Drain fluid biomarkers for prediction and diagnosis of clinically relevant postoperative pancreatic fistula: A narrative review
2022-11-30NadyaRykinaTameevaJaswinderSamraSumitSahniAnubhavMittal
Nadya Rykina-Tameeva,Jaswinder S Samra,Sumit Sahni,Anubhav Mittal
Nadya Rykina-Tameeva,Jaswinder S Samra,Sumit Sahni,Anubhav Mittal,Northern Clinical School,University of Sydney,St Leonards 2065,Australia
Abstract Clinically relevant postoperative pancreatic fistula (CR-POPF) has continued to compromise patient recovery post-pancreatectomy despite decades of research seeking to improve risk prediction and diagnosis.The current diagnostic criteria for CR-POPF requires elevated drain fluid amylase to present alongside POPFrelated complications including infection,haemorrhage and organ failure.These worrying sequelae necessitate earlier and easily obtainable biomarkers capable of reflecting evolving CR-POPF.Drain fluid has recently emerged as a promising source of biomarkers as it is derived from the pancreas and hence,capable of reflecting its postoperative condition.The present review aims to summarise the current knowledge of CR-POPF drain fluid biomarkers and identify gaps in the field to invigorate future research in this critical area of clinical need.These findings may provide robust diagnostic alternatives for CR-POPF and hence,to clarify their clinical utility require further reports detailing their diagnostic and/or predictive accuracy.
Key Words: Biomarkers;Clinically relevant postoperative pancreatic fistula;Diagnosis;Drain fluid;Prediction
INTRODUCTION
Pancreatic cancer represents a grave diagnosis in which incidence closely parallels mortality[1].Manifesting as adenocarcinomas,neuroendocrine tumors,acinar carcinomas,colloid carcinomas,pancreatoblastomas and solid-pseudopapillary neoplasms,pancreatic cancer is predicted to be the second most diagnosed cancer by 2030[2].Both the challenges of early diagnosis and treatment contribute to its dismal prognosis,whereby its failure to manifest symptoms early and resistance to conventional treatments leaves surgery as the only curative option[3,4].The greatest contributor to the burden of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC) which occurs in 90% of cases and has the highest fatality rate of all solid tumors[3,5].The majority of PDACs develop in the head of the pancreas (60%-70%) and require pancreaticoduodenectomy.The remainder arise in the body and tail (15% of cases each) which require a distal pancreatectomy to excise the tumor[6] (Figure 1).As only 20%-25% of PDAC patients are diagnosed with resectable disease,maximising their surgical outcomes is of utmost importance,particularly as 5-year survival can improve from < 7% without surgery[3] to 39% after surgery[7].Necessarily,this involves minimising surgical complications,not only to improve recovery,but to avoid increasing the challenges of cancer which already include compromised nutrition,immunity,metabolism as well as mental and financial wellbeing[8-10].Clinically relevant postoperative pancreatic fistula (CR-POPF) has persisted as the leading cause of postoperative morbidity and mortality despite decades of improving pancreatectomy techniques and perioperative care[11-15].Affecting up to 50% of cases[16],CR-POPF has been shown to increase readmission rates,length of stay,health-related costs and particularly relevant for pancreatic cancer patients,potentiate recurrence and delay the delivery of adjuvant therapy,both of which can compromise the curative intent of surgery[17-22].
CR-POPF definition
POPF was initially stratified into grades A-C[23],with grade A since being reclassified by the International Study Group on Pancreatic Surgery (ISGPS) as a biochemical leak in favour of recognising the clinically relevant grades B and C[24].CR-POPF is diagnosed once drain amylase on postoperative day (POD) three exceeds three times the upper limit of normal for serum amylase and the patient develops a clinically relevant change in their condition,necessitating intervention (Figure 2).Grade B fistulae are characterised by prolonged drainage exceeding three weeks,pharmaceutical interventions,additional imaging and infections.Grade C sequelae are more severe,potentiating sepsis,organ failure and in up to 35% of cases,death[25].This definition does not require imaging to confirm a diagnosis of CR-POPF,particularly as intra-abdominal fluid collections may be transiently increased after surgery.Imaging,however,may be necessary for planning interventions in confirmed cases[26].Recently,non-contrastenhanced computed tomography paired with machine learning has been shown capable of evaluating pancreatic texture to predict CR-POPF,doing so with a sensitivity of 0.96 and specificity of 0.98[27].Similarly,transabdominal pancreatic ultrasound elastography has been associated with CR-POPF,occurring more in patients with softer parenchyma (P= 0.002)[28].

