Effect of Xingnao-Jianshen granules in treating AIS patients: study protocol for a non-randomized controlled intervention trial
2022-12-03ChunYuMaMiaoLiuHaoYuZhangMinYangXinNaWangDeXiZhao
Chun-Yu Ma ,Miao Liu ,Hao-Yu Zhang ,Min Yang ,Xin-Na Wang ,De-Xi Zhao
1Department of Traditional Chinese Medicine,Changchun University of Chinese Medicine,Changchun 130117,China.2Department of Geriatrics,The Fifth Affiliated Hospital Sun Yat-Sen University,Zhuhai 528406,China.3Central Hospital in Panyu District,Guangzhou University of Chinese Medicine,Guangzhou 511486,China.4School of Traditional Chinese Materia Medica,Jilin Agricultural Science and Technology University,Jilin 130118,China.5Department of Neurology,Jilin Province Hospital of Chinese Medicine:First Affiliated Hospital to Changchun University of Chinese Medicine,Changchun 130021,China.
Abstract Objective:Acute ischemic stroke (AIS) is characterized by high morbidity and high mortality.In recent years,complementary and alternative medicine has gradually been widely accepted and applied.At present,traditional Chinese medicine therapy and standard treatments are used for the treatment of AIS.Xingnao-Jianshen prescriptions (XNJS) is an effective prescription for the clinical treatment of AIS,but there is a lack of large-scale clinical evidence to confirm its clinical efficacy.Therefore,our team designed this protocol to evaluate the initial therapeutic effect of XNJS.Methods:The protocol for a non-randomized controlled trial is designed in which 72 eligible patients will be allocated to one of two groups.The control group (n=36) will receive standard treatment for AIS,the test group (n=36) will receive XNJS and standard treatment.Patients will be recruited after stroke onset and will receive the intervention continuously over 10±1 days,with a follow-up period of 90 days.The primary outcome will be the change in the NIHSS,BI,mRS scores.All outcome measures will be assessed at inception,after the intervention (10±1 days),and at the follow-up(90 days).The results will be disseminated to the public through peerreviewed journals and academic conferences.Discussion: The study will provide evidence of the preliminary effects.
Keywords: Xingnao-Jianshen prescriptions;AIS;non-randomized controlled intervention trials;study protocol
Introduction
Acute stroke manifests as interruption of blood flow or bleeding in the central nervous system [1],including ischemic stroke,hemorrhagic stroke and subarachnoid hemorrhage.Ischemic stroke (cerebral infarction) is the most common type,accounting for approximately 70% of all strokes,and it is also one of the main causes of death and disability for adults over 60 years old[2,3].It occurs after thrombosis of a blood vessel in the brain or neck and is a devastating disease with complex pathophysiology [4].From 1990 to 2010,in low-and middle-income countries,the number of first-time IS patients increased by 37%,and mortality increased by 21% [5].
A European paper on the epidemiology of stroke and trends in the 21st century predicts that the absolute number of strokes is expected to increase dramatically in the coming years due to an aging population: By 2025,1.5 million Europeans will have a stroke each year.In addition to primary outcomes,stroke patients are at increased risk of poor outcomes in the first year after the event [6].In addition,due to factors such as accelerated population aging,changes in dietary structure,and increased work pressure,there is an increasing prevalence of stroke among younger individuals.This finding coincides with an increase in the prevalence of vascular risk factors and substance abuse among young adults [7].Strategies to improve preventive measures are therefore needed.Intravenous thrombolysis with recombinant tissue plasminogen activator is currently an effective method for the treatment of AIS [8].However,due to the narrow time window for thrombolysis and the presence of bleeding complications,only a minority of patients can receive thrombolysis[9].
