Shenweifang-containing serum inhibits transforming growth factorβ1-induced myofibroblast differentiation in normal rat kidney interstitial fibroblast cells
2022-11-17LINJiaruWANGLiCHENBoOUSantaoQINJianhuaFANJunming
LIN Jiaru,WANG Li,CHEN Bo,OU Santao,QIN Jianhua,FAN Junming
LIN Jiaru,Department of Nephrology,the Affiliated Hospital of Southwest Medical University,Luzhou 646000,China;Nephropathy Clinical Medical Research Center of Sichuan Province,Luzhou 646000,China;Chengdu University of Traditional Chinese Medicine,Chengdu 610075,China
WANG Li,CHEN Bo,Central Laboratory,the Affiliated Chinese Medicine Hospital of Southwest Medical University,Luzhou 646000,China;
OU Santao,Department of Nephrology,the Affiliated Hospital of Southwest Medical University,Luzhou 646000,China
QIN Jianhua,Department of Nephrology,the Affiliated Hospital of Southwest Medical University,Luzhou 646000,China
FAN Junming,Southwest Medical University,Luzhou 646000,China;Department of Nephrology,the Affiliated Hospital of Southwest Medical University,Luzhou 646000,China;Chengdu University of Traditional Chinese Medicine,Chengdu 610075,China;Central Laboratory,Affiliated Chinese Medicine Hospital of Southwest Medical University,Luzhou,Sichuan 646000,China;Chengdu Medical College,Chengdu,Sichuan 610500,China
Abstract OBJECTIVE: To investigate the efficacy of Shenweifang(SWF)-containing serum on transforming growth factor(TGF)-β1–induced fibroblast-myofibroblast transition in normal rat kidney interstitial fibroblast cells (NRK-49F).METHODS: Sprague-Dawley rats were gavaged with one of five solutions: (a) saline;(b) saline plus low-dose SWF;(c) saline plus medium-dose SWF;(d) saline plus highdose SWF;and (e) saline plus valsartan.NRK-49F cells were treated with TGF-β1 and cultured using serum from the gavaged rats.RESULTS: TGF-β1 treatment increased the expression of α-smooth muscle actin,proliferating cell nuclear antigen,collagenⅠ,Smad3,mitogen-activated protein kinase (MAPK) 10,and c-Jun N-terminal kinase (JNK) 3 and induced abnormalities in cell morphology,cell cycle progression,and cell proliferation.CONCLUSIONS: SWF-or valsartan-containing serum corrected (or partially corrected) TGF-β1–induced abnormal changes in this in vitro system.SWF-containing serum reversed abnormalities in morphology,cell cycle progression,and proliferation in TGF-β1–treated NRK-49F cells,probably by blocking the TGF-β1/Smads and TGF-β1/MAPK/JNK pathways.
Keywords: fibrosis;transforming growth factor beta1; Smad proteins;mitogen-activated protein kinases;JNK mitogenactivated protein;kinases drug-containing serum;Shenweifang
1.INTRODUCTION
Chronic kidney disease (CKD) affects 11% of the adult population in developed countries,including the United States and Australia.1In China,the prevalence of CKD is approximately 10% in the adult population.2From 1990 to 2013,the mortality rate in the Chinese CKD population increased by 147%.3Some reports presented an estimated annual cost of approximately one trillion dollars for dialysis,a common treatment option for CKD globally.4Despite the widespread prevalence of CKD and its substantial burden on patients and the healthcare system,an extremely limited number of specific therapies (e.g.,inhibitors of the angiotensin receptor and its ligand) have been developed to prevent organ failure in CKD.5Of note,notwithstanding the etiopathology,interstitial fibrosis is a well-characterized outcome of all types of progressive CKD.6Studies expounding on the underlying mechanism of renal fibrosis would offer potential host targets for treating CKD.
