Profile of biological characterizations and clinical application of corneal stem/progenitor cells
2022-12-02PeiXiYingMinFuChangHuangZhiHongLiQingYiMaoShengFuXuHuiJiaYuChenCaoLiBingHongLiYangCaiXiGuoRuBingLiuFankeMengGuoGuoYi
Pei-Xi Ying, Min Fu, Chang Huang, Zhi-Hong Li,Qing-Yi Mao, Sheng Fu, Xu-Hui Jia, Yu-Chen Cao, Li-Bing Hong,Li-Yang Cai, Xi Guo,Ru-Bing Liu, Fan-ke Meng, Guo-Guo Yi
Abstract
Corneal stem/progenitor cells are typical adult stem/progenitor cells. The human cornea covers the front of the eyeball, which protects the eye from the outside environment while allowing vision. The location and function demand the cornea to maintain its transparency and to continuously renew its epithelial surface by replacing injured or aged cells through a rapid turnover process in which corneal stem/progenitor cells play an important role. Corneal stem/progenitor cells include mainly corneal epithelial stem cells, corneal endothelial cell progenitors and corneal stromal stem cells. Since the discovery of corneal epithelial stem cells (also known as limbal stem cells) in 1971, an increasing number of markers for corneal stem/progenitor cells have been proposed, but there is no consensus regarding the definitive markers for them.Therefore, the identification, isolation and cultivation of these cells remain challenging without a unified approach. In this review, we systematically introduce the profile of biological characterizations, such as anatomy, characteristics, isolation, cultivation and molecular markers, and clinical applications of the three categories of corneal stem/progenitor cells.
Key Words: Corneal epithelial stem cells; Corneal endothelium stem cells; Corneal stromal stem cells;Bioengineering; Gene markers
INTRODUCTION
The cornea acts as a structural barrier to protect the eye from the outside environment. Transparency of the cornea allows the light to enter the retina and has a very good ability to refract. The cornea is elliptical-shaped horizontally, 11.5-12.0 millimetres long horizontally and 10.5-11.0 millimetres long vertically[1]. The thickness of the cornea increases gradually from the centre (approximately 0.5 millimetres) to the periphery (approximately 1 millimetre)[2]. The cornea accounts for two-thirds of the refractive power of the eye, which is why corneal integrity is important for the maintenance of vision[3].
The cornea is composed of cellular and acellular components. Cell components include epithelial cells, keratocytes and endothelial cells as well as neural and immune cells[4]. The cell-free components include collagen and glycosaminoglycans. Corneal epithelial cells are derived from the epidermal ectoderm, while stromal cells and endothelial cells are derived from the neural crest. The corneal layer includes the epithelium, Bowman membrane, stroma, Descemet membrane and endothelium (Figure 1).
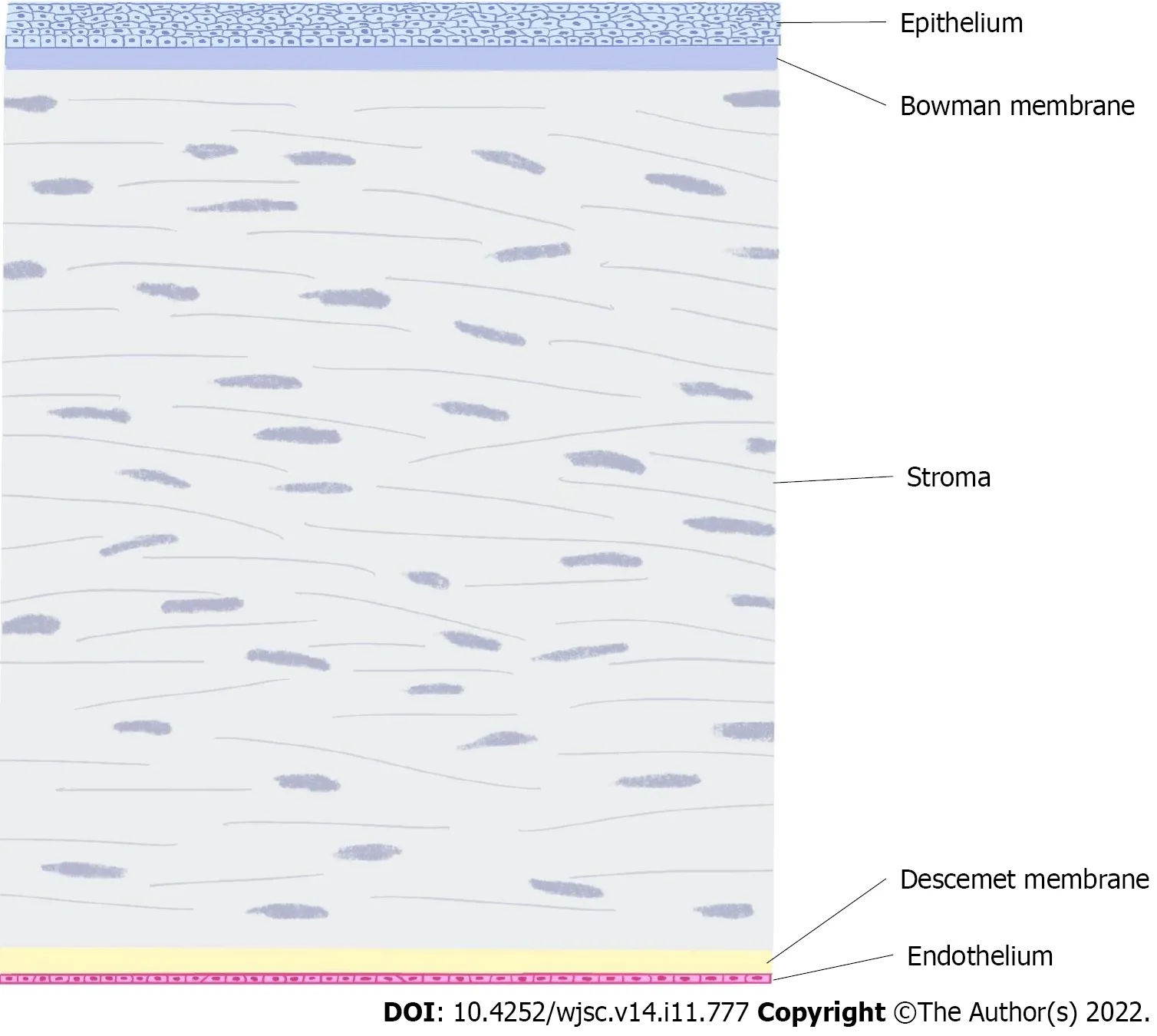
Figure 1 Corneal stratification. The corneal layer includes the epithelium, bowman membrane, stroma, Descemet membrane and endothelium.