Figure 1 Resection and reconstruction phases of different pancreatic surgeries.
Pathophysiology of CR-POPF:Pancreatic fistulae often occur in pancreata with preserved exocrine function in which pancreatic enzymes are released and activated,damaging tissues,and potentiating systemic complications.Such glands are characterised by soft texture and at least normal acinar cell density at the surgical margin,both of which have been associated with CR-POPF after pancreatoduodenectomy and distal pancreatectomy[29-33].In advanced PDAC,obstructive pancreatitis may develop[34],contributing to a firm parenchyma.Recently,neoadjuvant therapy has been explored as a potential protector against CR-POPF[35],being shown to favour a more fibrotic and acinar-deplete parenchyma[36].However,pancreatic texture has been shown to not predict CR-POPF after distal pancreatectomy[37],emphasising the clinical importance of the distinct risk profiles for both resection types.
CR-POPF can develop following the reconstruction phase of surgery (Figure 1).In pancreatoduodenectomy,fistula is often attributed to failure of the pancreatoenteric anastomosis whereby pancreatic fluid destined for the duodenum leaks into the abdomen[38].Leakage can also occur from the gland itself,in what is referred to as a parenchymal leak[39].In distal pancreatectomy,increased pressure in the pancreatic duct due to obstruction at the sphincter of Oddi has been thought to result in pancreatic juice leakage[40,41].As distal resections do not cause downstream obstruction of the pancreatic duct,they are not predisposed to leakage in the same way as pancreases after pancreatoduodenectomy.Splenic preservation has been seen effective in preventing CR-POPF,owing this to the avoidance of pancreatic ischemia secondary to splenic vessel ligation[42,43].Indeed,the higher morbidity inherent to multi-visceral resection is avoided in spleen-preserving distal pancreatectomy.Moreover,the former facilitates shorter operative times,which may be advantageous given that operations exceeding 480 min were at greater risk of developing pancreatic fistula (P= 0.02)[44].This finding however did not persist in pancreatoduodenectomy patients[31,45].Whilst the location of the tumour matters in determining the surgical approach,the size of the tumour has not been shown to influence the development of CR-POPF in pancreatoduodenectomy patients[46] but has so in distal pancreatectomy patients undergoing staple closure (P= 0.009,univariate analysis)[47].
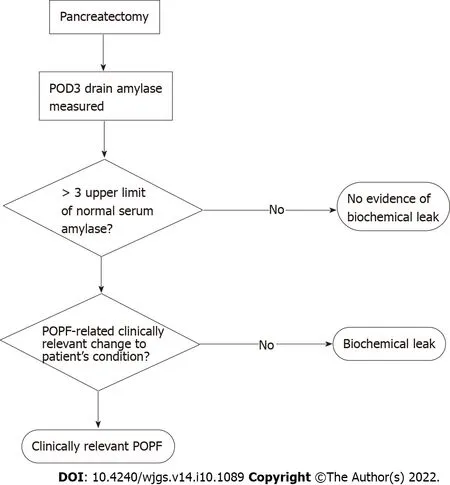
Figure 2 Current standard pathway for the diagnosis of clinically relevant postoperative pancreatic fistula.
Drain biomarkers for CR-POPF: Predictive biomarkers have commonly been investigated in both drain fluid and blood.The operative placement of drains and close relationship of drain fluid to the pancreas highlights its potential as a convenient biofluid capable of reflecting CR-POPF risk.Hence,the present review synthesises all drain fluid biomarkers identified for the prediction and diagnosis of CR-POPF.