Traditional Chinese medicine (TCM) is widely used in Asia as a complementary therapy to modern medicine for AIS [10,11].For example,in China,TCM that activates blood circulation is widely used in the treatment of ischemic stroke.XNJS was created by Professor Ren Jixue,a master of traditional Chinese medicine,and is an effective prescription for the treatment of stroke.Phlegm-heat and fu-organism syndrome is one of the common types of AIS.It is a combination of "internal wind","internal fire" and "phlegm dampness"and "blood stasis" syndrome elements (the smallest unit of the syndrome).XNJS can give a way out to abnormal products (including"internal wind","internal fire","phlegm dampness"and "blood stasis").The application of XNJS in the treatment of AIS conforms to the principle of TCM syndrome differentiation and treatment ent.Therefore,it can be used for the treatment of AIS,especially with"phlegm-heat and fu-organism syndrome".For other syndromes,herbs can be added or subtracted or the dosage can be adjusted.However,there is a lack of large-scale clinical evidence to confirm the clinical efficacy of XNJS.Therefore,we designed the trial to assess preliminary effects (the control group received standard treatment for AIS,and the test group received XNJS and standard treatment.)and to inform sample size calculations for future studies on the treatment of AIS.Our hypothesis is that the effect of this method is not lower than that of the standard treatment recommended by the guidelines.
Materials and methods
Study design
This trial will be a non-randomized,controlled,intervention study of AIS with phlegm-heat and fu-organism syndrome.The patients will be allocated to two parallel treatment groups using a 1:1 ratio.To compare the clinical efficacy of XNJS and routine treatment (test group) and routine treatment (control group) by observing the effects of XNJS on the recovery of neurological function,ability of daily life,improvement of syndrome elements and time of first defecation in patients with AIS.We will recruit patients who have sustained AIS and present with constipation,according to predefined inclusion and exclusion criteria,between April 2021 and October 2022.