Transforming growth factor (TGF)-β has been proposed as a key player in renal fibrosis,7and it has been reported to activate Smad proteins (e.g.,Smad2,Smad3,and Smad7),a group of intracellular transduction molecules that can activate or inhibit the expression of certain genes/proteins involved in the development of renal fibrosis.8-11Furthermore,TGF-β can activate the mitogen-activated protein kinase (MAPK) signaling pathway,including c-Jun N-terminal kinase (JNK),p42/44 MAP kinases (ERK),and p38,and subsequently affect the development of renal fibrosis.12-14In particular,a group of Chinese medicines has been demonstrated to be effective in preventing and/or treating renal fibrosis associated with CKD progression.15,16Shenweifang(SWF),a Chinese medical formula containing seven major ingredients [Huangqi (Radix Astragali Mongolici),Sanqi (Radix Notoginseng),Danggui (Radix Angelicae Sinensis),Niuxi (Radix Achyranthis Bidentatae),Kunbu(Thallus Laminariae Japonicae),and Dahuang (Radix Et Rhizoma Rhei Palmati)],has long been used to prevent and treat CKD.17,18In a clinical trial of 96 patients with CKD,82 (85.42%) exhibited improvement in the CKD status (e.g.,reduced renal fibrosis and decreased levels of urinary protein,blood urea nitrogen,and serum creatinine) after 4 months of oral SWF therapy.19
During our studies on SWF,we observed that the renal expression of TGF-β1 was decreased by oral SWF administration in mice (unpublished data).Previously,we investigated the mechanisms underlying SWFinduced improvement in renal fibrosis in an animal model.This study was conducted to determine the effects of SWF-containing serum on the TGF-β/Smad and TGFβ/MAPK pathways in normal rat kidney interstitial fibroblast cells (NRK-49F).
2.METHODS
2.1.Ethics approval
All study protocols were approved (ID: SYXK-Chuan-2013-065) by the Animal Care and Use Committee of the Affiliated Traditional Medicine Hospital of Southwest Medical University (Luzhou,China).All applicable international,national,and/or institutional guidelines for the care and use of animals were followed.
2.2.Preparation of SWF-containing serum
The SWF formula is composed of seven Chinese medicinal ingredients (voucher specimens were deposited in the Southwest Medical University Plant Herbarium,Luzhou,China).Each 146 g of SWF contains 50 g of Huangqi (Radix Astragali Mongolici),(specimen ID: 1503090),10 g of Sanqi (Radix Notoginseng),(specimen ID: 160116-2),10 g ofDanggui (Radix Angelicae Sinensis,(specimen ID: 1503049),30 g ofNiuxi (Radix Achyranthis Bidentatae),(specimen ID:1407058),30 g ofKunbu (Thallus Laminariae Japonicae),(specimen ID: 1404204),10 g of Muli(Concha Ostreae),(specimen ID: 1410042),and 6 g of Dahuang (Radix Et Rhizoma Rhei Palmati),(specimen ID: 1502068).The therapeutic standard dose in humans is 2.17 g/kg body weight per day.In this study,wellmixed SWF powder was administeredviagavage to rats together with saline at 5 (low-dose SWF gavage),10(medium-dose SWF gavage),or 20 (high-dose SWF gavage) times the standard human dose.Specific pathogen-free male Sprague-Dawley (SD) rats (n=40;3 months old,average body weight,250 g) were randomly allotted into one of five treatment groups (n=8 each) as follows: (a) saline only;(b) saline containing low-dose SWF;(c) saline containing medium-dose SWF;(d) saline containing high-dose SWF;and (e) saline containing valsartan (13 g/kg body weight per day,which is 10 times the standard human dose).Gavage was performed twice daily for 3 consecutive days (3 mL of solution per dosage).Three days after gavage,the rats were fasted for 12 h.Then,a final gavage was conducted,and the rats were anesthetized using sodium pentobarbital(intraperitoneal injection) 1 h later for blood sampling (4 mL from each rat)viathe abdominal inferior vena cava under aseptic conditions.Blood samples were refrigerated overnight and then centrifuged (1006.2 ×gat 4 ℃ for 15 min) to collect serum.All serum samples from rats in the same gavage group were merged and inactivated at 56 ℃ (water bath for 30 min).Thereafter,the serum samples were filtered through a microporous membrane under aseptic conditions.The filtrate was aliquoted and stored at-80 ℃ until use.
2.3.Cell culture
NRK-49F cells (ATCC,USA) were digested and seeded into 96-well plates at a density of 4 × 104cells/mL.After the cells became adherent,the culture medium was switched to serum-free medium,and the cells were synchronized for 24 h.Then,the serum-free medium was replaced with the test medium (see below),and the cells were cultured for 72 h.