Recovery after corneal injury depends mainly on the regeneration of stem cells or progenitor cells.Corneal trauma and disease are highly associated with fibrosis, which can easily lead to severe visual impairment. In particular, the lesions of the central part of the cornea will seriously affect vision. After the corneal epithelium is damaged, it can regenerate without scarring[3]. However, when the damage affects Bowman’s layer, its ability to regenerate is very limited, and Bowman’s layer will be replaced by scar tissue. The stromal layer plays an important role in maintaining corneal transparency and resisting intraocular pressure and is also repaired by scar tissue after injury, resulting in the loss of transparency.The injury of Descemet’s membrane can be regenerated by endothelial cells. In the past, damage to the endothelium was generally believed to be difficult to regenerate and could only cover the deficit left by injury through cell enlargement and migration. Although still controversial, in recent years, there has been increasing evidence supporting the existence of corneal endothelial progenitor cells[5-7].
At present, the most commonly used method to treat corneal injury is corneal transplantation, and research on stem cells or progenitor cells will provide great help. In 1971, Wolosin et al[8] proposed the concept of limbal stem cells (LSCs), suggesting that epithelial corneal stem cells exist in the limbal gland.LSC deficiency (LSCD) is a serious disease that causes permanent corneal injury and visual loss due mainly to various kinds of corneal injuries and chronic immune diseases. LSCD has become a hot research direction in recent years, and new treatments are emerging. LSCs play an important role in repairing various kinds of corneal injuries and chronic immune diseases. In 2005, Du et al[9] found the first stem cell-like precursor cells of human corneal stromal cells. Similarly, corneal endothelial progenitors with the ability to self-renew and differentiate into mature corneal endothelial cells have also been identified, although corneal endothelial cells were widely believed not to be able to regenerate in humans. These stem cells have attracted great attention from the public, and an increasing number of people have devoted themselves to the study of their anatomy, physiology, immunology, cell culture and isolation.
It is essential to understand the anatomy, characteristics, methods of isolation and cultivation,molecular markers and therapeutic potential and applications of corneal stem/progenitor cells. We briefly discuss the three types of stem/progenitor cells of the cornea in this review.
CORNEAL EPITHELIAL STEM CELLS
Anatomy
Human corneal epithelial stem cells have been proven to be situated in the basal layer of the limbal epithelium, so they are also known as LSCs[10]. Pathologically, it is generally believed that the anterior limbus is situated in the plane connecting the end of the Bowman membrane and the Descemet membrane, while the posterior limbus is located in the plane passing through the iris root and perpendicular to the ocular surface. In addition, ophthalmic surgeons should be proficient in the gross anatomy of the limbal, which is the incision for most cataract and glaucoma surgeries. Surgically,limbus is known as the grey or blue zone because this transition zone shows a blue-grey appearance when viewed externally after the conjunctiva has been reflected away from the limbus[11].
Moreover, due to high vascularization and neuralization, the limbus is nutrient-rich and perceptive,and protected from potential ultraviolet (UV) damage by melanin pigmentation[4]. The corneal epithelial stem cell region is only 1.5-2.0 mm wide in the basal layer of the corneal epithelium, a small portion of the entire tissue, and is estimated to account for 0.5% or less to 10.0% of the total number of cells in palisades of Vogt[8,12,13] (Figure 2).
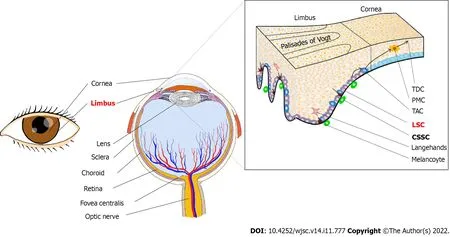
Figure 2 Location of limbal stem cells and corneal stromal stem cells. Limbal stem cells (LSCs) are located at the base of the limbus and are in close contact with niche cells, including melanocytes. LSCs are symmetrically divided into two identical cells in the horizontal plane or asymmetrically differentiated into another LSC and a transient amplifying cell (TAC) in both vertical and horizontal planes. Then, TACs are divided into postmitotic cells (PMCs) as they migrate centripetally. The PMCs are then differentiated into terminally differentiated cells (TDCs) and shed from the corneal surface. Corneal stromal stem cells are in the anterior stroma subjacent to the epithelial basement membrane, in regions where the basement membrane has muslimah and folds termed the Palisades of Vogt.LSC: Limbal stem cell; TAC: Transient amplifying cell; PMC: Postmitotic cell; TDC: Terminal differentiated cell; CSSC: Corneal stromal stem cell.
Cell migration is one of the most basic elements of epithelial homeostasis. A mathematical analysis of the kinetics of maintenance of corneal epithelial mass confirms that the corneal epithelium can be maintained by the centripetal migration of epithelial cells of limbal origin[14,15]. Currently, it is widely accepted that LSCs generate transient amplifying cells (TACs), which then migrate centripetally and anteriorly to give rise to differentiated corneal epithelial cells that eventually fall off the surface of the cornea, as proposed in the X-Y-Z hypothesis[16,17]. LSCs are particularly important in maintaining corneal epithelial homeostasis and normal corneal wound healing. In addition, acute damage to the central cornea can be repaired by the proliferation and migration of central corneal epithelial cells[18].
Characteristics
Low differentiation with a primitive phenotype: Stem cells have long been recognized to be primitive cells with little differentiation. In 1986, Schermer et al[19] proposed the limbal location of corneal stem cells based on differentiation-related expression of 64K keratin. Immunohistochemical data showed that limbal basal cells were the least differentiated of all corneal epithelial cells. Moreover, cytokeratin K3 and K12, as specific markers of the corneal epithelial phenotype, were not expressed in limbal basal epithelial cells. Therefore, many studies have also confirmed the low differentiation phenotype of LSCs[20,21]. Many studies have shown that some materials or cells [such as Frizzled 7, HC-HA/PTX3 and human limbal melanocytes (hLM)] can maintain the low differentiation state of LSCs[22] (Niche regulation of limbal epithelial stem cells: HC-HA/PTX3 as a surrogate matrix niche) (Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments).
Long cell cycle and high proliferative potential: A long cell cycle indicates low mitotic activity. Most stem cells are in a state of steady growth, so the proliferation rate is extremely low[23]. However, the high proliferative potential of stem cells can be activated under injury or in vitro culture. Taking advantage of several animal models and ex vivo human limbal cultures, the existence of slow-cycling and label-retaining cells in the basal layer of the limbal basal epithelium was proven by previous studies[24-27]. Multiple studies on proliferation potential in the presence of injury, absence of injury, in vitro culture, or differentiation-inducing agents have further supported the idea that corneal epithelial stem cells are located in the limbus[28-32]. Recent studies by Sagga et al[33] quantified the proliferative dynamics of LSCs during corneal wound healing. They found that the cell circulation of central corneal epithelial cells in young mice (4.97 d ± 0.50 d) was significantly slower than that in ageing mice (3.24 d ±0.20 d). In wounded eyes, the proportion of LSCs entering S phase within 24 h in young mice increased 7 times compared with that in uninjured mice, but no significant increase was observed in aging mice.The contribution of LSCs in regenerative ophthalmology has demonstrated that LSCs can also be used to reconstruct the entire corneal epithelium in the case of severe ocular surface injury[34]. Additionally,the location of corneal epithelial stem cells in the limbus may account for the relative superiority of limbal neoplasms and the rarity of corneal epithelial tumours[35-37].