To reduce potential bias caused by non-randomization,we held large expert seminars,a unified standard of diagnosis and treatment plan was formulated for the two participating hospitals.Strict inclusion and exclusion criteria were established.Strict training was given to the participants,and a system of review scale was developed for superior physicians to minimize the degree of bias.(See Figure 1 for the research flow chart and Table 1 for the research plan.)
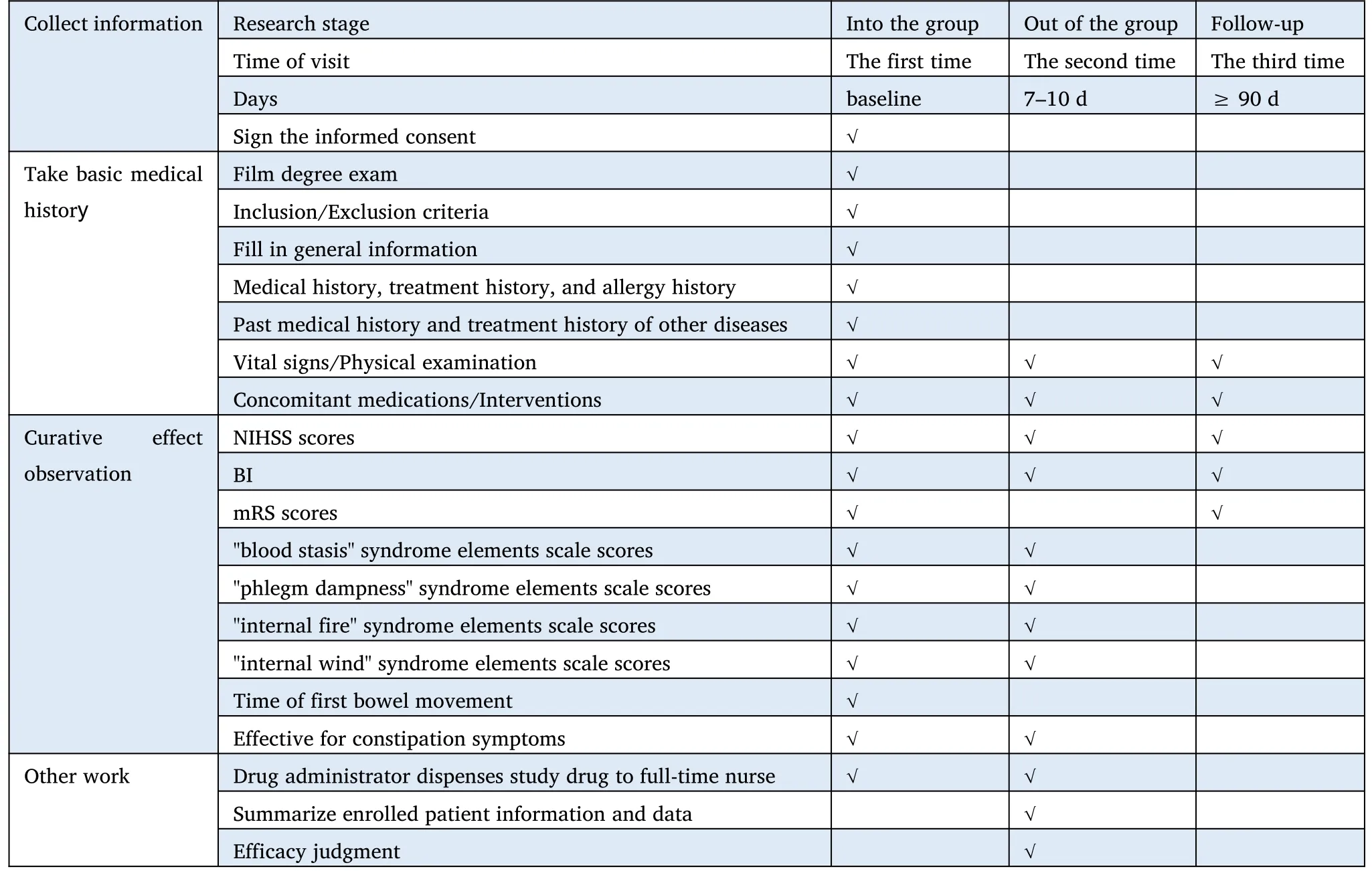
Table 1 Flow chart of Clinical trial study of Xingnao-Jianshen prescriptions for ischemic stroke (experimental phlegm-heat and fu-organism)
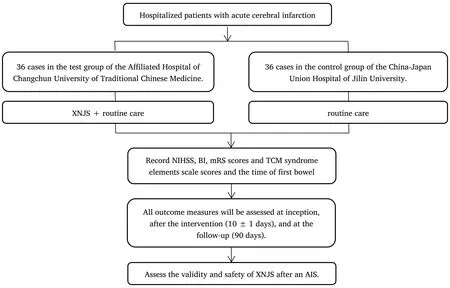
Figure 1 Flow diagram of the study selection process
Ethics and registration
The trial was registered in the China Clinical Trial Registration Center(No.ChiCTR2100045760) and fully complies with the principles of the Helsinki Declaration and Good Clinical Practice (GCP) guidelines.The clinical trial will recruit 72 participants after the participants provide informed consent,including a 10 ± 1 day treatment period and a 90 day follow-up period.They can stop or withdraw from the trial at any time during the study.All information collected will be kept strictly confidential.Ethical approval of this trial has been granted by the Research Ethics Committee of the Affiliated Hospital of Changchun University of Traditional Chinese Medicine (No.CCZYFYLL2021zhunzi-019).All the participants signed informed consent form.
Patients
Diagnostic basis.The Western medicine diagnostic criteria of IS will refer to the ‘Guidelines for the diagnosis and treatment of AIS in China’ (2018 edition) [12].The Chinese medicine diagnostic criteria of IS will refer to the ‘Guiding Principles for Clinical Study of New Chinese Medicines’[13].
The diagnostic criteria of phlegm-heat and fu-organism syndrome in TCM will refer to the ‘Guiding Principles for Clinical Study of New Chinese Medicines’.
Inclusion criteria.Patients will be recruited for this study if they meet all of the following criteria: (1) 40 ≤age ≤80 years;(2)consistent with the Western and Chinese medicine diagnostic criteria of AIS;(3) meet the diagnostic criteria of TCM symptom differentiation(phlegm-heat and fu-organism syndrome);(4)course of disease of 1-14 days;(5) 4 ≤National Institute of Health Stroke Scale(NIHSS) score ≤15;and (6) the patient or the entrusted immediate family signed the informed consent.