TGF-β1-treated NRK-49F cells were used as a myofibroblast differentiation model as reported previously.20To determine the appropriate TGF-β1 concentration for inducing myofibroblast differentiation,NRK-49F cells were cultured for 72 h with different concentrations (0,1.25,2.5,5,10,20,50,and 100 ng/mL)of recombinant human TGF-β1 (Peprotech,Cranbury,NJ,USA).A TGF-β1 concentration of 10 ng/mL was deemed appropriate on the basis of proliferation(measured at 12,24,48,and 72 h after treatment,as described further),and morphological changes in these TGF-β1-treated NRK-49F cells were recorded after 12,24,48,and 72 h of treatment using an inverted-phase contrast microscope (S8APO,LEICA,Solms,Germany).Thereafter,NRK-49F cells were cultured under different conditions for 48 h as follows: (a) Control group,cells received no treatment (cultured in 10% fetal bovine serum);(b) TGF-β1 group,cells were treated with TGFβ1 (10 ng/mL) and 10% normal rat serum (rats were given salineviagavage);(c) TGF-β1 plus low-dose SWF rat serum group,cells were treated with TGF-β1(10 ng/mL) and 10% low-dose SWF rat serum (rats were given low-dose SWFviagavage);(d),TGF-β1 plus medium-dose SWF rat serum group,cells were treated with TGF-β1 (10 ng/mL) and 10% medium-dose SWF rat serum (rats were given medium-dose SWFviagavage);(e) TGF-β1 plus high-dose SWF rat serum group,cells were treated with TGF-β1 (10 ng/mL) and 10% high-dose SWF rat serum (rats were given highdose SWFviagavage);(f) TGF-β1 plus valsartan rat serum group,cells were treated with TGF-β1 (10 ng/mL)and rat serum containing 10% valsartan (rats were given valsartanviagavage);and (g) medium-dose SWF rat serum group,cells treated with 10% medium-dose SWF rat serum alone (rats were given medium-dose SWFviagavage).
2.4.Determination of cell proliferation
The proliferation of NRK-49F cells was determined using a CCK-8 kit purchased from KGI Biotechnology Development Co.,Ltd.(Nanjing,China).Briefly,10 μL of CCK-8 and 100 μL of serum-free culture medium were added to each well of the culture plate.The plate was incubated at 37 ℃ for 2-4 h,and the absorbance of each well was recorded at 490 nm.The rate of proliferation was calculated according to the manufacturer’s instructions.An increase in the optical density indicated increased cell proliferation.
2.5.Cell cycle distribution
The cell cycle distribution was analyzed using flow cytometry following propidium iodide (PI) staining.Briefly,cells were seeded and allowed to adhere overnight.Then,the medium was changed to SFNcontaining complete medium for 24 h (at 37 ℃).The cells were then sampled,washed [with phosphatebuffered saline (PBS)],and fixed (with 70% ethanol) for PI staining (treated for 30 min with 80 μg/mL RNase A and 50 μg/mL PI).These PI-stained NRK-49F cells were subjected to flow cytometry (BD FACSCanto™,USA)to determine the cell cycle distribution.
2.6.Immunofluorescent assay (IFA)
NRK-49F cells were plated onto coverslips (2000-5000 cells each) and fixed with 10% paraformaldehyde for 10 min.After washing thrice with PBS (5 min each),the fixed cells (on the coverslip) were permeabilized for 10 min in 2.5% Triton X-100 (Sigma,shanghai,China).The cells were washed again with PBS (three washes for 5 min each at room temperature) and blocked with 0.5 mL of 10% goat serum for 1 h.Then,the coverslips(with adherent cells) were incubated with 20 μL of primary antibody (diluted to 1∶100,dropped onto the sealed membrane with cells face down) overnight at 4 ℃.After three washes with PBS (5 min each at room temperature),the secondary antibody (20 μL,diluted to 1∶400,dropped onto the sealed membrane with the cells facing down) was applied accordingly.Following incubation for 1 h,cells were washed with PBS again(three washes for 5 min each) and stained with DAPI(Sigma) for 5 min.Thereafter,the cells were washed with PBS (three times for 5 min each),dried with Kimwipes,and covered by slides with anti-fluorescence quenching agents (cells facing down on the anti-fluorescence quenching slides).The stained slides were stored at-20℃ in the dark and scanned using an upright fluorescence microscope (Nikon 80i,Japan).Primary antibodies to α-smooth muscle actin (α-SMA),fibronectin,and proliferating cell nuclear antigen (PCNA) were purchased from Abcam (USA),Boster Biological Technology Co.,Ltd.(Wuhan,China),and Bioworld (USA),respectively.Goat anti-mouse immunoglobulin G (IgG)and goat anti-rabbit IgG secondary antibodies [horseradish peroxidase (HRP)-conjugated] were purchased from Bioworld.
2.7.Real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from NRK-49F cells using TRIzol reagent (Invitrogen,USA).The cDNA was then reconstituted using a commercial kit (Thermo Fisher Scientific,Fermentas,USA).Quantitative RT-PCR was performed using primers (Table 1) synthesized by Shanghai Biological Engineering Technology and Service Limited Company (Shanghai,China).The RTPCR procedure was as follows: 95 ℃ for 10 min,40 cycles of 95 ℃ for 15 s,55 ℃ for 15 s,and 72 ℃ for 20 s,and a final 7-min extension at 72 ℃.The mRNA expression of α-SMA,fibronectin,PCNA,and collagenⅠwas quantified using the 2-ΔΔCtmethod (GAPDH served as an internal control).

