,at doses of 0.1%and 1%(weight:weight).Vertical bars indicate standard deviations of the means(n=4,degree of freedom=55).Different letters above or below the bars indicate significant differences based on the Duncan’s test at P<0.05.Three-and two-way analysis of variance results are shown in Tables II and III,respectively.CK=control with no soil amendment.
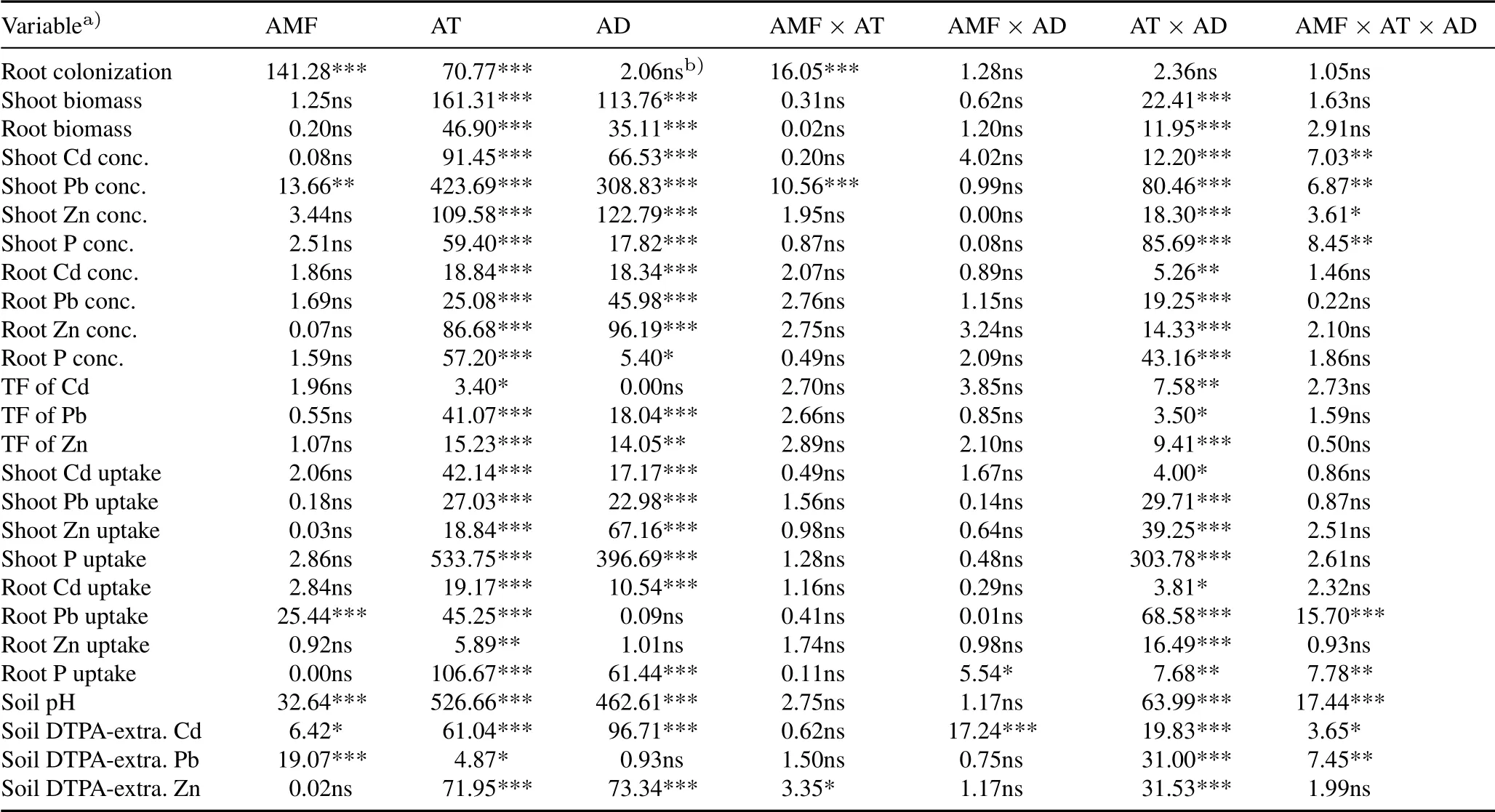
TABLE IISignificance levels(F values)of arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ inoculation,amendment type(AT),amendment dose(AD),and their interactions on measured variables by a three-way analysis of variance
Overall,the TFs of Cd,Pb,and Zn were not influenced by AMFinoculation,but changed by soil amendments(Fig.4,Tables II and III).All three amendments decreased the TFs of Pb and Zn,and 1%HAP produced the most pronounced effects.The TFof Cd only decreased in the soil with 1%HAP.