Drain amylase
Drain amylase has been extensively explored given its evaluation being embedded in the current diagnostic pathway (Figure 2).Where diagnostic cut-offs have been defined for drain amylase,many measures of accuracy beyond sensitivity and specificity have been reported[48-57] (Table 1).While postoperative evaluation of drain amylase are common,earlier assessments may represent a simple way to improve the utility of drain amylase observations.Particularly,intraoperative measures provide an immediate assessment of pancreatic exocrine function and hence,its propensity to secrete erosive enzymes and predispose the pancreas to leak and subsequent CR-POPF.Indeed,Nahmet al[32] reported a significant association between intraoperative amylase concentration and CR-POPF with an area under the receiver operating characteristic curve (AUC) of 0.76 (P= 0.004) in their cohort of 61 pancreatectomy patients.The accuracy of intraoperative amylase has also been evaluated in surgeryexclusive cohorts with de Reuveret al[58] reporting an AUC of 0.83 in their cohort of 62 pancreatoduodenectomy patients.Wanget al[59] investigated this time point in 40 distal pancreatectomy patients,obtaining a sensitivity and specificity of 0.846 and 0.889 respectively for a cut-off of > 3089 U/L.These studies indicate that the enzymatic leak which can catalyse the development of a CR-POPF begins at the time of surgery,presenting an opportunity to expediate the diagnostic pathway which currently begins on POD3.

Table 1 Drain amylase accuracy evaluated beyond sensitivity and specificity
To better facilitate earlier diagnosis of CR-POPF,the sensitivity and specificity of POD1 drain amylase has been widely reported with cut-offs ranging from 282 U/L - 5000U/L[34,49,60-79].Beyond discrete cut-offs,Hirakiet al[80] found median drain amylase concentration in a prospective study of 30 pancreatoduodenectomy patients to have a sensitivity and specificity of 0.933 and 0.867 respectively.Moreover,Kühlbreyet al[81] found POD1 drain amylase to effectively predict CR-POPF after pancreatectomy returning AUCs of 0.829 (P< 0.001) and 0.637 (P< 0.01) for pancreatoduodenectomy and distal pancreatectomy respectively.This was corroborated by Wüsteret al[82] who reported similar AUCs of 0.830 and 0.854 respectively.POD1 drain amylase concentrations have been noted as significantly higher in CR-POPF patients[32,83],correlated with CR-POPF following univariate analysis[84] and identified as an independent risk factor for CR-POPF after pancreatoduodenectomy[49,85-87].In contrast,in a cohort of 74 pancreatectomy patients,no significant differences in POD1 drain amylase were found in patients who did and did not develop CR-POPF[88].However,this study may have been underpowered as only nine (12.2%) patients developed CR-POPF.
The accuracy of POD2 drain amylase has been less explored with all reports evaluating pancreatoduodenectomy patients alongside POD1 drain[65,69] or serum amylase[57].Sensitivity has been reported by two independent studies as 0.88,with the specificity of 0.83 in Ansorgeet al’s work[69] surpassing Caputo’s group’s specificity of 0.74 when cut-offs of 314 U/L and 368 U/L were used respectively[65].Odds ratios of 35 and 29 have further been reported in prospective studies[89,90].While measuring on POD2 does allow greater time for the biochemical leak to develop thereby enhancing diagnostic accuracy,it does require a change to monitoring protocols which predominantly sample drain fluid on odd PODs (e.g.,POD1,POD3 or POD5).Moreover,this relatively unexamined timepoint reflects current preferences to either assess drain amylase early on POD1 or to abide by the recommended testing day of POD3.