Capacity for unlimited self-renewal and error-free proliferation: According to the result of division,the division of stem cells is divided into symmetric division and asymmetric division. Symmetric division occurs when a stem cell divides into two identical daughter stem cells, while asymmetric division occurs when a stem cell divides into two different cells. Lamprecht et al[38,39] first reported asymmetric division of mammalian corneal epithelial stem cells. Using differences in cell size, nuclear chromatin condensation, and cytoplasm density as criteria for histological analysis, they found that mitosis of corneal epithelial stem cells can be classified according to the position relationship between the spindle axis and basal lamina: Vertical mitosis (spindle axis at 60-90 degrees from basal lamina);oblique mitosis (spindle axis at 30-60 degrees from basal lamina); horizontal mitosis (spindle axis 0-30 degrees from basal lamina). Among these relationships, the daughter cells produced after horizontal mitosis were all located in the basal lamina, and their morphology and proliferation ability were similar to that before mitosis. The daughter cells produced after vertical mitosis and oblique mitosis were different in morphology and proliferation potential. The cells located in the basal lamina still had proliferation ability, while the cells located above the basal lamina developed towards the direction of terminal differentiation. The asymmetric division of corneal epithelial stem cells not only contributes to the replenishment of the stem cell pool but can also renew the corneal epithelium in time to cope with accidents or diseases such as corneal injury. This process of division does not allow errors because any genetic error at the stem cell level will continue to be transmitted to the entire cell clone, leading to abnormal differentiation and cellular dysfunction.
Morphological criteria: Both confocal microscopy and flow cytometry have shown that the smallest cells are located in the limbal basal epithelium rather than the corneal basal epithelium[40]; electron microscopy showed that limbal epithelial basal cells were characterized by immature cells, such as small cells, rich in tonofilaments in the cytoplasm, euchromatin-rich nuclei, almost undetectable nucleoli and a high nuclear-cytoplasmic ratio[41].
Isolation and cultivation
There are numerous of ways to isolate cells, one of which uses enzymes. In 2010, Yamamoto et al[42]cultured human corneal epithelial cells in serum-free medium by an enzyme-treated cell culture method. The corneal epithelium was separated from the limbus of the corneal peripheral area, and the corneal endothelium was removed. The limbus was then carefully separated from the underlying matrix with 0.25% collagenase and Accumax (cell aggregation disintegration medium). Finally, corneal epithelial cells were cultured in serum-free PCT corneal epithelial medium. The results showed that compared with human corneal epithelial cells (HCEC2 cells), the survival rate of the corneal epithelial cells obtained in this experiment increased significantly after being cultured on CNT-20 medium to the sixth generation, indicating that the enzyme separation method could maintain cell viability.
Recent studies have shown that flow cytometry and immunofluorescence activation can be used to isolate cells. Shaharuddin et al[43] isolated and identified LSCs using an optimized limbal side population (LSP) regimen, including an optimized Hoechst concentration, Hoechst incubation time and inhibitor concentration. After preoptimization, cells isolated from tissues were bound to a DNA-binding dye, Hoechst 33342, mediated by a TP-binding box (ABC) transporter. Then, two types of cells, Hoechst Blue (SP) and Hoechst Red (non-SP), were obtained by flow cytometry analysis and fluorescentactivated cell sorting. Compared with non-SP cells, the expression of the stem cell markers ABCG2, Δ Np63 and SOX2 was significantly higher in SP cells according to the immunofluorescence assay.Shaharuddin et al[43] demonstrated that the LSP protocol identifies and enriches LSCs by preoptimizing key parameters.
Based on previous studies, Nam et al[44] cultured corneal epithelial cells on the canine amniotic membrane. Under a light microscope, the cultured cells fused 100% after 7-8 d, and 5-8 Layers of epithelial cells were formed on the amniotic membrane. K3 (a corneal epithelial-specific marker) was observed in all cultures by immunofluorescence, while ABCG2, P63, and vimentin (a stem cell marker)were observed only in the basal layer. The results showed that beagle corneal epithelial cells could be cultured on the canine amniotic membrane, and the basal layer cells might be stem cells. Moriyama et al[45] cultured equine LSCs in standard supplemental hormone epithelial medium (SHEM). Cells isolated from LEC tissue extracted from the limbs were cultured in standard SHEM. Immunostaining showed positive p63, CK14, and negative CK3 cells, similar to the expression pattern of limbal epithelial basal cells, suggesting that LSCs could be obtained by this method.
In addition, different temperatures also affect the separation from the cells. Nam et al[46] compared the effects of different media (canine amniotic membrane, heterotopic collagen gel, temperaturesensitive culture dish) on corneal stem cell culture. Cells were isolated from beagle corneal epithelial cells and cultured on canine amniotic membrane, collagen gel and temperature-sensitive culture dishes.Ki-67, K3, ABCG2 and P63 were used as indices. Immunofluorescence and real-time quantitative polymerase chain reaction (RT-PCR) were used to observe the culture time and the number of cell layers adhered to and fused. The specific results are shown in Table 1.
RT-PCR showed that the expression level of ABCG2 mRNA was 9.9 times larger on the canine amniotic membrane than on the atelocollagen gel and 7.2 times larger than on the temperatureresponsive culture dish. The expression level of P63 on atelocollagen gel was 2.8 times and 3.2 times higher than that on canine amniotic membrane and temperature-responsive culture dishes, respectively.The atelocollagen gel-cultured corneal epithelium showed morphology similar to the normal corneal epithelium and retained more stem cells/progenitors than the canine amniotic membrane and temperature-responsive culture dish.
Compared to the canine amniotic membrane, the human amniotic membrane (HAM) is the most common scaffold for culture, both experimentally and clinically. Kim and Tseng[47] were the first to use HAM for clinical treatment. They transplanted HAM alone into corneas with mild LSCD that did not require LSC treatment to promote re-epithelialization. Subsequently, researchers began to study the possibility of HAM as an amplification carrier of LSCs. From the early coculture with the mouse 3T3 fibroblast feeding layer[48], animal-free amplification methods have been designed to avoid the risk of zoonosis[49,50]. With the deepening of the research, HAM was found to have increasing advantages in the aspect of corneal transplantation, including anti-inflammatory, antimicrobial, antiangiogenesis,antifibrosis, secretion of neurotransmitters and growth factors, finally reducing scar formation,stimulating the epithelialization and differentiation of corneal epithelial cells as well as enhancing adhesion and preventing apoptosis[51,52]. Today, HAM can play a role in a variety of corneal injury diseases or postoperative treatment, including refractory corneal ulcers and corneal epithelial defects,malignant tumours or pterygium resection[53].