Table 1 Primers used in real-time polymerase chain reaction
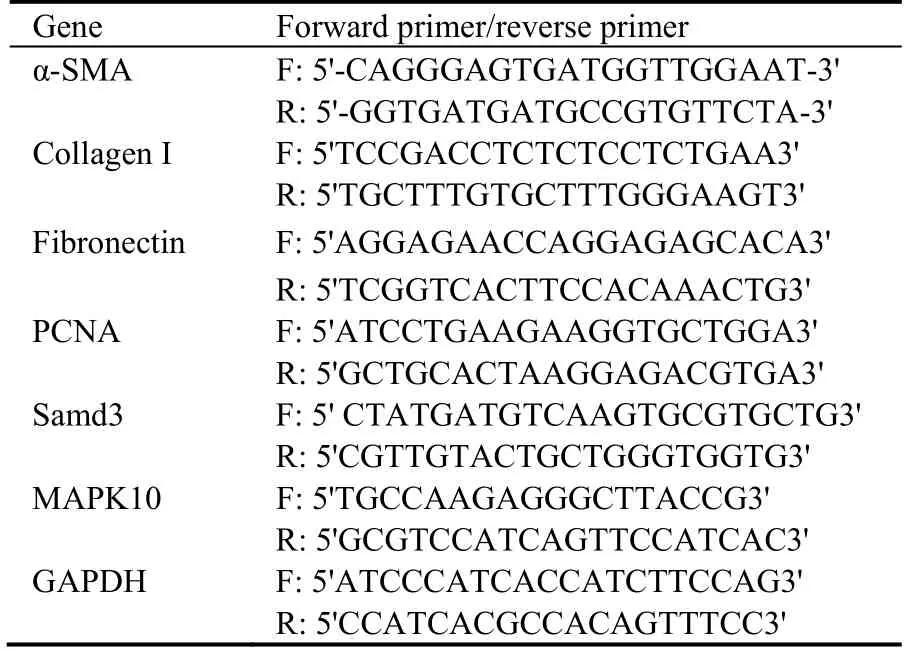
Table 1 Primers used in real-time polymerase chain reaction
2.8.Western blotting
The protein component of the cells was extracted and determined using a BCA protein assay kit.This protein component was electrophoresedvia5%-12% SDSPAGE and transferred electrophoretically to polyvinylidene difluoride (Immobilon) membranes.These membranes were incubated with primary antibodies(diluted to 1∶3000) overnight at 4 ℃.The secondary antibodies (HRP-conjugated,diluted to 1∶3000) were applied for 2 h at room temperature.Then,the membranes were washed,probed,and autoradiographed.Antibodies to α-SMA,JNK-3,and collagenⅠwere purchased from Abcam.The antibodies to fibronectin and PCNA were purchased from Boster Biological Technology Co.,Ltd.and Bioworld,respectively.Antibodies to phosphorylated Smad3 (p-Smad3) and JNK were purchased from Cell Signaling Technology and Signal way Antibody,respectively.Goat anti-mouse IgG and goat anti-rabbit IgG secondary antibodies were purchased from Bioworld.The band density was quantified using Quantity One 4.6.3 and then normalized to the GAPDH content as a loading control.
2.9.Statistical analysis
One-way analysis of variance (ANOVA) was performed to assess the between-group differences using SPSS 17.0(SPSS Inc.,Released 2008.,SPSS Statistics for Windows,Version 17.0.,Chicago,IL,USA).Subsequently,the post hoc Student-Newman-Keuls test was conducted when the outcome of one-way ANOVA was significant (P <0.05).Data are presented as the mean ±standard error of mean.
3.RESULTS
3.1.Development of TGF-β1-induced myofibroblast differentiation in NRK-49F cells
Control cells exhibited a spindle shape and close arrangement.Conversely,TGF-β1-treated cells had a short spindle or polygonal shape with an increased cell volume and cytoplasmic content.Moreover,TGF-β1-treated cells had larger nuclei (circular or oval) than control cells (Supplementary Figure 1).We reveals that the addition of TGF-β1 increased (P <0.05) the proliferation of NRK-49F cells.The highest cell proliferation rate was observed in cells treated with 10 ng/mL TGF-β1 for 48 h (Supplementary Figure 2).