The close relationship of POD3 amylase to the ISGPS definition has resulted in few explorations of its true diagnostic performance[24].Following pancreatoduodenectomy,POD3 drain amylase has been noted to be significantly higher in CR-POPF patients[91].Diagnostic cut-offs for POD3 drain amylase have ranged between 26 U/L and 1026 U/L for distal pancreatectomy cohorts[54,56,92,93],93-2820 U/L for pancreatoduodenectomy cohorts[55,56,83,84,94-99] and 200-3000 U/L in studies analysing the biomarker in pancreatectomy patients[16,56,75,100-103].When cut-off accuracy was reported,sensitivity ranged from 0.316-1.00 and specificity,from 0.631-0.968[16,55,75,84,92,94-98,101-103],showing drain amylase alone does not completely include or exclude CR-POPF.This reinforces the importance of clinically relevant sequelae developing for accurate diagnosis as stipulated by the consensus definition[24].POD4 drain amylase was found by Kosakaet al[104] to be significantly elevated in CR-POPF patients after pancreatoduodenectomy later defining a cut-off of 646 U/L as having an AUC of 0.87[105].After distal pancreatectomy,Suzumuraet al[106] identified ≥ 1200 U/L as the predictive cut-off and Hiyoshi’s group reported a sensitivity and specificity of 0.938 and 0.7 for a cut-off of ≥ 800 U/L[107].POD5 drain amylase has been significantly correlated with CR-POPF post-pancreatoduodenectomy[108] where after distal pancreatectomy,a cut-off of > 1000 U/L was significantly associated with CR-POPF[109],with the cut-off of > 538 U/L by Coaylaet al[98] predicting CR-POPF with a sensitivity and specificity of 0.86 and 0.91 respectively.
Median drain amylase levels post-pancreatoduodenectomy have been observed as significantly higher in CR-POPF patients[110] on POD1[52],POD2[57] and POD3[69].Similarly,Moskovicet al[111] found median drain amylase concentration post-pancreatectomy to be significantly elevated on PODs 1-6 in CR-POPF patients.However,this offered no diagnostic advantage and given the wide day range,would prevent diagnosis on a designated day and limit early intervention.Both median and statistically derived cut-offs are limited in their ability to determine specific patient risk as they are summarised from entire patient cohorts which exhibit a spectrum of risk profiles.
Studies stratifying CR-POPF risk using drain amylase have been few and pancreatoduodenectomy exclusive.On POD1,Sutcliffe’s group reported drain amylase < 2000 U/L excluded grade C POPF with a negative predictive value (NPV) of 0.99[112],whereas Caputoet al[113] found POD1 drain amylase ≥ 807 U/L to significantly predict grade C POPF with a sensitivity and specificity of 0.727 and 0.644 respectively.However,Chibaet al[114] did not find drain amylase to be a significant grade C POPF risk factor during the first postoperative week,owing this potentially to the difficulty of ensuring adequate pancreatic juice drainage post-operatively.Drain amylase was similarly examined by Liet al[115] to better identify low and high-risk patients.Here,a POD1 cut-off of 921.7 U/L (AUC = 0.85) had a sensitivity and specificity of 0.789 and 0.828 whereas their POD3 cut-off of 4021.5 U/L had overall higher accuracy favouring specificity at 0.954 (sensitivity = 0.778).In low-risk patients undergoing PD,Newhooket al[56] reported a POD1 cut-off of 661 U/L and POD3 cut-off of 141 U/L could completely exclude CR-POPF when drain amylase was below these levels (sensitivity = 1).Amongst high-risk PD patients,the POD1 and POD3 cut-offs to exclude CR-POPF were < 136 U/L and < 93 U/L respectively.Whilst these cut-offs ensure no false negative results,they may be rarely encountered and hence,rarely utilised.As such,clinicians may prefer higher cut-offs,compromising on sensitivity,to clarify the danger of higher amylase levels more likely to be encountered in clinical practice.These risk-stratified approaches are a welcome advance on previous reports which have predominantly derived predictive cut-offs from entire patient populations,limiting targeted risk prediction.Similar investigations should be conducted in distal pancreatectomy cohorts to define risk-specific cut-offs in these patients.As such,accounting for the operation,patient risk and corresponding predictive drain amylase levels will help refine CR-POPF diagnosis,ultimately decreasing complication rates.