The above studies (Table 2) provide a variety of methods for the isolation and culture of LSCs.Enzymatic dissociation is one of the most common methods to disperse tissues into single cells[54-56].According to the differences in cell and interstitial composition between different tissues, trypsin or collagenase digestion is used to achieve cell separation. Compared with separating cells with only enzymes, the LSP protocol preoptimizes the concentration of dyes and inhibitors, staining time, etc.,which is beneficial to the identification and enrichment of LSCs. In the cultivation of LSCs, a variety of substances can be used as culture media for LSCs, such as media supplemented with hormones, animal materials (canine amniotic membrane), HAM, collagen gels and temperature-responsive culture dishes.Compared with animal materials and temperature-responsive culture dishes, cells cultured in collagen gel are more similar in morphology to normal corneal epithelial cells and contain more stem cells.

Table 1 Culture conditions of limbal stem cells
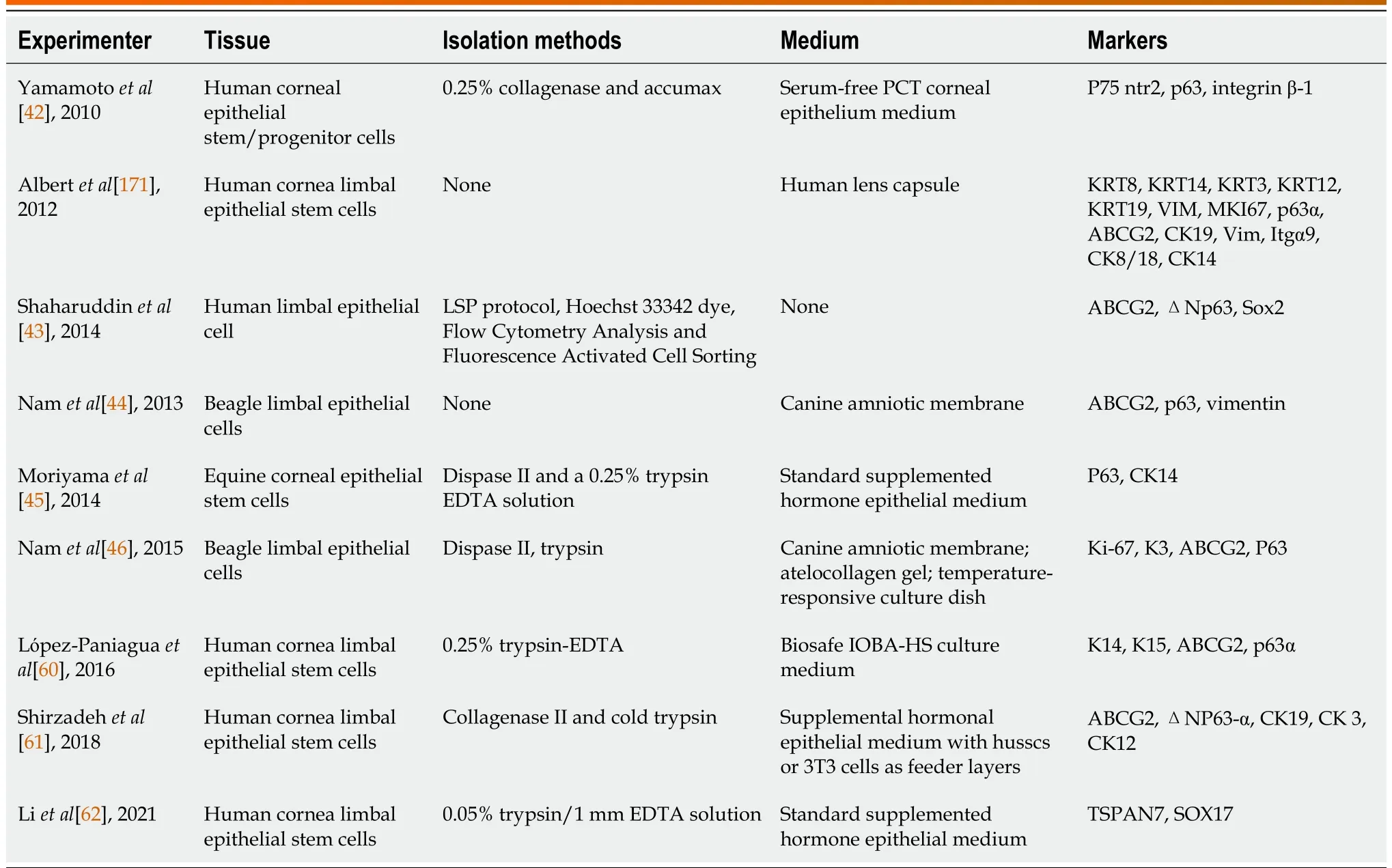
Table 2 Methods for the isolation of limbal stem cells
However, there are some disadvantages in culturing LSCs with HAM and animal materials. For example, the amniotic membrane has obvious biodegradation and immunosuppressive properties during transplantation. Its thickness and variable transparency are also disadvantages. Moreover, the use of feeder cells or a composite medium containing growth factors or animal materials may also cause biological contamination or other safety issues(such as prion transfer or unknown diseases)[57-62].Therefore, more improvements or alternative methods are needed.
Molecular markers for LSCs
Cytoskeletal proteins: In recent years, corneal transplantation has become a research hotspot of corneal epithelial stem cell gene markers. Moriyama et al[45] studied the eye tissues of 12 adult thoroughbred horses. They used immunohistochemical staining for negative markers (CK3) and positive markers (P63,CK14)[63] to determine the distribution and culture of equine corneal epithelial stem cells (CESCS). The experimental results showed that P63 played an important role in the proliferation of human keratinocytes and was expressed in basal cells of various human epithelial tissues and was a stem cell marker[64]. Moreover, the results indicated that 13,14,24-26,31,33 CK14[65] is a useful indicator for the identification of epithelial progenitor cells with basal cell activity, as well as a marker of stem cells. In addition, among these stem cell markers, antihuman P63, antiCK14, and CK3 antibodies can cross-react with equine corneal epithelial cells[66]. These research results are of great significance for follow-up clinical treatment.
Cytosolic proteins: Lyngholm et al[67] used a proteomics method combining two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and liquid chromatography-tandem mass spectrometry (LCMS/MS) to study the difference in protein expression between human central corneal epithelium and limbal epithelial cells. A total of 25 different proteins were identified. Superoxide dismutase 2 (SOD2) is expressed in the basal limbal epithelial cell population; heat shock protein 70 protein 1 (HSP70.1) and annexin I are expressed at higher levels in the limbal epithelium, but there is also a small amount of expression in the central epithelium. They also found that SOD2 appears almost exclusively in the limbal epithelium of the basal cornea. SOD2 induces phosphorylation and activation of mitogenactivated protein kinases (MAPKs) by regulating H2O2and activates signalling molecules, including extracellular regulatory kinases (ERKs) and c-Jun N-terminal kinase (JNK)[68].