3.2.Effect of SWF-containing serum on morphology,proliferation,and cell cycle progression
As presented in Supplementary Figure 3,control cells were long,spindle-shaped,and arranged closely.However,TGF-β1 treated cells were short,spindleshaped,or polygonal-shaped,and they had a higher cell volume and cytoplasmic content as well as a larger nucleus (circular or oval) compared with the findings in control cells.Interestingly,the use of SWF-or valsartancontaining serum corrected (or partially corrected) the TGF-β1-induced abnormalities of cell morphology.Without TGF-β1 treatment,cells treated with SWFcontaining serum had a normal morphology.Supplementary Figure 4 reveals that the cells treated with TGF-β1 plus high-dose SWF rat serum had a lower (P <0.05) cell proliferation rate than those cells treated with TGF-β1 alone.Compared with the findings in control cells,TGF-β1-treated cells exhibited a smaller G0/G1 population (P <0.01) and a larger S-phase population(P <0.01) (Figure 1).Interestingly,compared with the findings in TGF-β1-treated cells,cells treated with TGFβ1 plus SWF-containing rat serum had a larger G0/G1 population (P <0.01) and a smaller S-phase population(P <0.01).

Figure 1 Effect of Shenweifang (SWF)-containing serum on the cell cycle distribution of transforming growth factor (TGF)-β1-treated normal rat kidney interstitial fibroblast cells (NRK-49F)
3.3.Effect of SWF-containing serum on α-SMA,fibronectin,and PCNA protein expression
The results of IFA illustrated that TGF-β1-treated cells exhibited higher α-SMA,fibronectin,and PCNA expression than control cells.However,the use of SWFor valsartan-containing serum corrected (or partially corrected) the TGF-β1-induced changes of α-SMA,fibronectin,and PCNA expression (Figure 2).
3.4.Effect of SWF-containing serum on the mRNA expression of α-SMA,fibronectin,PCNA,collagenⅠ,Smad3,and MAPK10