The majority of drain amylase investigations have reported the biomarker at singular timepoints,with others considering its accuracy across multiple PODs.Tzedakis’ group found in their cohort of pancreatectomy patients that drain amylase elevated beyond three times the upper limit of normal on POD1 and POD3 had a sensitivity of 0.974 and NPV of 0.971[101].Similarly,Linnemannet al[116] reported a NPV of 0.95 in pancreatoduodenectomy patients with a peak drain amylase of 1000 U/L on PODs 1-3.These studies evidence a superior ability to exclude CR-POPF which may justify the additional monitoring of drain amylase which differs from the popular,singular day approach.Hence,early,and continued monitoring can strengthen the identification and selection of low-risk patients for accelerated recovery pathways.In pancreatoduodenectomy cohorts,numerous reports have investigated changes in drain amylase across the postoperative period.This measure possesses the potential to reflect existing and imminent CR-POPF risk.Dugalicet al[110] reported a moderate decline of < 50% between POD 1 and POD3 to be significantly associated with CR-POPF.Seemingly supporting these findings,Koizumiet al[117] found a notable decrease in drain amylase between POD1 and POD5 in patients without CR-POPF.This suggests the relative persistence of elevated drain amylase may be predictive of CR-POPF,a finding which corroborates reports of drain amylase being significantly elevated in CR-POPF patients during this time period[118,119].However,Furukawaet al[120] identified a decline of pancreatic amylase of greater than 80% between POD1 and POD3 to be predictive of CRPOPF after pancreatoduodenectomy.Further into the postoperative period,Kuharaet al[121] appear to support this in identifying a decrease in drain POD5 amylase to a third of the POD3 level to be a significant risk factor for CR-POPF.To bolster day-specific tracing of CR-POPF risk,future studies should clarify these discrepancies and quantify the drain amylase changes that would indicate impending CR-POPF.Similar investigations in distal pancreatectomy cohorts are also warranted.Nobuokaet al[122] evaluated CR-POPF risk by considering the product of drain amylase and volume.This combined variable was found to be significantly higher in CR-POPF patients on POD1 and POD7.Extending this,Okanoet al[119] evaluated the product of drain amylase and volume on POD3 and POD1 in ratio.Here,patients who did not develop CR-POPF had significantly lower values.Together,these indicate that involving drain volume in the assessment of CR-POPF risk may provide opposite findings to when drain amylase is exclusively evaluated,persisting at elevated levels and potentially decreasing,respectively.
Drain lipase
Drain lipase has gained momentum as a potential accompaniment or replacement for drain amylase in diagnosing CR-POPF given its similar ability to capture the exocrine function of the remnant pancreas.Moreover,serum lipase assists in acute pancreatitis diagnosis[123,124],a postoperative complication which itself has been shown to independently predict CR-POPF[125,126].Lipase drives intraperitoneal lipolysis which can exacerbate systemic inflammation and trigger multi-organ dysfunction specifically as the subsequent high systemic unsaturated fatty acid levels can cause mitochondrial toxicity[127],lipotoxicity[128] and kidney[129,130] or liver damage[131].Diagnostic cut-offs have ranged from 4.88 U/L to 1000 U/L with the majority exploring both pancreatoduodenectomy and distal pancreatectomy patients[100-102,132].However,pancreatoduodenectomy[97] and distal pancreatectomy[107] exclusive studies have also been conducted.Amongst these reports,the sensitivity and specificity has ranged from 0.8-0.938 and 0.649-0.95,respectively[97,100-102,107,132].Suzukiet al[133] reported POD1 drain lipase levels to be an independent risk factor for CR-POPF (P= 0.037).Tzedakiset al[101] further considered the evolution of drain lipase and its relation to CR-POPF risk with sustained elevation of drain lipase across POD1 and POD3 having a sensitivity of 0.948 which was then confirmed in their validation cohort.
In the way of risk stratification,Frymermanet al[134] identified the combination of elevated POD3 and POD5 drain lipase (> 5000 U/L) and soft pancreatic texture to be predictive of grade C fistula.As this combination includes the most widely reported risk factor for CR-POPF,soft parenchyma,the contribution of elevated drain lipase to overall grade C risk remains unclear.Hence,drain lipaseexclusive risk stratification requires further investigation particularly during the early postoperative period as the majority of the aforementioned studies evaluated drain lipase on or after POD3.