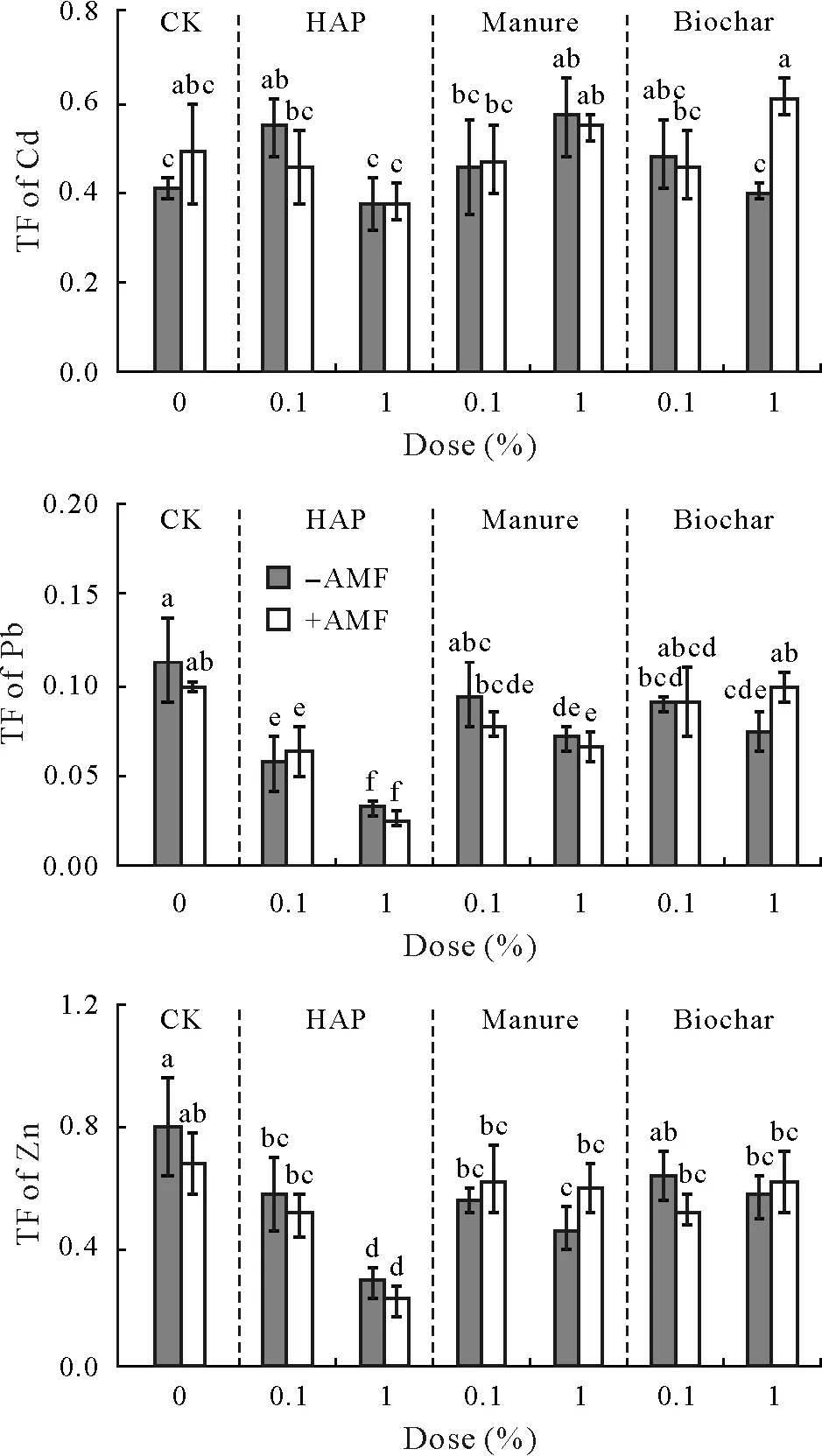
Fig.4 Translocation factor(TF)of Cd,Pb,and Zn in sweet sorghum plants uninoculated(−AMF)and inoculated(+AMF)with arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ under three soil amendment treatments,including hydroxyapatite(HAP),manure,and biochar,at doses of 0.1%and 1%(weight:weight).Vertical bars indicate standard deviations of the means(n=4,degree of freedom=55).Different letters above the bars indicate significant differences based on the Duncan’s test at P<0.05.Three-and two-way analysis of variance results are shown in Tables II and III,respectively.CK=control with no soil amendment.
Uptake of heavymetals in plant tissues
The uptake of heavy metals by plants was also calculated(Fig.5).In most cases,heavy metal uptake by plants varied significantly with the amendment type and dose(Table III).Compared to CK,0.1%and 1%HAP increased Cd uptake in shoots and roots,but 1%HAP greatly decreased plant Pb and Zn uptake;0.1%manure did not influence Pb and Zn uptake in plants,whereas 1%manure increased Cd uptake in shoots,and Pb and Zn uptake in roots.Biochar did not change shoot Cd,Pb,and Zn uptake,but decreased root Pb uptake at a dose of 0.1%.Compared to the non-inoculation treatments,AMFinoculation did not influence shoot Cd,Pb,and Zn uptake,and root Cd and Zn uptake,but decreased root Pb uptake in most cases(Fig.5,Tables II and III).