Figures 3 and 4 reveal that TGF-β1 treatment increased(P <0.01) the mRNA expression of α-SMA,fibronectin,PCNA,collagenⅠ,Smad3,and MAPK10 in NRK-49F cells.Interestingly,the use of SWF-containing serum(low,medium,or high dose) or valsartan-containing serum corrected (or partially corrected,P <0.01) the TGF-β1-induced upregulation of α-SMA and collagenⅠmRNA expression.Medium-and high-dose SWFcontaining serum corrected (or partially corrected,P <0.05) the TGF-β1-induced increases of fibronectin and PCNA mRNA expression.Low-dose SWF-containing serum corrected (or partially corrected,P <0.05) the TGF-β1-induced upregulation of PCNA mRNA expression.Medium-dose SWF-containing serum corrected (or partially corrected,P <0.05) the TGF-β1-induced upregulation of Smad3 mRNA expression.High-dose SWF-containing serum corrected (or partially corrected) the TGF-β1-induced upregulation of Smad3(P <0.01) and MAPK10 mRNA expression (P <0.05).Valsartan-containing serum corrected (or partially corrected,P <0.05) the TGF-β1-induced upregulation of Smad3 mRNA expression.
3.5.Effect of SWF-containing serum on fibronectin,α-SMA,collagenⅠ,PCNA,Smad2/3,JNK-3,p-Smad3,and JNK-3 protein expression
As shown in Figures 5,6,TGF-β1 treatment increased (P<0.01) the protein expression of fibronectin,α-SMA,collagenⅠ,PCNA,p-Smad3,and JNK-3.The use of SWF-containing serum (low,medium,or high dose) or valsartan-containing serum corrected (or partially corrected,P <0.01) the TGF-β1-induced upregulation of collagenⅠ,p-Smad3,and JNK-3 expression.Mediumdose SWF-containing serum also corrected (or partially corrected) the TGF-β1-induced upregulation of PCNA(P <0.05),α-SMA (P <0.01),and fibronectin expression (P <0.05).High-dose SWF-or valsartancontaining serum corrected (or partially corrected)the TGF-β1-induced upregulation of PCNA (P <0.05),α-SMA (P <0.01),and fibronectin expression(P <0.01)

Figure 2 Effect of Shenweifang (SWF)-containing serum on the protein expression of α-smooth muscle actin (α-SMA),fibronectin,and proliferating cell nuclear antigen (PCNA) in transforming growth factor (TGF)-β1-treated normal rat kidney interstitial fibroblast cells (NRK-49F)