In addition, the Notch-1 gene plays an important role in corneal stem cell transplantation, and related research on this gene has also been reported in recent years. For example, Thomas et al[69] obtained human corneoscleral tissue from the Doheny Eye & Tissue Transplant Library through explant culture and primary culture and performed cross-sectional and full-thickness analysis. Their experiment studied mainly the expression of Notch 1 in the basal epithelium of the limbus, and the results showedthat Notch-1 was expressed in the area of limbus stem cells. The antigenicity of Notch-1 is more obvious in the cell mass, mainly in the fence of Vogt. There is almost no Notch-1 in the centre of the cornea. In addition, ABCG2[70] and Notch1 double staining showed that some ABCG2-positive cells coexpressed Notch-1 in the limbus basal epithelium, suggesting that Notch-1-expressing cells may be a unique subgroup with stem cell characteristics. This result suggests that further research and characterization of the Notch pathway will provide valuable clues for clinical research on stem cell transplantation.
Neuronal markers: In addition, the interaction between KLF, PAX-6 and ESE-1 has important research value in human corneal development and physiology. The regulation of KLF family members by K12 is closely related to cell growth, proliferation and proliferation. GKLF/KLF4 participates in p53 transactivation of p21 WAF1/Cip1 promoter induction, cyclin D1 promoter activity inhibition and cell proliferation inhibition, which indicates that this protein may play a direct role in negative growth control[71]. A recent study found that KLF4 can block the epithelial to mesenchymal transition (EMT) of human corneal epithelial cells (HCECs) by inhibiting the canonical TGF-β signalling pathway.Therefore, KLF4 plays a crucial role in the maintenance of HCEC homeostasis and has the potential to prevent the formation of corneal fibrosis scars[72].
Pinin: Pinin (PNN) is an exon junction component (EJC) that is important in the differentiation of corneal epithelial cells and can act as a stabilizer for the corneal epithelial phenotype. Using RNA-seq to obtain the results, PNN knockout-upregulated genes included a large number of genes related to cell migration and ECM remodelling processes, such as MMPs, ADAMs, HAS2, LAMA3, CXCRs, and UNC5C. Genes knocked down by PNN gene knockout included IGFBP5, FGD3, FGFR2, PA X6, RARG,and SOX10[73].
Recent research on LSC deficiency
Causes of LSC deficiency: LSCD is a pathological condition caused by dysfunction and/or insufficient quantity of LSCs, which is marked by impaired barrier function of the limbus and the replacement of corneal epithelium by conjunctival epithelial cells[74,75]. Direct damage to LSCs and/or destruction of their niche microenvironment is the causes of their pathogenesis. Loss of stem cells due to severe damage to the limbal can result in permanent corneal epithelial defects and vision loss due to the conjunctiva[76]. Moreover, although LSCD can be divided into unilateral LSCD and bilateral LSCD,their causes are similar. Burns are the most common cause, followed by Stevens-Johnson syndrome(SJS), atopic keratoconjunctivitis (AKC)/vernal keratoconjunctivitis (VKC) and mucous membrane pemphigoid (MMP). Since congenital aniridia is a disorder of two eyes caused by haploinsufficiency of the Pax6 gene, it is only the cause of bilateral LSCD[77]. Additionally, the primary burns are alkali burns. Thermal burns, acid burns, radiation burns and others account for only a small percentage[78,79]. The conditions that lead mainly to LSCD include two main categories, hereditary LSCD and acquired LSCD, as shown in Table 3[80-115].
Diagnosis of LSCD: The main symptoms and signs of LSCD are[75,116]: (1) Nonspecific symptoms,including vision loss, photophobia, lacrimatorrhea, blepharospasm, redness associated with chronic inflammation, and recurrent episodes of pain caused by epithelial lesions; (2) signs: Under a slit-lamp biomicroscope, the corneal epithelium shows three grades of damage including mild, moderate and severe/total LSCD: Mild grade: Dull or irregular corneal surface without light reflex, opaque corneal epithelium and deterioration of limbal palisades of Vogt; moderate grade: A vortex pattern of abnormal epithelium with fluorescein staining, superficial vascularization of the cornea and peripheral pannus;and severe grade: Thick fibrous pannus formation, chronic keratitis, scarring and corneal conjunctivization; and (3) the migration of conjunctiva and goblet cells to the corneal surface was confirmed by impression cytology.
In addition, there are some new advances in the diagnosis of LSCD involving in vivo laser scanning confocal microscopy and anterior segment optical coherence tomography.
Basic treatment principles and methods of LSCD: Treatment strategies for LSCD can generally be divided into two categories: (1) Conservative treatment, including conservative nonsurgical options and conservative surgical options; and (2) Invasive treatment, which means transplantation aimed at repairing the structure and function of the corneal epithelium[117] (Table 4).