Fig.5 Shoot(above X-axis)and root(below X-axis)Cd,Pb and Zn uptake in sweet sorghum plants uninoculated(−AMF)and inoculated(+AMF)with arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ under three soil amendment treatments,including hydroxyapatite(HAP),manure,and biochar,at doses of 0.1%and 1%(weight:weight).Vertical bars indicate standard deviations of the means(n=4,degree of freedom=55).Different letters above or below the bars indicate significant differences based on the Duncan’s test at P<0.05.Three-and two-way analysis of variance results are shown in Tables II and III,respectively.CK=control with no soil amendment.
Soil pH
In comparison with the soil before plant growth,soil pH increased substantially after plant harvest.Compared to CK,soil pH was significantly increased in the HAP,manure,and 1%biochar treatments,with the highest values obtained in the 1%HAP treatment;however,soil pH was not altered by 0.1% biochar(Fig.6).Among all the treatments,the soil with inoculated plants and 1%HAP had the highest soil pH.The AMFinoculation alone increased soil pH,but exhibited no consistent impacts when soil amendments were applied.Two-way ANOVA results showed that AMFinoculation,soil amendments,and their interactions all had significant impacts on soil pH(Table III).Furthermore,there were significant tripartite interactions among AMFinoculation,amendment type,and amendment dose on soil pH(Table II).