Exclusion criteria.The exclusion criteria will be as follows: (1)patients diagnosed with other brain diseases based on head CT or MRI,such as cerebral hemorrhage,brain tumor,brain trauma,transient ischemic attack (TIA);(2) comatose patients;(3) those who need or have undergone thrombolysis or endovascular treatment;(4) allergic constitution,allergic to the test drug or its related components;(5)after treatment,the blood pressure is still <90/60 mmHg or ≥220 mmHg/120 mmHg;(6) combined with severe liver and kidney damage,the levels of ALT,AST,Cr and BUN are 1.5 times higher than normal values;(7) those with other comorbidities and complications that affect drug evaluation,such as severe cardiac insufficiency,severe mental illness,depression and dementia after stroke,and cerebral hemorrhage after cerebral infarction;(8) previous ischemic stroke mRS score ≥ 2;(9) disabled patients specified by law(blindness,deafness,muteness,intellectual disability,mental disorder,etc.),physical disabilities caused by other diseases that affect the evaluation of neurological deficits;(10) those with bleeding tendency or severe bleeding within 90 days;(11) suspected or actual history of alcohol,drug abuse,or other conditions that,in the investigator's judgment,reduce the likelihood of enrollment or complicate enrollment;(12) menstruating women,pregnant and breastfeeding women,those with a positive pregnancy test or those who plan to have children recently;(13) those who are participating in other clinical trials or who have participated in other drug clinical trials for less than 90 days.
Sample size
Since no randomized controlled trials (RCTs) have previously investigated the topic,the study will provide effect size data for sample size calculations for subsequent large RCTs.For practical reasons,a sample size of 36 per group for a total of 72 participants will be recruited based on the hospital's outpatient and inpatient censuses throughout the study period.
Interventions
All the participants will receive standard treatment in accordance with the “Guidelines for diagnosis and treatment of ischemic stroke in China”.All these treatments will be well documented in the CRF.During the study period,the CRF will also keep detailed records of the patients' medications for other underlying medical conditions.However,during the entire study period,any traditional Chinese medicine with similar effects to XNJS will not be allowed.
The treatment group is the standard treatment recommended in the guidelines plus XNJS (Jiangyin Tianjiang Pharmaceutical Co.,Ltd.,Jiangyin,China).Including Niuhuang (Bos taurus domesticus),Shexiang (Moschus berezovskii),Honghua (Carthamus tinctorius),Lingyangjiao (Saiga tatarica),Shuizhi (Hirudo nipponica),Puhuang(Typha angustifolia),Chuanxiong (Ligusticum chuanxiong,Dahuang(Rheum palmatum),Shichangpu (Acorus tatarinowii),Jianghuang(Curcuma Longa),Dannanxing (Arisaema cumBile),Difuzi (Kochia scoparia).Take 100 mL with water,orally twice a day.All medicines were provided by the prescription pharmacy and Western medicine pharmacy in the treatment area of the Affiliated Hospital of Changchun University of Traditional Chinese Medicine.
Recruitment and baseline assessment
Patients with AIS will be recruited from the Encephalopathy Center of the Affiliated Hospital of Changchun University of Traditional Chinese Medicine in Changchun,four wards of the Department of Neurology,China-Japan Union Hospital of Jilin University.Recruitment strategies will include publishing recruitment advertisements on social media,online publications,and at community centers.Following written informed consent,eligible patients will be asked to complete a general information form,including personal information,medical history,treatment history and allergy history,past medical history and treatment history of other diseases.A neurologist will determine the NIHSS,BI,mRS scores and TCM syndrome elements scale scores.
Outcomes
Primary outcome.The primary outcomes will be NIHSS,BI,and mRS scores.These scores will be measured at the end of the 10 ± 1 day treatment period with a follow-up period of 90 days and compared with baseline,and changes will be compared between groups.
Secondary outcomes.The time of the patient's first defecation should be recorded,and the effective rate of the treatment of constipation symptoms should be determined with reference to the ‘Guiding Principles for Clinical Study of New Chinese Medicines’.
Safety reporting
Adverse events (AEs) were defined as any adverse or unexpected signs,symptoms,or illnesses related to the trial treatment during the study period between the two groups.Investigators should take appropriate steps to ensure patient safety and follow up all AEs until disease returns to normal or stable.Serious adverse events (SAEs) are events that may lead to permanent or severe disability or may even be life-threatening.In the event of SAEs,the principal investigator should be notified by telephone the first time(within 24 hours).The Principal Investigator reports to the Safety Monitoring Board and consults with relevant experts to develop a treatment plan.During the study,all reported or observed AEs or SAEs will be recorded in the CRF,including the nature of the event,time of onset,duration,intensity,severity (symptoms and signs),assessment of its cause,treatment options (how the event resolved or did not resolve during treatment),and outcomes.