Table 3 Causes of limbal stem cell deficiency
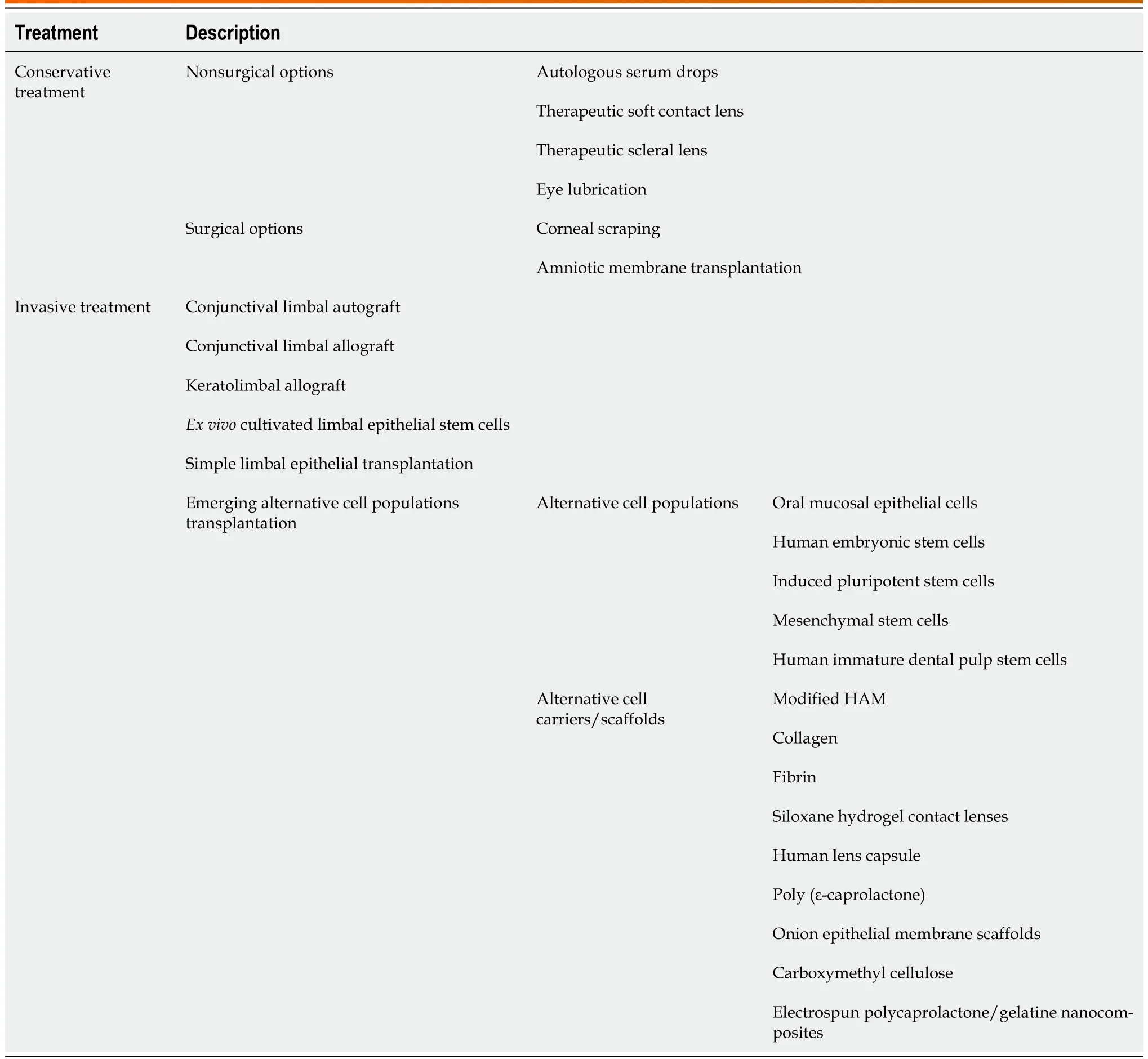
Table 4 Treatment strategies for limbal stem cell deficiency
Conservative treatment: (1) Conservative nonsurgical options: Autologous serum drops[118],therapeutic soft contact lenses[119], therapeutic scleral lenses and eye lubrication[114]; (2) conservative surgical options: Corneal scraping[120] and amniotic membrane transplantation (AMT)[53,121]. AMT is effective in patients with partial or mild LSCD[122]. However, long-term debilitated vision remained in those with severe LSCD caused by burns[123]. In recent years, LSC deficiency has been successfully treated by direct transplantation of a portion of healthy limbal tissue or LSC, even some other alternative cells populations.
Transplantation: (1) Conjunctival limbal autograft (CLAU)[124,125]; (2) conjunctival limbal allograft(CLAL)[86,126]; (3) keratolimbal allograft (KLAL)[127,128]; (4) ex vivo cultivated limbal epithelial stem cells (CLET); (5) simple limbal epithelial transplantation (SLET); and (6) alternative cell population transplantation. In CLAU, CLAL and KLAL, since the long time for limbus transplantation from the donor to the stem cell deficient eye, healthy donor eyes have an increased risk of LSCD. In addition, the application of immunosuppressants in CLAL and LSCD may increase the risk of infection or cancer.CLET is a technique in which autologous or allogeneic LSCs are cultured on a carrier for transplantation, which greatly reduces the incidence of LSCD in healthy donors[129,130]. In addition,since LSCs cultured ex vivo do not differentiate into Langerhans cells, the incidence of immune rejection will also be decreased. SLET is a technique to evenly distribute autologous limbal tissue and attach it to HAM for transplantation. SLET not only holds the advantages of CLET in reducing the incidence of LSCD and immune rejection but also avoids the difficulties of ex vivo culture technology, which achieves higher economic benefits[131-133]. However, the risk of disease transmission is increased due to the application of carriers such as the HAM[134].
Emerging alternative therapies for LSCD: In addition to the above traditional treatments for LSCD performing limbal tissue or stem cell transplantation, advances in tissue engineering have led to thedevelopment of a growing number of emerging therapies, including alternative cell populations and alternative cell carriers/scaffolds.
Alternative cell populations: Compared with traditional transplants, the application of alternative cell populations avoids corneal donor shortages and the risk of disease transmission, graft rejection and tumours (due to immunosuppressant application) associated with allografts, which makes it possible to replace traditional transplants. Currently, the alternative cell populations include oral mucosal epithelial cells[135,136], human embryonic stem cells (HESCs)[137], induced pluripotent stem cells (iPSCs)[138,139], mesenchymal stem cells (MSCs)[140,141], human immature dental pulp stem cells[142,143], etc.Among these alternatives, MSCs and iPSCs are of vital importance and will be described in detail.
MSCs are a population of proliferative and multipotent stem cells present in various tissues throughout development. In the cornea, MSCs are natural residents of the LSC niche and can modulate immune response via paracrine action[144]. Additionally, MSCs have been shown to reduce neovascularization, stromal opacification, inflammation, and corneal oedema in animal models of LSCD secondary to chemical or mechanical injury, which offers advantages in corneal reconstruction[145,146].The therapeutic effect of MSCs in regenerative corneal therapy can be attributed to direct cell replacement[147], differentiation into corneal epithelial-like cells[148] and secretion of soluble factors to regulate tissue wound repair, inflammation, angiogenesis and the immune response. Many studies have shown that MSCs have a wide range of applications, whether in vivo or in vitro, to help repair corneal epithelium[148-151], corneal stroma[152,153] and even corneal endothelium[154]. Therefore, MSCs have the potential to be differentiated into corneal cell types and can be potential candidates for regeneration of the cornea.
iPSCs are a population with pluripotent capacity to differentiate into many cell types and are generated from embryonic or adult body cells[155,156]. In 2006, the iPSC technique was first reported by Takahashi and Yamanaka, who dedifferentiated mouse fibroblasts into embryonic stem cell-like cells,which they named “iPSCs”[155]. iPSCs have self-renewing and multidirectional differentiation potential, which could generate corneal organoids expressing markers of adult corneal tissue[157] and displaying similar features of the developing cornea[158]. iPSCs are easy to obtain, and their autologous transplantation can avoid immune rejection, which has good prospects in the treatment of LSCD in the future.
Alternative cell carriers/scaffolds: HAM is the most commonly used cell carrier for ocular surface reconstruction. However, due to the disadvantages of HAM (such as high thickness, variable transparency, biodegradation and immunosuppression), it is urgent for us to find alternative cell carriers/scaffolds to substitute for HAM. At present, alternative cell carriers/scaffolds contain modified HAM, collagen[159,160], fibrin[161], siloxane hydrogel contact lenses, human lens capsules, poly (εcaprolactone)[162], onion epithelial membrane scaffolds[163], carboxymethyl cellulose (CMC)[164],electrospun polycaprolactone/gelatine nanocomposites[165] and other emerging materials. The modified HAM will be described in detail below.