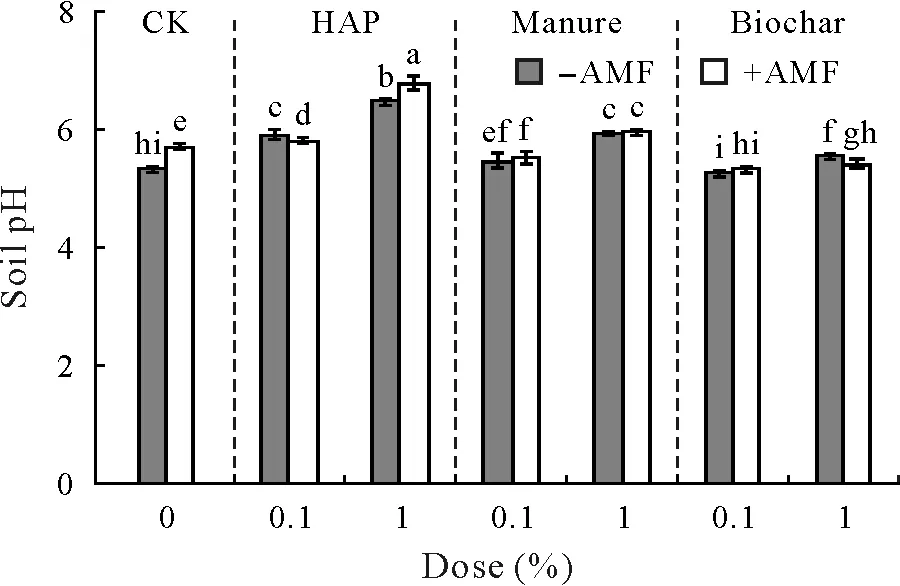
Fig.6 Soil pH after harvest of sweet sorghum plants uninoculated(−AMF)and inoculated(+AMF)with arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ under three soil amendment treatments,including hydroxyapatite(HAP),manure,and biochar,at doses of 0.1%and 1%(weight:weight).Vertical bars indicate standard deviations of the means(n=4,degree of freedom=55).Different letters above the bars indicate significant differences based on the Duncan’s test at P<0.05.Three-and two-way analysis of variance results are shown in Tables II and III,respectively.CK=control with no soil amendment.
Bioavailabilityof heavymetals in soil
In most cases,all three soil amendments decreased the DTPA-extractable Cd concentrations in the soil(Fig.7).However,only HAP and manure were effective in decreasing the concentrations of DTPA-extractable Pb and Zn.Compared to the non-inoculation treatments,AMFinoculation decreased DTPA-extractable Pb concentrations when applied independently,and decreased DTPA-extractable Cd concentrations when applied in combination with 1% HAP andmanure.Overall,1%HAP was the most effective treatment for the immobilization of Cd,Pb,and Zn in the soil.Compared to CK,DTPA-extractable concentrations of Cd,Pb,and Zn in 1%HAP-amended soils decreased by 31%–43%,30%–38%,and 22%–23%,respectively.Two-way ANOVA results showed that AMFinoculation had significant interactions with HAP and manure on soil DTPA-extractable Cd and Pb concentrations(Table III),while three-way ANOVA results showed significant tripartite interactions among AMF inoculation,amendment type,and amendment dose(Table II).

Fig.7 Soil DTPA-extractable Cd,Pb,and Zn concentrations after harvest of sweet sorghum plants uninoculated(−AMF)and inoculated(+AMF)with arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ under three soil amendment treatments,including hydroxyapatite(HAP),manure,and biochar,at doses of 0.1%and 1%(weight:weight).Vertical bars indicate standard deviations of the means(n=4,degree of freedom=55).Different letters above the bars indicate significant differences based on the Duncan’s test at P<0.05.Three-and two-way analysis of variance results are shown in Tables II and III,respectively.CK=control with no soil amendment.
