A cross-sectional study of post-COVID syndrome at a tertiary care center in Turkey
2022-11-09EfraimGuzelOyaBaydarToprakBurakMeteYaseminSaygdegerBenguCurukSedatKuleci
Efraim Guzel, Oya Baydar Toprak, Burak Mete, Yasemin Saygıdeger, Bengu Curuk, Sedat Kuleci
1Cukurova University, Faculty of Medicine, Department of Chest Diseases, Adana, Turkey
2Cukurova University, Faculty of Medicine, Department of Public Health, Adana, Turkey
ABSTRACT
KEYWORDS: Post-COVID syndrome; COVID-19; Symptoms;Pulmonary function tests; Risk factors
1. Introduction
As the COVID-19 pandemic enters its third year, the World Health Organization reported that more than 610 million individuals have been infected with the virus, with more than 6.5 million deaths[1].COVID-19 is typically asymptomatic or advances with modest illness symptoms in the acute stage, but it may potentially evolve to severe forms which can lead almost to death in a group of patients.Furthermore, survivors have been seen to have persisting symptoms of various organs and systems which can linger for weeks or months[2]. Post-COVID Syndrome (PCS) has been defined as signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis; it usually presents with clusters of symptoms, often overlapping, which can fluctuate and change over time and can affect any system in the body; PCS might be considered before 12 weeks while the possibility of an alternative underlying disease is also being assessed[3].
Dyspnea is known to be the most often reported respiratory symptom after COVID-19. The frequency of persistent dyspnea varied from 5%-81% in hospitalized patients and 14% in nonhospitalized patients, according to studies reporting respiratory symptoms after 1 to 12 months following COVID-19[4-11]. Cough is less prevalent, although it can last for weeks or months after SARS-CoV-2 infection and has been observed in a wide range(2%-42%) of patients[5,7-9,12]. While numerous researches have effectively documented the symptoms of the post-COVID period,data on the incidence of these symptoms is reported in a wide range. Uncertainties regarding when the respiratory dysfunction may develop in which patients, when the resulting impairment will improve, or the predisposing factors of this dysfunction still continue. The goal of this study was to determine the long-term symptoms that may arise as a result of the COVID-19, to assess the relationship between these symptoms and pulmonary functions, and to identify the risk factors for post-COVID symptoms in patients who had an acute COVID-19 either at home or in the hospital at least four weeks after recovery.
2. Subjects and methods
2.1. Ethical approval and participants consent statement
This is a cross-sectional study that was approved by the local institutional ethics committee of Cukurova University, Adana,Turkey (approval No. 117/2021). An informed consent statement was required to be assigned by the participants. All procedures performed in the study involving human participants were in accordance with the ethical standards of the hospital, national research committee and the 1964 Helsinki declaration.
2.2. Study design and participants
This cross-sectional study was conducted at Cukurova University Faculty of Medicine Balcali Hospital, Department of Chest Diseases between December 2020 and May 2021. A total of 123 individuals who had COVID-19 and followed-up either at home or in the hospital participated the study at least after four weeks of acute infection. The patients were separated into two groups:
Group 1: the participants in long-COVID first three months;
Group 2: the participants in post-COVID period after three months to 1 year of acute infection.
The severity of COVID-19 disease has been assessed in the literature using several parameters[4,13]. This study categorized the diseases severity as:
Mild disease: less than 25% uptake on thorax computed tomography (CT) and outpatients;
Moderate disease: 25%-49% CT involvement or hospitalized or with low flow oxygen support and not admitted to the intensive care unit;
Severe disease: more than 50% CT involvement or high flow oxygen support or treatment in the intensive care unit.
2.3. Variables and measurement
The data collection forms were used to record sociodemographic characteristics, previous history of post-COVID symptoms,momentary history and physical examination signs, previous and novel laboratory and radiographic findings, medical therapies employed during the acute infection and in post-COVID period.Extensive biochemical tests including routine complete blood count,C-reactive protein, procalcitonin, ferritin, liver and kidney function tests were documented. According to the Charlson Comorbidity Risk Index, the participant’s comorbidities were classified as “0 points low, 1-2 points moderate, 3-4 points high, and 5 points and above very high risk”[14].
Thorax CT/high resolution computed tomography was performed in all patients in the radiological evaluation. Major CT images have been described in international standard terminology defined by the Fleischner Society dictionary and in the peer-reviewed literature on viral pneumonia using terms such as ground glass opacity, crazy pavement pattern, and consolidation[5,16]. A semiquantitative scoring system was used to quantitatively predict the pulmonary involvement of all these abnormalities based on the area of interest[17]. Lung involvements were evaluated over 6 lobes by considering the lingula as a separate lobe and 0=no involvement,1=1%-24% involvement, 2=25%-49% involvement, 3=50%-75%involvement and 4≥75% involvement classified[17]. Image analysis was evaluated by expert radiologists in our institution using the institutional digital database system (HBYS Mergentech PACS,version v3.22.03.1-20220314).