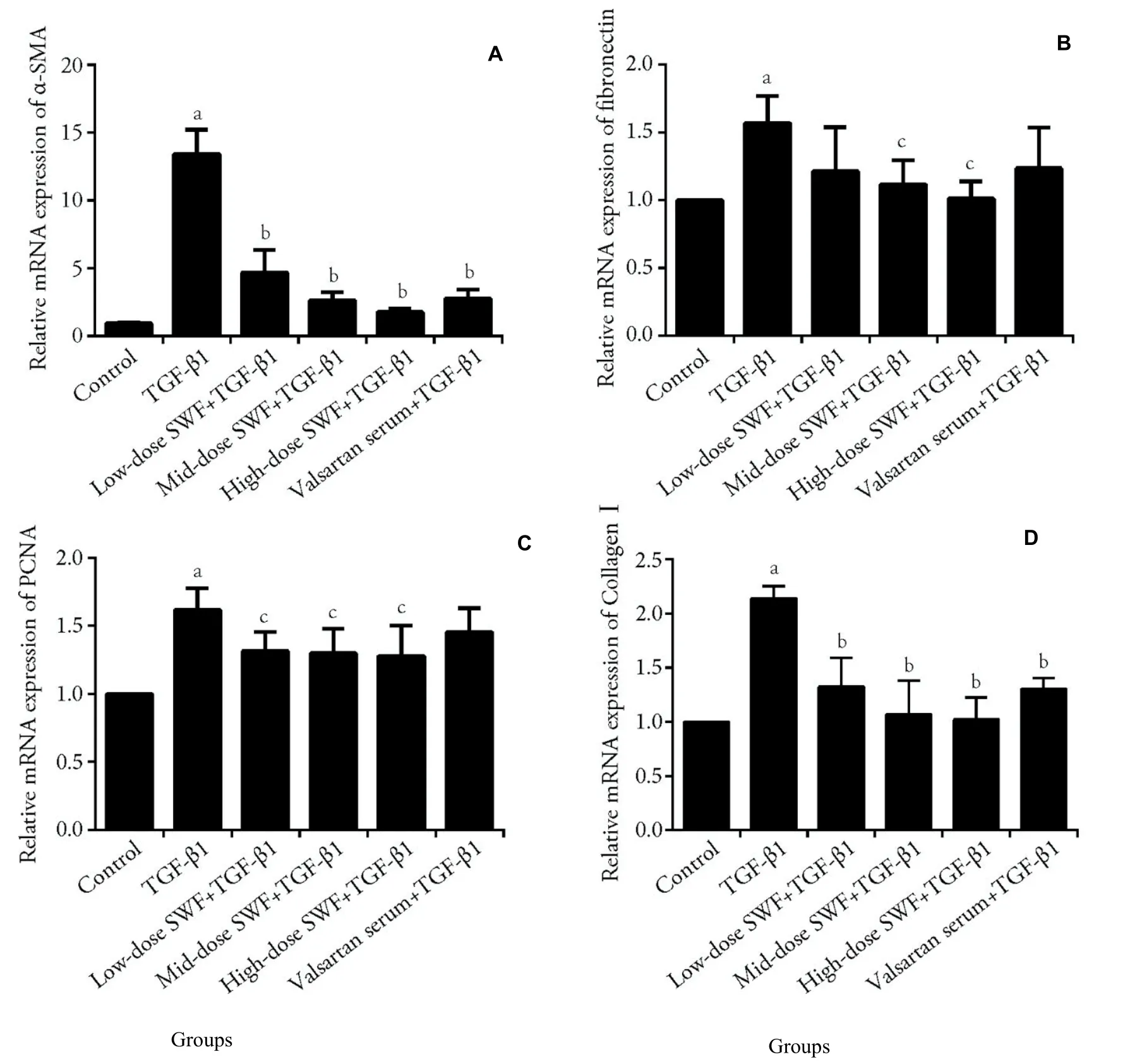
Figure 3 Effect of Shenweifang (SWF)-containing serum on the mRNA expression of α-smooth muscle actin (α-SMA),fibronectin,proliferating cell nuclear antigen (PCNA),and collagen I in transforming growth factor (TGF)-β1-treated NRK-49F cells

Figure 4 Effect of Shenweifang (SWF)-containing serum on the mRNA expression of Smad3 and MAPK-10 in transforming growth factor(TGF)-β1-treated NRK-49F cells
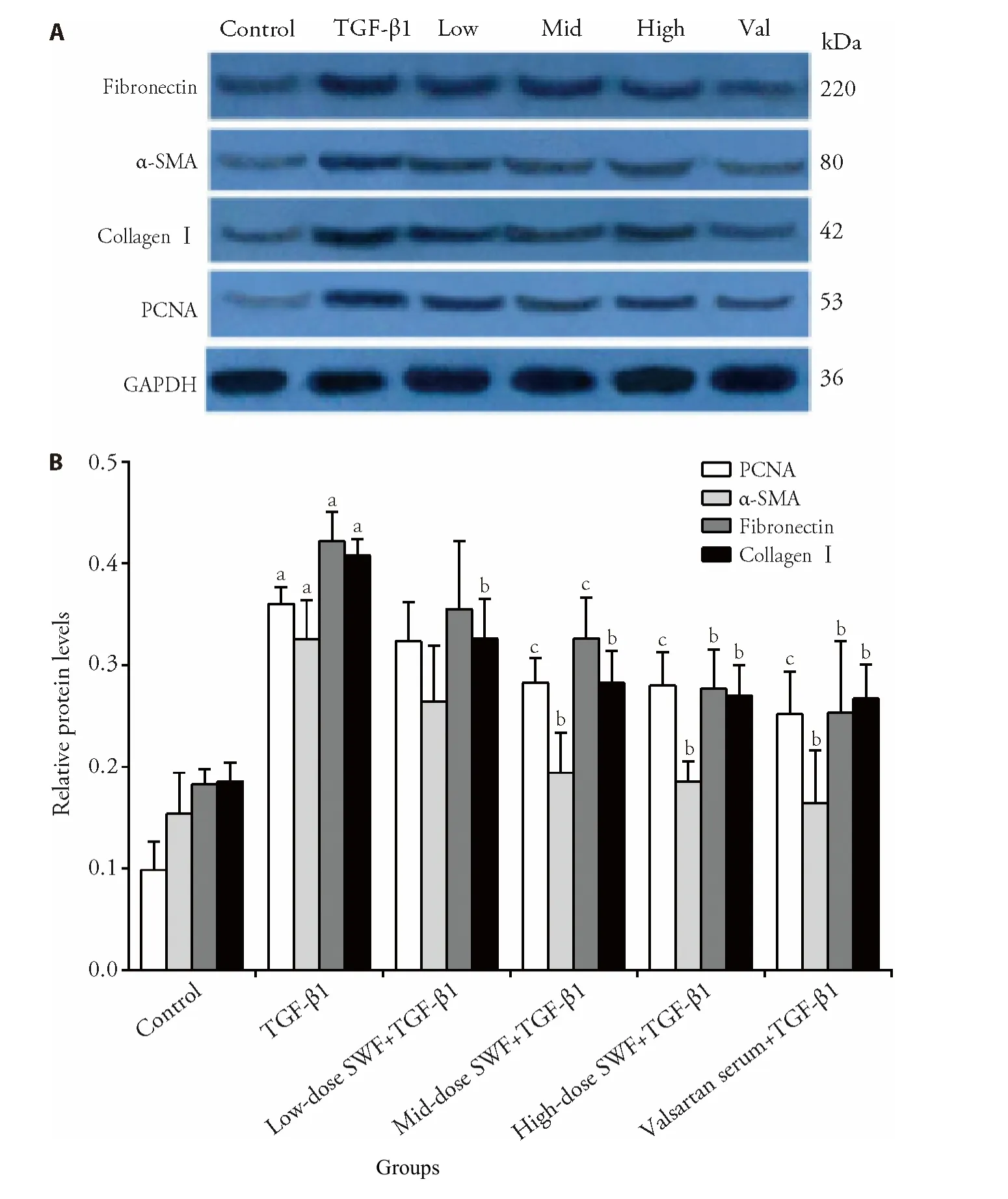
Figure 5 Effect of Shenweifang (SWF)-containing serum on the protein expression of α-smooth muscle actin (α-SMA),fibronectin,proliferating cell nuclear antigen (PCNA),and collagen I in transforming growth factor (TGF)-β1-treated NRK-49F cells

Figure 6 Effect of Shenweifang (SWF)-containing serum on the protein expression of Smad2/3,phosphorylated Smad3 (p-Smad3),c-Jun Nterminal kinase (JNK),and JNK-3 in transforming growth factor (TGF)-β1-treated NRK-49F cells
4.DISCUSSION
Similar to the effects of valsartan-containing serum,SWF-containing serum corrected (or partially corrected)cell morphology and cell cycle distribution abnormalities in TGF-β1-treated NRK-49F cells.Furthermore,highdose SWF-containing serum decreased TGF-β1-induced increases in cell proliferation.Valsartan is a highly selective antagonist to angiotensin Ⅱ type 1 receptors,which are involved in renal fibrosis.21Valsartan therapy has been revealed to be effective in reducing renal fibrosis regardless of the underlying causes.22-25The current result is consistent with our previous understanding of reports in the literature.17-19and suggests that SWF might have the same mechanism as valsartan in preventing or treating CKD.In normal cells without TGF-β1 treatment,the application of SWFcontaining serum had no harmful effect on cell morphology.In other words,SWF-containing serum is presumably nontoxic/safe for cell growth.