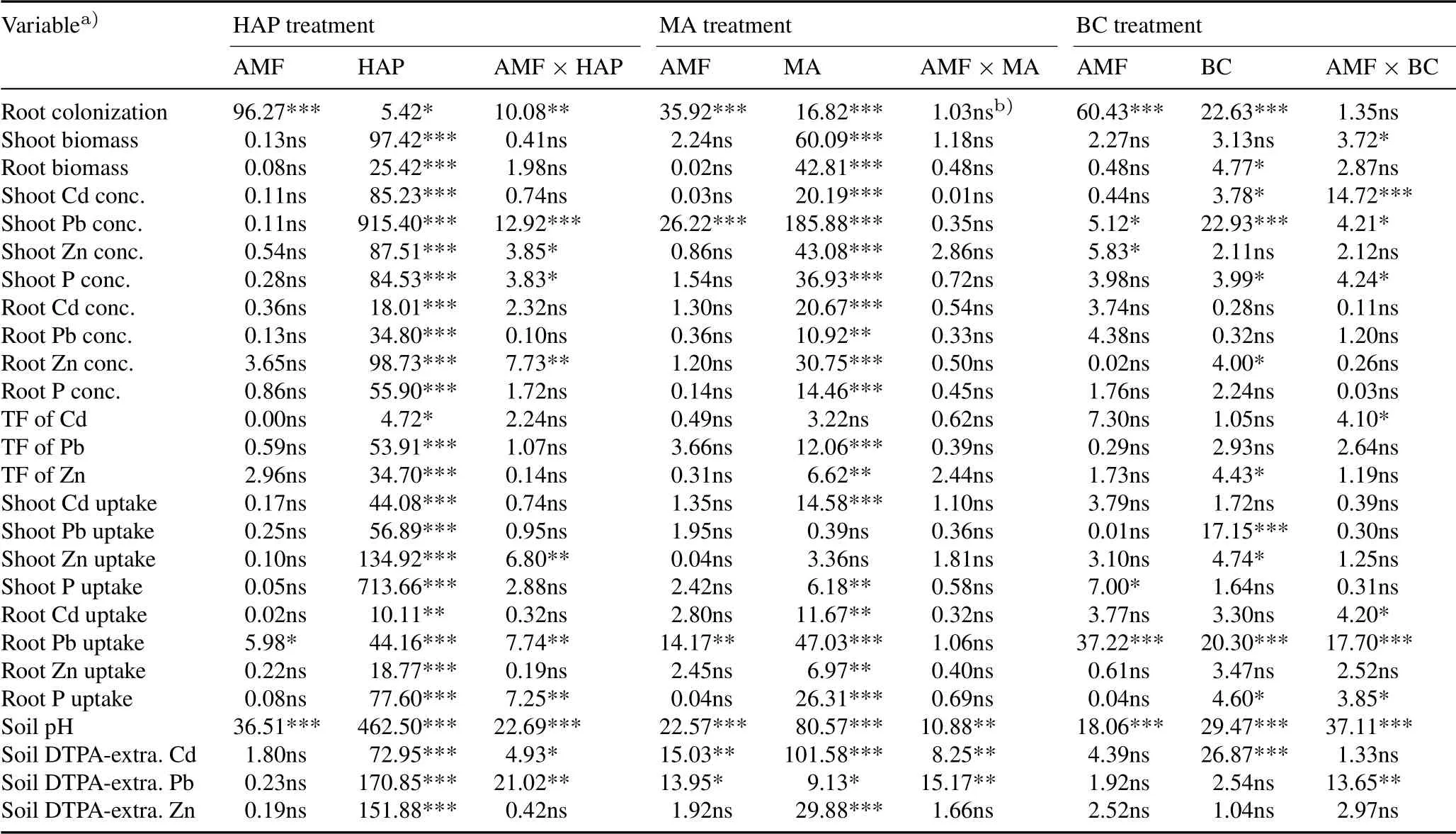
TABLE IIISignificance levels(F values)of arbuscular mycorrhizal fungus(AMF)Acaulospora mellea ZZ inoculation,soil amendments(hydroxyapatite,HAP;manure,MA;and biochar,BC),and their interactions on measured variables by a two-way analysis of variance
DISCUSSION
AMFcolonization under heavymetal contamination
Good colonizing capacity is necessary for the successful application of AMFspecies.Plants belonging to the genusSorghumare widely known to easily form symbioses with AMF(Wang FYet al.,2019;Shiet al.,2020).In the present study,root colonization in non-inoculated plants confirmed that indigenous AMFwas present in the soil contaminated with combined heavy metal(loid)s.In fact,a few AMF species belonging to Glomeraceae and Acaulosporaceae were observed(unpublished data).They can colonize sweet sorghum successfully,implying the excellent infectivity of these AMF.AMFoccur in environments polluted withvarious heavy metals artificially and naturally(Ghre and Paszkowski,2006;Wang,2017b).However,in some polluted sites without vegetation(and related AMF),it is of great importance to introduce effective AMFstrains to help plant colonization at these sites.