Follow-up
A follow-up phone call will be conducted 90 days after leaving the group.During this time,they will receive the usual health care and secondary prevention provided to all other ischemic stroke patients.During the follow-up period,laxatives will be permitted as emergency medication.
Data management and quality control
To ensure the quality of this trial,the trial coordination committee and the general director will be responsible for solving related problems.To ensure data reliability,all patient personal information will be collected and stored in a separate storage room and regularly monitored by a good data management and monitoring committee(Quality Control Department of Clinical Trials,Affiliated Hospital of Changchun University of Traditional Chinese Medicine).
Statistical analysis
The collected data were statistically analyzed using SPSS 26.0 software.Chi-square test was used for counting data.Mean ±standard deviation () was used for measurement data,independent sample t test would be used for normal distribution,and Mann-WhitneyUtest would be used for skewness distribution.Differences were considered statistically significant whenP<0.05.
Discussion
Stroke is an acute cerebrovascular disease characterized by sudden coma,unconsciousness,hemiplegia,slanted tongue and speech difficulties without coma.Stroke has high morbidity,mortality and morbidity in the world,and it is the third most common cause of death in Western countries [14,15].On the one hand,the damage to the brain and body caused by stroke is multi-link,multi-path and multi-factor.Current studies have found that cerebrovascular disease can be attributed to a small proportion of single-gene genetic diseases[16].The occurrence and development of stroke may be related to the toxic effects of excitatory amino[17,18],glutamate [19] and other metabolites,activation of apoptotic genes,and inflammatory responses [20].On the other hand,stroke is a complex disease that is often accompanied by a series of neuropsychological symptoms in addition to neurological symptoms and signs[21].Therefore,stroke is not a single neurological disorder but a manifestation of an underlying systemic problem.Treatment for a single link is not enough to deal with stroke itself and the series of problems it produces.
As a supplementary and complementary medicine,traditional Chinese medicine has attracted increasing attention and has gradually been widely accepted and applied.At present,many proprietary Chinese medicines are used in combination with conventional Western medicine for the treatment of AIS.Compound Chinese medicine has been used in China and some Asian countries for thousands of years,and research in recent years has revealed its significant advantages in the treatment of complex diseases.Studies have shown that the treatment of cerebrovascular disease by traditional Chinese medicine compounds may be related to various effects,such as anti-oxidative free radical damage,inhibiting the release of excitatory amino AISds,maintaining intracellular calcium balance,inhibiting the activation of apoptotic genes,and promoting vascular endothelial growth and nerve regeneration [22].Promoting the recovery of neurological function is the key to improving the prognosis of stroke patients.Integrative medicine therapy has become an optimized method to improve neurological function in patients with AIS.Relevant Chinese treatment guidelines note that Chinese patent medicines can play an important role in the treatment of AIS [23].A number of clinical systematic reviews have reported that Chinese patent medicine adjuvant therapy for AIS can improve clinical efficacy.For example,an RCT showed that the total effective rate of adding the Chinese patent medicine Naoxintong Capsule for AIS was higher than that of routine treatment.A study that included 58 meta-analyses showed that the clinical efficacy of Naoxintong capsule combined with conventional Western medicine in the treatment of cerebral infarction was 22% higher than that of Western medicine alone,and it could significantly reduce the neurological deficit score and hemorheological indexes of patients,making it more secure [24].Danhong injection has remarkable curative effect and is a representative traditional Chinese medicine injection for the treatment of AIS.In addition,Danhong injection and other drugs have also been approved by the China Food and Drug Administration for the treatment of AIS [25-27].