Renal fibrosis is a typical characteristic of end-stage CKD.In healthy individuals,most renal interstitial fibroblasts are in the resting state.In cases of inflammation and medical/pro-fibrosis factor stimulation,renal interstitial fibroblasts in the resting state can be induced to develop into matrix-producing myofibroblasts.Large quantities of α-SMA are secreted during this fibroblast-myofibroblast transition.Therefore,α-SMA has long been used as the key predictor of CKD progression (renal fibrosis).26In this study,α-SMA expression (both mRNA and protein) in NRK-49F cells was significantly increased by TGF-β1 treatment.Interestingly,the addition of SWF-containing serum,as well as valsartan-containing serum,effectively reversed the TGF-β1-induced release of α-SMA.Similarly,SWF-and valsartan-containing serum decreased the abnormal expression of fibronectin and collagen Ⅰin TGF-β1-treated cells.Thus,it can be concluded that SWF-containing serum could prevent (or at least partially prevent) the induction of the fibroblastmyofibroblast transition by TGF-β1in vitro.
Furthermore,SWF-containing serum prevented the TGF-β1-induced fibroblast-myofibroblast transition,probably through Smad2 and Smad3,which directly determine the processes mediating renal fibrosis.26-30It has been repeatedly demonstrated that knocking out Smad3 could prevent TGF-β1-induced renal fibrosis.28,31-35In the current study,TGF-β1 treatment significantly increased Smad3 mRNA and protein expression in NRK-49F cells.In particular,TGF-β1-induced Smad3 expression was decreased by the addition of SWF-and valsartan-containing serum.These results indicate that (a)the TGF-β1/Smad 3 signaling pathway is a host-targeting pathway for preventing/treating renal fibrosis and (b)SWF and valsartan share the same mechanism (targeting Smad3) in preventing/treating renal fibrosis.
In this study,significant changes were observed in the TGF-β1/MAPK signaling pathway (e.g.,p38,JNK,and ERK proteins),a non-Smad signal that is involved in renal fibrosis.36-38Strategies targeting the activation and transcription of JNKs (MAPK8,MAPK9,and MAPK10)have been revealed to be equally effective in controlling the progression of CKD.38As expected,the mRNA expression of MAPK10 and the protein expression of JNK-3 were increased by TGF-β1 stimulation.More interestingly,all of these abnormal changes were decreased by the addition of SWF-or valsartancontaining serum.Therefore,we hypothesize that the SWF-containing serum prevents TGF-β1-induced renal fibrosis through (or partially through) the TGFβ1/Smad3 and TGF-β1/MAPK signaling pathways.Future research should investigate (a) the active components of SWF and (b) the interactions between the TGF-β1/Smad3 and TGF-β1/MAPK signaling pathways.Drug-containing sera have long been used to investigate the pharmacological mechanisms underlying the efficacy of Traditional Chinese Medicines.39-41In this study,SWF-containing serum was collected from rats.Moreover,the cell line used (NRK-49F) is a rat renal interstitial fibroblast line.Therefore,no interspecies interference existed in the currentin vitrosystem.In previous studies,drug-containing sera were generally prepared by gav-aging the animals with the corresponding drug twice daily for 3 d,and blood samples were collected 1 h after the last gavage.39In this study,SWF-containing serum was prepared in the same manner,and the results clearly demonstrated its efficacy in preventing the TGF-β1-induced fibroblastmyofibroblast transition.In the currently available literature,it is controversial whether drug-containing sera should be inactivated before use.Some reports stated that the complements and antibodies in fresh serum could affect thein vitrocell system.Thus,we inactivated SWF-containing serum,and it worked well in NRK-49F cells.In this study,low-,medium-,or highdose SWF-containing serum suppressed the TGF-β1-induced fibroblast-myofibroblast transition.Future studies should attempt to determine the appropriate dose of SWF for animal gavage.
In conclusion,SWF-containing serum blocked (or partially blocked) the TGF-β1/Smads and TGFβ1/MAPK/JNK pathways and reversed changes of morphology,cell cycle progression,and proliferation in TGF-β1-treated NRK-49F cells.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Flavonoids from traditional Chinese herbs for diabetes in rats: a network Meta-analysis
- In-vitro and in-vivo pharmacological screening of Iris albicans
- Detailed approach toward the anti-hyperglycemic potential of Sterculia diversifolia G.Don against alloxan-induced in vivo hyperglycemia model
- Efficacy of Bushen Culuan decoction (补肾促卵方) on ovarian follicle and follicular granulosa cells in mice with premature ovarian insufficiency induced by tripterygium wilfordii polyglycoside
- Efficacy of Wumei Baijiang prescription (乌梅败酱方) on regulatory T cells/ helper T cells Immune balance in mice with ulcerative colitis
- Therapeutic effects of salidroside vs pyrrolidine dithiocarbamate against severe acute pancreatitis in rats