Another finding was that AMFinoculation significantly increased root colonization rate,irrespective of indigenous AMF,implying that the introduced AMFstrain may have a strong tolerance to multi-metal contamination.This is similar to our previous report thatA.melleaZZ is tolerant to contaminants(Wang and Miao,2006;Wanget al.,2007a,2011).This fungal strain originated from coalmine spoil and may have developed great ecological adaptability to environmental pressures during its evolution process.Different AMF species differ in their tolerance to toxic metals.As discussed by Khalidet al.(2021),it is necessary to elucidate how AMF communities react to heavy metal stress,which may play a crucial role in phytoremediation.One study found that AMFcommunity diversity and colonization were closely associated with plant diversity and vegetation establishment in Pb-contaminated soil(Schneideret al.,2016),suggesting that increasing AMFdiversity may facilitate phytoremediation strategies.Another study found that inoculation with an AMFconsortium was more effective than a single species for phytoremediation using maize(Wanget al.,2007b).In thelong run,it is important to introduce more AMFspecies for revegetation and phytoremediation of heavy metal-polluted sites with few or no AMFspecies.
AMFeffects on plant growth and phytoremediation
Unexpectedly,AMFinoculation did not promote the growth of sweet sorghum,independent of soil amendments(Fig.2).Although a number of studies have confirmed that AMFcan improve plant tolerance and growth under heavy metal contamination conditions(Cabralet al.,2015;Wang,2017b),AMFinoculation effects can vary depending on various factors such as plant genotype and tolerance,AMForigin and tolerance,plant-AMFcompatibility,and environmental conditions.
There are several reasons for the finding in this study.The first may be competition from indigenous AMF.Compared to the introduced strain,indigenous AMFcommunities may have developed higher adaptability to the surroundings,and thus they may have stronger competitiveness.In sterilized soil,excluding indigenous AMF,A.melleaZZ showed significant plant growth-promoting effects on maize and soybean(Wang and Miao,2006;Wanget al.,2007a).Second,since AMFbelong to obligate biotrophs depending on host plants for photosynthates,their effects on plant growth are not always positive(Johnsonet al.,1997).Mycorrhizal effects are generally more significant for plant species and cultivars with lower tolerance to environmental stress(Repettoet al.,2003;Merloset al.,2016).Sweet sorghum is a plant species with a high tolerance to heavy metal toxicity(Zhuanget al.,2011),and the plants in our current experiment exhibited no obvious toxicity symptoms,indicating that they did not suffer from the stress caused by the heavy metal(loid)s.Ultra and Manyiwa(2021)found that the mycorrhizal effects on threeAcaciaspecies grown in Cu-Ni mine soil were plant genotype-specific:beneficial forAcacia luederitzii,but not beneficial forA.albidaandA.tortilis.Third,AMFcan benefit plants by facilitating P acquisition from the soil,which varies greatly with soil fertility,particularly with regard to available P(Smithet al.,2011).Plant growth promotion by AMFis generally maintained in soil with poorly available P(Bolan,1991),but tends to disappear in soils with highly available P(Smith and Read,2008).As the soil in our current experiment was not deficient in P(particularly in soil receiving HAP),AMFinoculation did not improve plant P nutrition(Figs.S2and S3,see Supplementary Material for Figs.S2 and S3).Hence,the benefits of AMFinoculation may be concealed.