Drain culture
The extent and character of drain fluid infection has been explored in surgery-specific and all-inclusive analyses of CR-POPF patients (Table 2).Pancreatoduodenectomy has been more extensively explored,with infection of the ascitic fluid and surgical site potentially explained by preoperative bile duct infection[135,136].Moreover,the construction of the gastrointestinal anastomosis exposes the pancreas to the densely colonised duodenum and jejunum,causing intra-abdominal translocation of species that is further facilitated by bile and pancreatic outflow[137].

Table 2 Investigations of drain culture across different pancreatic surgery cohorts

Table 3 Recommendations for future research
Investigations in pancreatoduodenectomy cohorts:A significantly higher prevalence of CR-POPF in pancreatoduodenectomy patients with positive drain culture has been widely reported[138-144],where internal and preoperative biliary drainage,elevated drain amylase,combined colectomy and a longer duration of surgery have been identified as significant risk factors for contaminated drain fluid[140,145].
Kimuraet al[146] identified contaminated drain fluid on POD1 and POD3 to be an independent risk factor for CR-POPF which has since been corroborated in the early postoperative period PODs 1-3[139],POD1[147,148] and POD3[142,149].The accuracy of POD1 drain culture was reported by Hataet al[145] as having a sensitivity of 0.45 and specificity of 0.813 resulting in a positive predictive value (PPV) of 0.479,with specificity (0.99) similarly prevailing over sensitivity (0.32) in Morimotoet al’s analysis of POD3 drain fluid smear tests which reported a superior PPV of 0.89[142].
The great diversity of microorganisms in post-pancreatoduodenectomy drain fluid is evident in the wide identification ofEnterococcus[137-139,141,148,150-153],Enterobacter[138,141,148,151,152],Pseudomonas[151,153,154],Klebsiella[137,153],Methicillin-resistantStaphylococcus aureus[153],Candida[150,151],CitrobacterandEscherichia coli(E.coli)[137,155].Yanget al[139] identified fungi,Staphylococcus,Enterococcus,Pseudomonas,Acinetobacter,Stenotrophomonas,E.coliandKlebsiellasignificantly more often in their CR-POPF patients.E.colihas specifically been implicated in bacterobilia whereby its colonisation of the bile stent has been significantly associated with grade C POPF (P= 0.028,odds ratio = 4.07)[156].
During the first postoperative week,Chibaet al[114] found gram-positive bacteria to predominate in grade B POPF patients while gram-negative rods were identified an independent predictor for grade C fistula.As McMillan’s group isolated gram-negative organisms more commonly than gram positive (78.3%vs68.1% respectively)[157],this could indicate that infections,being more commonly comprised of high-risk bacteria,predispose patients to more severe POPF.Indeed,Yamashitaet al[154] isolatedPseudomonas aeruginosaexclusively in CR-POPF patients and identified the bacteria as the source of proteases which activated trypsin from trypsinogen.Belmouhand’s group corroborated this latter finding and further identified drainEnterobacter cloacaeas a source of trypsin-activating proteases thereby contextualising the role of gram-negative rods in CR-POPF development[150].
Nagakawaet al[141] found the bacteria detected on POD1 and POD3 to be similar in CR-POPF patients.This taken with the consistent number of non-intestinal bacterium observed on POD3 and POD7 highlights an opportunity for early risk assessment on POD3 as clinicians could anticipate a CRPOPF diagnosis when diagnostic bacteria are first detected[149].Hence,the concurrent assessment of drain culture alongside POD3 drain amylase may assist earlier CR-POPF diagnosis,potentially reducing the reliance on complication development as is stipulated by the current consensus definition.Hence,patient safety will be increased as despite developing CR-POPF,patients will not have to endure challenging sequelae prior to diagnosis.
Beyond individual microorganisms,Demiret al[158] reported patients presenting with both CR-POPF and positive drain culture had significantly more polymicrobial infections with De Pastena’s group noting the number of CR-POPF patients with polymicrobial infections to be significantly higher than those with biochemical leak (P= 0.003)[159].The prevalence of polymicrobial infections in CR-POPF patients has ranged from 0.478-0.681,however their association with the complication has not been noted[157,159,160].Belmouhand’s group did not find polymicrobial drain fluid infections to be associated with anastomosis leakage[150],neither did Maatmanet al[161] find this for any postoperative complication.