Modified HAM is initiated by chemical modification of HAM to avoid or mitigate the above shortcomings. Chemically modified HAMs have been developed using cross-linking agents, including glutaraldehyde[166], carbodiimide[167,168] and aluminium sulfate. Compared with glutaraldehyde,carbodiimide has lower cytotoxicity and the addition of L-lysine enhances the mechanical and thermal strength, the ability to support LESCs and the enzyme digestion resistance of HAM[169]. Additionally,aluminium sulfate cross-linked HAM remains sterile and shows increased tensile strength during 12 mo of storage[170].
Albert et al[171] cultured LSCs in animal-free medium-that is, the lens capsule with human serum as the only growth supplement. The results showed that the viability of LSCs cultured on human LC was greater than 97% at the two checkpoints (day 7 and day 14), and the percentages of early apoptotic cells and late apoptotic cells were lower. Immunofluorescence showed that the resulting cells maintained their pluripotency by maintaining p63, ABCG2, CK19, Vim and Itg9 and low ck3/12 expression. The presence of differentiation characteristics (positive for CK8/18 and CK14) also indicates its potential for orthotopic differentiation into the corneal epithelium. All the above results indicated that LSCs could be cultured from lens capsules using human serum as the only growth supplement without the disadvantage of animal medium.
CORNEAL STROMAL STEM CELLS
Anatomy
The stroma, which makes up approximately 90% of the cornea, is a collagenous mesenchymal tissue composed of approximately 200 orthogonally oriented lamellae. Each lamella is made up of long uniform collagen fibrils lying parallel to one another with regular interfibril spacing, which is essential in rendering the tissue transparent[172]. Many fibroblast-like cells distributed in the matrix are commonly known as corneal stromal cells. The corneal stroma is derived from the neural crest, which is the source of mesenchymal tissue in the head and neck. Corneal stromal cells have extensive cytoplasmic processes in contact with similar processes in neighbouring cells.
By staining with ABCG2 and PAX6 proteins, corneal stromal stem cells were observed largely in the transitional zone between the cornea and sclera known as the limbus[173]. More specifically, CSSCs are in the anterior stroma subjacent to the epithelial basement membrane, in regions where the basement membrane has Muslimah and folds termed the Palisades of Vogt (Figure 2)[173,174].
Characteristics
Under normal physiological conditions, corneal stromal cells remain stationary in mitosis and maintain a highly ordered layer of collagen and proteoglycan, which are essential for providing corneal transparency. In addition, they are often characterized by molecular markers, including aldehyde dehydrogenase (ALDH), crystallins, CD133, and CD34[175]. When the cornea is injured corneal stromal cells are activated and lose the expression of cellular markers and adopt fibroblasts and myofibroblasts to form scar phenotypes.
Bioengineering corneal tissue with stromal stem cells
The highly ordered hierarchical ultrastructure of the corneal matrix, which exhibits exceptional biomechanical properties and optical transparency, makes it one of the most challenging steps in engineering human corneal tissue[176]. Fortunately, in 2005, Du et al[9] identified the first stem cell-like human corneal stromal cell precursors. The study found that in serum-free medium, human corneal stromal stem cells (hCSSCs) differentiate into cells expressing a gene profile similar to the profile of human keratocytes and secrete multilayered lamellae with orthogonallyoriented collagen fibrils similar to the corneal stroma, when cultured as floating pellets in the absence of rigid scaffolding or substratum[177].
Furthermore, some studies have found that CSSCs may not only have the ability of immune privilege but also have the potential to provide direct cell therapy pathways. The ability of CSSCs to exhibit immune privilege makes them excellent candidates for the generation of bioengineered corneal stromal constructs. Du et al[175] found that after direct injection into mouse corneas with scars, both the organization and transparency of the cornea were successfully restored without eliciting an immune T-cell response. Similarly, Ghoubay et al[178] developed a mouse model of corneal stromal scarring induced by liquid nitrogen (N2) application. Through direct injection of mouse or human corneal stromal stem cells in this model, they found that the transparency of the injured cornea was improved,the inflammatory response disappeared, recipient corneal epithelial cells grew collagen type III stromal content, corneal rigidity and stromal haze decreased, stromal ultrastructure was restored, and vision was improved. Their work concluded that corneal stromal stem cells could reverse the formation of mechanism scars and had the ability to promote the regeneration of transparent stromal tissue. Someone has investigated the mechanism and found that corneal stromal stem cells inhibited neutrophil infiltration on injured corneas by secreting TSG-6, thereby reversing scar formation[179].
CORNEAL ENDOTHELIAL CELLS AND CELL PROGENITORS
Anatomy
Corneal endothelial cells (CECs) are important for maintaining corneal transparency. The corneal endothelium is derived from the neural crest and forms a monolayer of hexagonal cells[180]. In the past,the corneal endothelium has been thought to be different from the corneal epithelium in that once the mature single-cell layer is formed, corneal endothelial cells lose their ability to proliferate. Instead of regenerating into new cells to replace dead or damaged cells, the wound can only be repaired by the expansion and migration of endothelial cells around the damaged area, resulting in a decrease in the density of endothelial cells by 0.3%-0.6% per year[181].
However, corneal endothelial cell progenitors, similar in part to stem cells, have been found to have the ability to self-renew and differentiate into mature effector cells. The progenitors are thought to be situated in the posterior limbus, a transitional area (also known as Schwalbe’s ring region) from the periphery of the endothelium and Schwalbe’s line to the anterior portion of the trabecular meshwork(TM). These hypothesized corneal endothelial cell precursors give rise to corneal endothelial cells and trabecular cells[5-7]. However, progenitor cells and stem cells are not exactly the same and cannot be substituted for each other. The self-renewal ability of progenitor cells is limited, which results in apoptosis of progenitors at the end of differentiation. Nevertheless, in contrast with the previous view that corneal endothelial cells cannot proliferate at all, the discovery of corneal endothelial progenitor cells has profound implications.
Characteristics
Different cell densities: The density distribution of corneal endothelial cells is uneven. B H Schimmelpfennig divided the collected corneas into two groups for staining. He found that in both 19 corneas stained with Orcein and 22 corneas stained with Alizarin Red, the cell density of the peripheral corneal endothelium was approximately 23.5% higher than that of the central corneal endothelium[182,183].This difference in density suggests that the smaller peripheral endothelial cells can migrate to the centre by increasing the area, which is conducive to the repair of the corneal endothelium. Meanwhile, the possibility that progenitor cells may exist in the peripheral transition region to provide differentiated endothelial cells is also suggested[5].