The AMFcan contribute to phytostabilization by facilitating the chelation/immobilization of toxic metals in their structures,mycorrhizal roots,and soil(Ghre and Paszkowski,2006;Wang,2017b).However,most laboratory studies have been conducted using sterilized soil without the interference of indigenous microorganisms,especially AMFand other plant-associated microbes.Unsterilized soil may represent a more realistic condition.Currently,in unsterilized soil with indigenous AMF,inoculation contributes little to heavy metal uptake and translocation in plants.However,AMFinoculation substantially influenced soil pH(Fig.6,Table III),which may further contribute to heavy metal immobilization(Table SI,see supplementary material for Table SI).For instance,AMFinoculation alone increased soil pH and decreased available Pb concentration in the soil,implying positive contribution from the inoculated fungal strain.The extraradical mycelia of AMFhave been shown to induce alkalinization,thereby enhancing the pH of substrates(Janoukovet al.,2006;Janoukov and Pavlkov,2010).Our previous studies also found higher soil pH and lower available metal concentrations in AMF-inoculated soil contaminated with single or multiple metals(Wanget al.,2007a,2012,2013,2018),but these were all conducted using sterilized soil.Our current findings further highlight the potential application of AMFin phytostabilization under realistic scenarios.
Amendment effects on phytoremediation and plant growth
Our present results showed that HAP,manure,and biochar were generally effective in the immobilization of multiple metals(Fig.7),following the order of HAP>manure>biochar.Several mechanisms can explain the higher immobilization efficiency of HAP.First,the immobilization of metals by HAP is mainly due to the formation of phosphate precipitates with much lower solubility(Zenget al.,2017;Leeet al.,2018;Sunet al.,2018).However,complexation and adsorption,instead of precipitation,may dominate the immobilization of heavy metals by manure and biochar(Khalidet al.,2017;Heet al.,2019).Second,soil pH is a key factor in determining heavy metal solubility(Chuanet al.,1996;Zenget al.,2011).Higher soil pH may lead to lower heavy metal toxicity.Pearson’s correlation analysis showed that soil pH was negatively correlated with heavy metal availability,but positively correlated with plant growth(Table SI).HAP has higher liming effects on soil pH than manure and biochar(Fig.6),and consequently functions as a liming amendment to decrease the solubility of heavy metals.Third,HAP may facilitate the phytostabilization of heavy metals by improving plant P nutrition(Figs.S2 and S3)and fitness.As an inorganic P fertilizer,the solubility of HAP is relatively low at a higher pH,but increases rapidly with decreasing soil pH<6(Lindsay,1979).As the soil we used is acidic with a low pH of 5,HAP has a high phytoavailability.Comparatively,manure and biochar have a much lower fertility,which may result in fewer benefits for plants.
However,the effects of soil amendments on the phytoaccumulation of heavy metals are generally dependent on the type of amendment,plant traits,metal concentration,and metal bioavailability(Evangelouet al.,2007;Sharma and Nagpal,2018;Hasanet al.,2019).Overall,HAP and 1%manure increased shoot Cd uptake,and thereby could be used to enhance the phytoextraction of Cd(Fig.5).However,1%HAP greatly decreased shoot Pb and Zn uptake,leadingto much lower phytoextraction of Pb and Zn.Compared to Pb and Zn,Cd usually has higher bioavailability and translocation factor in paddy soil,and can cause“Cd-tainted rice”(Wanget al.,2015;Wang S Het al.,2016).HAP may be a feasible amendment for the phytoextraction of Cd from paddy soils.Furthermore,HAP decreased the concentrations of Cd,Pb,and Zn in the shoots,suggesting its potential in the safe production of food crops(Sharma and Nagpal,2018).
High plant biomass is a prerequisite for successful phytoremediation programs.Soil amendments can benefit plantsviaboth direct nutritional functions and indirect impacts on heavy metal toxicity and soil properties.Compared to manure and biochar,HAP generally showed more significant effects on heavy metal immobilization,soil pH,and plant P nutrition.Unsurprisingly,HAP had an optimal effect on plant growth promotion.However,manure and biochar contain a considerable amount of organic matter or carbon-based materials,which may potentially improve soil structure and quality in the long run.