Rather than investigating polymicrobial infections as a risk factor for CR-POPF,risk stratification would be best assisted by the specific identification of problematic bacteria within polymicrobial drain fluid samples.Hence,an exploration of microorganisms associated with CR-POPF naturally assists in this.Abeet al[162] reportedCandidato be significantly associated with CR-POPF and an independent risk factor for grade C fistulae (P= 0.043) which supported McMillanet al’s findings whereCandidawas found in 87.3% of grade C cases for which microbiological data was available[157].Here,EnterococcusandStaphylococcuswere also detected,conflicting later findings by Belmouhand’s group who reported no significant difference in the severity of POPF when drain fluid was similarly contaminated[150].The commonly identifiedEnterococcusandEnterobacterspecies have been detected on POD1[146] and proposed to originate from bile[140].Abeet al[162] detectedEnterococcusandEnterobacterspecies in drain fluid with Yamashitaet al[138] specifically identifyingEnterococcus faecalisandEnterobacter cloacaeas precipitating CR-POPF.Interestingly,McMillanet al[157] found mortality to be significantly lower in patients withEnterobacterpositive cultures despite it being widely identified in the drain fluid of CRPOPF patients.The inconclusive relevance ofEnterobacter,EnterococcusandStaphylococcusto CR-POPF risk and concurrent identification ofCandidain drain fluid confirms the findings ofCandidaas characteristic of polymicrobial infections[150] and more likely to appear in grade C POPF[162].
Investigations in distal pancreatectomy cohorts: Similar to pancreatoduodenectomy studies,distal pancreatectomy patients with positive drain culture have been associated with significantly higher rates of CR-POPF[163,164] with positive drain culture being an independent risk factor for the complication before POD3[165] and on POD4[166].However,abdominal infection was not found to be a risk factor for CR-POPF by Satoet al[167] in their cohort of 49 patients which may have been underpowered.Yanget al[165] identifiedStaphylococcus,Enterococcus,Pseudomonas,Acinetobacter,Stenotrophomonas,E.coliandKlebsiella sppsignificantly more often in their CR-POPF patients.Here,74.2% of patients contaminated withStaphylococcusand 92.9% of patients withKlebsiellasubsequently developed CR-POPF.Looset al[137] similarly identifiedStaphylococcus spp.andEnterococcus spp.most frequently in the drain fluid of CR-POPF patients.Harinoet al[163] foundStaphylococcusnumbers to increase in patients when drains were removed after POD5,with Yang’s group reporting rapid increases in positive drain culture when drains remained between POD3 and POD7 with a prevalence of 21.6% and 73.3% respectively[165].Hence,earlier drain removal may assist in curbing the growth of bacteria and its subsequent role in CRPOPF development.
Yanget al[165] also found fungi to be isolated significantly more often in distal pancreatectomy patients who developed CR-POPF,while Abeet al[162] noted the absence ofCandidawhich contrasted findings after pancreatoduodenectomy.Hence,the distinct drain culture portfolios following each resection type facilitate the identification of specific high-risk bacteria,the predictive potential of which would be enhanced by identifying the day of earliest detection and strongest association with CR-POPF.Moreover,it should be investigated whether mere presence of certain bacteria is predictive of CR-POPF or if there is a level at which risk is higher.Here,additional understanding of the time course for bacterial growth would assist close monitoring of colony numbers to facilitate better complication anticipation and prevention.
Miscellaneous drain biomarkers
Other biomolecules:Drain lipase activity has been indirectly explored through alternate biomarkers for CR-POPF.Indeed,POD1 drain glycerol (> 800 μmol/L) has been associated with CR-POPF after pancreatoduodenectomy[168].Similarly,drain free fatty acid has been significantly associated with CR-POPF.In an ensuing rat model,intraperitoneal lipolysis resulted in greater pancreatic juice leakage which risks CR-POPF by eroding the parenchyma and irritating acute pancreatitis[131].Being products of lipolysis,drain glycerol and free fatty acids could serve as surrogate biomarkers for drain lipase,and hence CRPOPF.To effectively compare the predictive performance of these newer biomarkers however,a better understanding of their accuracy is required.To determine their clinical utility,their accuracy should also be compared against drain amylase and lipase.