The proliferative ability is related to the location: Several studies have demonstrated that the proliferative ability of peripheral endothelial cells is stronger than that of central corneal cells in terms of cell distribution, molecular expression, senescence status and mitotic activity[183-186]. In 2000, Senoo et al[187] found that corneal endothelial cells can enter and complete the cell cycle in vitro after corneal endothelial injury, regardless of donor age. However, corneal endothelial cells from older donors responded more slowly and to a lesser extent than cells from younger donors. Subsequently, Mimura et al[186] further investigated the replication capacity of human corneal endothelial cells (HCECs) in central and peripheral regions and between young and old donors. They divided the corneas into a younger group (donors younger than 30) and an older group (donors older than 50). Minichromosome maintenance (MCM)-2 (a marker of replication competence) and senescence-associated β-galactosidase activity (SA-β-Gal) (a marker for identifying senescent HCECs) were used for staining. They found that in corneas from elderly donors, significantly fewer HCECs migrated to the central wound than to the periphery. Compared with HCECs from the young group with little SA-β-Gal activity both in the central or peripheral regions, the SA-β-Gal activity of HCECs from the older group was easier to detect, and the SA-β-Gal activity of central HCECs was significantly higher than that of peripheral HCECs. In both the younger and older groups, there were more MCM-2-positive cells in the peripheral corneal injury area than in the central corneal injury area. In vitro, HCECs from the peripheral region can be shown to have a higher replication capacity than HCECs from the central region, regardless of donor age. Therefore,the peripheral cornea has been suggested to act as a physiological supply and store for corneal endothelial cells so that the central cornea can be continuously supplied[183].
Proliferation ability is related to age differences among donors: After counting the stained proliferation marker protein Ki67, Senoo et al[187] found that the number and peak value of Ki67-labelled cells in the old group were significantly lower than that in the young group, and the speed of the old group entering the cell proliferation cycle was significantly slower than the speed in the young group.After in vitro culture and staining for Ki67 count, Zhu et al[188] found that the density of cells with positive staining in the young group was twice as high as that in the old group, and the time required for them to enter the cell cycle was half as long as that in the old group; Konomi et al[189] found that the doubling time tended to be higher for cells from older donors. Some people proposed explanations for these findings. Joyce[190] believed that with increasing age, the number of corneal endothelial cells entering the proliferative and senile phases gradually increased, and the expression of CKIS also increased, leading to a decrease in cell proliferation activity and a significantly reduced response to mitotic agents. In addition, Joyce et al[191] found that increased concentrations of 8-hydroxy-2’-deoxyguanosine (8-OHDG), a cell oxidative stress product, resulted in decreased proliferation.
As a result, the density and proliferation of peripheral endothelial cells are higher than those of central endothelial cells, although the proliferation of endothelial cells decreases with age. This conclusion strongly suggests that there may be corneal endothelial cell progenitors in the periphery of endothelial cells (namely Schwalbe’s ring).
Molecular markers for corneal endothelial cell progenitors
As early as 2005, Whikehart et al[192] detected telomerase activity (a stem cell marker) in the peripheral cornea, and bromodeoxyuridine (BrdU), a marker of cell division, was observed in the trabecular meshwork (TM) and the posterior limbus. After mechanical injury to the corneal endothelium, BrdU fusion was increased and extended to the corneal endothelium. In 2007, McGowan et al[193] found that cells from Schwalbe’s Ring expressed stem cell markers (Nestin, alkaline phosphatase, and telomerase).Additional putative stem cell markers (OCT3/4, Wnt1) and differentiation markers (Pax6, Sox2) were observed after corneal injury. In 2019, Yam et al[194] found that the cells expressing progenitor cell markers (i.e., SOX2, Lgr5, CD34, Pitx2 and telomerase) were involved in Schwalbe’s Ring on the side of the corneal endothelium. In addition, many studies have found that corneal endothelial progenitors express p75NTR, SOX9, FOXC2, Twist, Snail, and Slug[195,196].
Transplantation and therapeutic potential
Corneal endothelial cells play an important role in maintaining the stability and transparency of the corneal environment. When obvious visual impairment causes irreversible damage, the best strategy is to replace it with allogeneic corneal endothelial cells. However, severe rejection and a global shortage of donor corneal tissue have led people to seek alternative sources of transplantable tissue. Parikumar et al[197,198] successfully used a transparent nanocomposite sheet to transplant donor human corneal and colorectal cells into a cow’s eye, and implanted HCECs within three hours after transplantation. Their experiment paved the way for further clinical research.
On the basis of previous studies, Frausto et al[199] used next-generation RNA sequencing technology to compare human corneal endothelial cells (evHCEnCs) cultured in vitro with primary human corneal endothelial cells (pHCEnCs) and the human corneal endothelial cell (HCEnC) transcription profile.Transcriptomics analysis shows that at the molecular level, pHCEnCs are the most similar to evHCEnCs and therefore represent the most feasible cell culture treatment for corneal endothelial cell dysfunction.
In addition, some studies have found that cells from other tissues may also be the source of CEC-like cells used to treat corneal endothelial diseases. Inagaki et al[200] successfully induced corneal endothelial cells from human skin-derived precursors (SKPs) and showed that the transplanted cornea also maintained the transparency and thickness of the cornea. Shen et al[201] obtained abundant CEC-like cells through the coculture of human SKPs and B4G12 cells. Similar to human CECs in morphology and characteristics, when CEC-like cells are transplanted into rabbit and monkey models of corneal endothelial dysfunction, they show excellent therapeutic effects. Shao et al[202] transformed human foetal bone marrow-derived colorectal progenitor cells (BEPCs) into corneal endothelial cells in vitro,which may be useful for repairing corneal endothelial dysfunction. Zhang et al[203] induced the differentiation of hESCs into periocular mesenchymal precursors (POMPs). Using lens epithelial cell conditioned medium, CEC-like cells were obtained from POMPs and successfully transplanted into the eyes of a rabbit CE dysfunction model to gradually restore corneal transparency. Chen et al[204] used a simpler method to generate HCEC-like cells from hESCs. This method can greatly reduce the production work of HCEC and has potential clinical application value.
CONCLUSION
In this review, we introduced the characteristics of corneal epithelial stem cells, corneal endothelial cell progenitors and corneal stromal stem cells in detail, identified their anatomical features of their location near the limbus of the cornea, discussed a variety of isolation and culture techniques and related molecular markers and summarized their application and potential in treatment (especially the treatment of LSCD). Research on corneal stem cells is of great value for corneal transplantation,regenerative medicine and bioengineered corneal grafts, especially in the era of scarce corneal donors,which will bring good news to patients with corneal diseases worldwide.
FOOTNOTES
Author contributions: Ying PX, Mao QY, Huang C, Fu M, and Yi GG designed the research study; Jia XH, Cao YC,Hong LB, Cai LY, Guo X, Liu RB, Meng FK, Fu M, and Yi GG provided help and advice; Ying PX, Fu M, Mao QY,Huang C, Li ZH, and Fu S wrote the manuscript; all authors have read and approved the final manuscript.
Conflict-of-interest statement: The authors declare no conflict of interest.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non
commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin: China
ORCID number: Min Fu 0000-0003-2638-1756; Guo-Guo Yi 0000-0001-5809-4896.
S-Editor: Chen YL
L-Editor: A
P-Editor: Chen YL