AMFand amendment interactions
Our results revealed different effects of soil amendments on AMFcolonization.Theoretically,soil amendments can cause double-edged effects on AMFcolonization and function.HAP can be considered an optimal example.First,the high available P in soil has a negative impact on AMF(Covacevichet al.,2007;Breuillinet al.,2010;Elbon and Whalen,2015).HAP can release,which is converted into phytoavailable forms(and)in the soil,thus leading to an inhibition of AMFcolonization.Second,HAP can immobilize available heavy metals,thereby decreasing their toxicity to AMF.Thus,HAP effects on AMF colonization represent a compromise between benefits and side-effects.Comparatively,due to their low content or low availability of P,both manure and biochar generally contribute little to soil available P and produce more benefits but no harm to AMF.As studied previously,under heavy metal contamination conditions,the addition of biochar and manure can increase root colonization by AMFby altering certain soil physicochemical properties,such as increasing pH and decreasing the availability of toxic metals(Wanget al.,2012,2013;Liu Let al.,2018;Zhuoet al.,2020).Overall,given the ubiquitous presence of AMF in plant roots,the eco-friendliness of soil amendments should be considered for use in assisting phytoremediation.
Several previous studies,including ours,have shown synergistic contributions to plant tolerance and soil remediation of heavy metals between AMFand soil amendments such as phosphate,manure,and biochar(Soares and Siqueira,2008;Wanget al.,2012,2013;Liu Let al.,2018).However,these studies were all conducted using sterilized soils,which are far from realistic soil conditions.Here,using realistic soil conditions,we also found some synergistic interactions between AMFinoculation and soil amendments on soil characteristics,such as soil pH and available Pb and Cd(Table III).In particular,the HAP-induced inhibition of root colonization by native mycorrhiza was offset by AMFinoculation(Fig.1,Table III).Therefore,when indigenous AMFcolonization is inhibited by contaminants or soil amendments,inoculation with exogenous AMFmay be an effective strategy for heavy metal phytoremediation assisted by soil amendments.Overall,AMFand soil amendments can synergistically contribute to phytoremediation using sweet sorghum.
CONCLUSIONS
Using unsterilized soil with environmentally realistic multiple heavy metal contamination,we tested the feasibility of AMFinoculation and soil amendments in phytoremediation with sweet sorghum.The remediation effects were dependent on the amendment type and dose.Among the three soil amendments,HAP was the most effective in promoting plant growth and heavy metal immobilization,particularly at the 1%dose.Manure and biochar also exerted positive effects on heavy metal immobilization,but only manure showed plant growth-promoting effects.Due to the presence of indigenous AMF,all plants showed typical mycorrhizal colonization.However,inoculation with exogenous AMFnot only promoted root colonization and alleviated the inhibition of HAP,but also increased soil pH and the immobilization of Pb and Cd,indicating the potential of AMFin phytostabilization under realistic scenarios.Overall,soil amendments,in particular HAP,produced positive effects and synergistic interactions with AMF inoculation on heavy metal phytostabilization with sweet sorghum.In conclusion,AMF combined with soil amendments may be an effective strategy for future heavy metal phytoremediation programs.
ACKNOWLEDGEMENT
This work was supported by the Shandong Provincial Key Research and Development Program of China(No.2019GSF109008),the Qingdao Special Funds for the Science and Technology Program of Public Wellbeing,China(No.20-3-4-29-nsh),and the Doctoral Foundation of Qingdao University of Science and Technology,China(No.0100229003).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Effect of indaziflam on microbial activity and nitrogen cycling processes in an orchard soil
- Spectral indices measured with proximal sensing using canopy reflectance sensor,chlorophyll meter and leaf color chart for in-season grain yield prediction of basmati rice
- Effect of Zn binding to phytate and humic substances on its uptake by wheat(Triticum durum L.)as affected by carbonates and Fe oxides
- Occurrence of polycyclic aromatic hydrocarbons in the estuarine sediments of the Taihu Lake and their associated toxic effects on aquatic organisms
- Nitrogen slow-release behavior of oxamide granules in two different types of paddy soils
- Effective alleviation of Cd stress to microbial communities in mining reclamation soils by thiourea-modified biochar amendment