Further,trypsin activation peptide (TAP) as a surrogate measure for protease activation has been explored.Xiuet al[169] found the TAP to drain amylase ratio in pancreatoduodenectomy patients to be significantly higher in CR-POPF patients,with this predictive measure being significantly higher when compared to distal pancreatectomy and biochemical leak patients.Wüster’s group identified TAP and chymotrypsin elevation to be uniquely associated with distal pancreatectomy and pancreatoduodenectomy CR-POPF patients,respectively[82].Irrespective of resection type,myeloperoxidase and trypsin activity were significantly elevated on PODs 1-2 and PODs 1-7,respectively.However,amongst the CRPOPF patients,elastase was not found to be significantly associated with the complication[82].Ansorgeet al[168] identified a significantly higher intraperitoneal lactate to pyruvate ratio in CR-POPF patients which increased significantly between POD1 and POD2 due to increased lactate and decreased pyruvate,thereby implicating metabolic disruption in the pathophysiology of CR-POPF.Hence,these emerging biomarkers may offer new opportunities for bolstering CR-POPF prediction particularly if combined with established risk factors in future predictive models.
Drain fluid appearance:Observations of “sinister” drain effluent are often relied upon to inform an assessment of CR-POPF risk[76] and were a criteria of the initial consensus definition[23].Abnormal drain fluid can be brown,green,milky or unusually clear[65].Non-serous fluid following pancreatoduodenectomy has been independently associated with CR-POPF on POD1,POD3 and POD4[170].However,Kosakaet al[105] did not find drain fluid colour to significantly differ between CR-POPF and non-CR-POPF pancreatoduodenectomy patients on POD4 on multivariate analysis agreeing with Suzumuraet al’s findings following distal pancreatectomy[106].Drain turbidity has been significantly correlated with drain fluid amylase on POD5 and beyond[108],suggesting its early observation could anticipate later development of CR-POPF.
CONCLUSION
This review revealed the potential for drain fluid biomarkers to overcome the limitations of the current diagnostic definition which necessitates a reactive management approach[24].Numerous future directions for drain fluid research include investigating and confirming the accuracy of drain biomarkers in novel and established contexts respectively (Table 3).Through this,reports of biomarkers can specifically detail the accuracy of surgery-specific,risk-stratified cut-offs to clarify their clinical utility.Hence,decisions regarding drain removal and further monitoring can accordingly be made to either expediate or protect patient recovery respectively.Clarifying the clinical utility of drain biomarkers,could also facilitate their inclusion as variables in predictive models alongside blood biomarkers and medical imaging.This would complement recent efforts in which predictive models have sought to improve and expediate diagnosis when compared to the evaluation of individual variables[171-173].As such,progress can continue to be made towards risk-stratifying patients according to pre- and intra-operative variables.
FOOTNOTES
Author contributions:Rykina-Tameeva N wrote the paper;Samra JS,Sahni S and Mittal A provided feedback and revised the paper;Sahni S and Mittal A have contributed equally as senior authors.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Australia
ORCID number:Sumit Sahni 0000-0002-2900-8845;Anubhav Mittal 0000-0003-3960-2968.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Immunoglobulin G4-related disease in the sigmoid colon in patient with severe colonic fibrosis and obstruction: A case report
- Cecocutaneous fistula diagnosed by computed tomography fistulography: A case report
- Can DKI-MRI predict recurrence and invasion of peritumoral zone of hepatocellular carcinoma after transcatheter arterial chemoembolization?
- Topological approach of liver segmentation based on 3D visualization technology in surgical planning for split liver transplantation
- Reconstructing the portal vein through a posterior pancreatic tunnel:New choice for portal vein thrombosis during liver transplantation
- Long-term efficacy and safety of cap-assisted endoscopic sclerotherapy with long injection needle for internal hemorrhoids