XNJS is an effective prescription for the clinical treatment of stroke.Among them,the active ingredient of the most important drug Puhuang,Puhuang flavonoid,can improve the activity of human umbilical vein endothelial cells under hypoxia,increase the content of nitric oxide (NO) and prostaglandin (PGI2) in cells,and reduce the content of ET-1.It has a protective effect on vascular endothelial cells damaged by hypoxia.Moreover,total flavonoids of Puhuang may inhibit Akt/mTOR to activate autophagy and achieve anti-inflammatory protective effects [28,29].Ligustrazine has a variety of cardiovascular and cerebrovascular pharmacological effects,such as protecting the vascular endothelium,resisting platelets,resisting ischemia-reperfusion injury,and resisting oxidative stress.Importantly,it can pass through the blood-brain or blood-eye barrier[30].In the study of Guan D et al.,it was found that the bioactive component tetramethylpyrazine in ligustrazine can reverse the hypoxic injury of PC12 cells in vitro,which may be related to the activation of the Nrf2/GCLc/GSH pathway and the inhibition of the expression of HIF1-αNOX2/ROS [31].Rhein is the main active ingredient in Chinese herbal decoction pieces of rhubarb [32].Rhein can reduce the degree of cerebral edema caused by cerebral ischemia injury,and its mechanism may be related to its inhibition of neuroinflammation mediated by microglia and downregulation of aquaporin 4(AQP4)protein expression[33].Modern pharmacological studies have found that crocin can inhibit the transport of G protein-coupled receptor kinase 2 (GRK-2) and prevent the phosphorylation of ERK1/2,thereby reducing secondary injury after ischemia[34].
The classic evidence-based scale results suggest that RCTs have become the gold standard available for evaluating the effects of medical interventions and are designed as unbiased experimental practices that greatly reduce the risk of systematic error [35].Of course,RCTs also have research limitations and deficiencies,especially in the field of disease research on this topic.AIS is dangerous and progresses rapidly,and complications and sequelae are also important considerations that cannot be ignored.In addition,the therapeutic effect of the combination of traditional Chinese medicine and Western medicine is being explored,so it is difficult to carry out a randomized,double-blind,multi-center clinical controlled trial in an ideal state.For example,the disadvantages mentioned in an article introducing "the advantages and limitations of randomized clinical trials of each type of stroke" are that RCTs require a lot of time and energy and increase the pressure of communication between doctors and patients [36].To carry out TCM clinical trials of stroke more effectively and make it feasible and authentic,non-randomized interventional trials can be designed.Many scholars at home and abroad also believe that it is indispensable.Non-randomized controlled trials are as important as RCTs,and in some specific cases,they can make up for the shortcomings of RCTs [37].Theoretically,RCTs have good internal validity but may have poor external validity,whereas non-randomized designs may do the opposite.Judging from the feasibility and authenticity of clinical trials,this clinical research method is gradually enriching the evidence-based basis for medical research.
We will combine the evaluation scale of modern medicine with the evaluation standard of traditional Chinese medicine,which can comprehensively evaluate the clinical efficacy from the perspective of traditional Chinese and Western medicine,which not only reflects the advantages and characteristics of traditional Chinese medicine but also reflects the evaluation of the efficacy of Western medicine.In addition,the use of non-randomized controlled studies can better ensure the external validity,feasibility and authenticity of this trial.
However,this study has some limitations,such as the lack of long-term effect assessment of the primary outcome measure of XNJS and the relatively short follow-up time.Due to limited time,the potential role of prescribing in reducing overall mortality and major vascular events remains uncertain.Therefore,future clinical trials should include longer follow-up.We took the patient's blood samples for further biochemical testing to discuss the possible mechanism and safety evaluation of XNJS's effectiveness in the treatment of AIS.Since the evaluation index of cerebral infarction recovery does not depend on radiation/pathology,it may be more meaningful to improve sequelae,restore neurological function,and relieve the painful symptoms of the patient.Later,randomized controlled trials with larger sample sizes will be considered.The pharmacological mechanism of XNJS components and the determination of some active compounds also need to be further studied.In addition,this pilot study also has shortcomings such as insufficient sample size,which will lead to biased results and reduce the reliability of clinical evidence.Finally,since this study is based on the standard treatment to observe the preliminary efficacy of XNJS,we are still unable to determine whether XNJS can be used as a complete replacement therapy for AIS.
杂志排行
Clinical Research Communications的其它文章
- Not just a sponge:novel functions of circRNAs in cholangiocarcinoma
- POEMS syndrome characterized by peripheral neuropathy as the first symptom: a rare case report
- Effectiveness of hip upslip correction method on ambulation and back pain in patients with lumbar discopathy:a case report
- Meta-analysis of the efficacy and safety of Shenkang injection combined with Western medicine in the treatment of chronic glomerulonephritis
- A study of ultrasonography for the treatment of Qingre Liangxue decoction on blood-heat psoriasis