Pulmonary function tests (PFTs) were performed in accordance with the American Thoracic Society (ATS) guideline with a calibrated Sensor Medics V-Max 20 Spirometer (Jaeger MS-PFT Analyzer Unit, Viasys Healthcare GmbH, Höchberg, Germany).Basal forced expiratory volume 1 second (FEV1) and forced vital capacity (FVC) measurements were made according to the ATS guideline. Total lung capacity was measured with helium dilution technique (Jaeger MS-PFT Analyser Unit) and Transfer Factor for Carbon Monoxide was measured with a single breath method. It is measured by a single breath technique where 10% helium and 0.3% carbon monoxide are rapidly inspired, held for 10 seconds and then expired with the measurement of the remaining carbon monoxide[18]. Test results are presented as percentage of predicted values. The results of pulmonary function tests are interpreted based upon the ATS/ European Respiratory Society recommendations[19].The experimental model of our study, in which we show the PFTs,symptoms and radiological features of 2 groups who applied to the outpatient clinic early and late in the post-COVID period, in a single picture, is explained in detail in Figure 1.
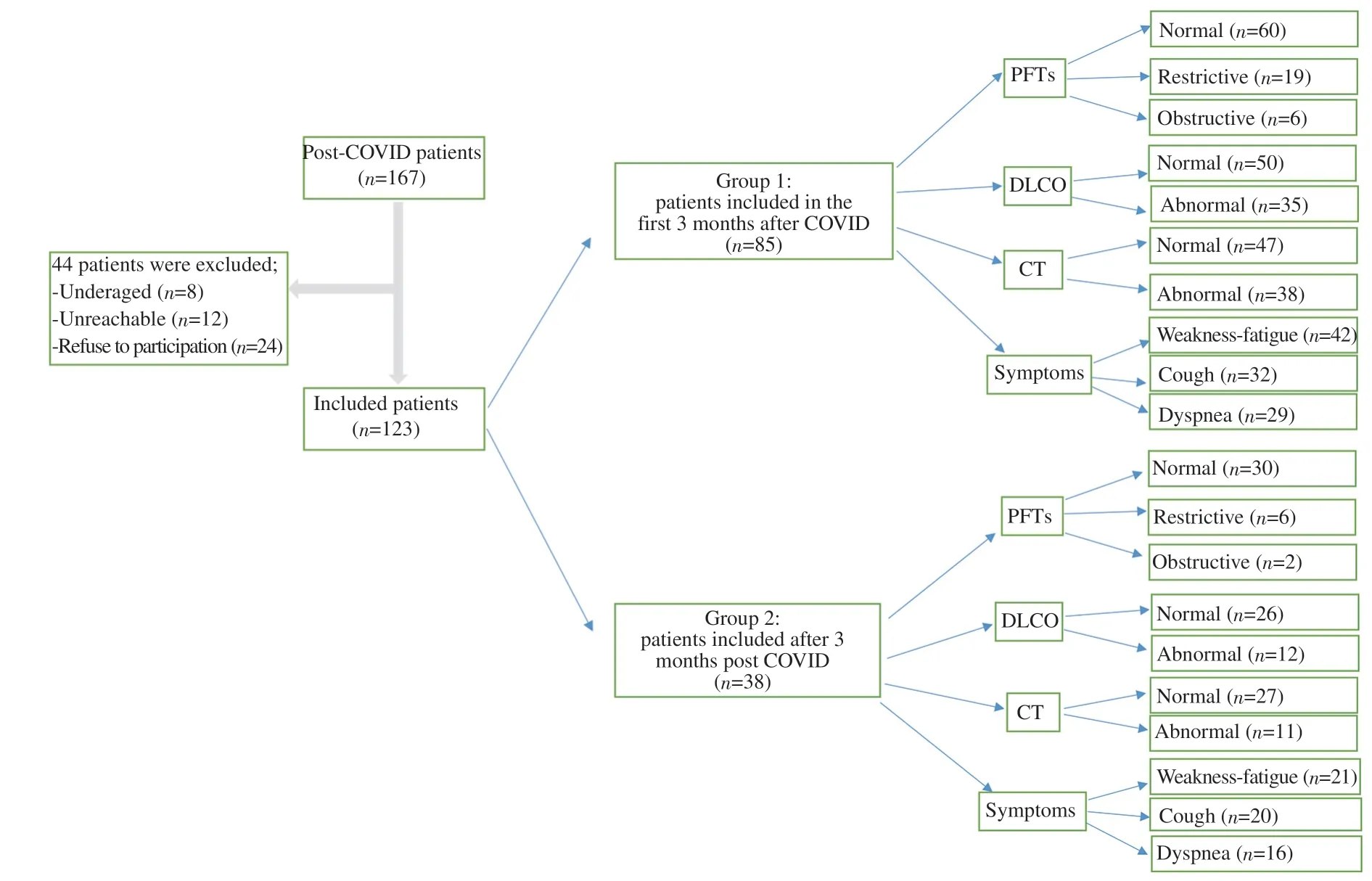
Figure 1. Registration and follow-up of post-COVID patients in the first three months and after three months.
2.4. Outcomes
Primary outcome:
-To determine the incidence of post-COVID syndrome and post-COVID pulmonary symptom frequencies;
-To identify the risk factors in post-COVID syndrome.
Secondary outcomes:
-To clearly document the incidence and characteristics of radiologic and pulmonary functional abnormalities in both the first three months and after three months of acute infection;
-To identify the long-term radiologic sequela of COVID-19.
2.5. Data analysis
SPSS22 program was used in the analysis of the data. Kolmogorov Smirnov test was used as the normal distribution test. T test, Mann Whitney U test, One Way ANOVA test, Kruskal Wallis test, Chisquare test were used in the analysis. Binary logistic regression test,Cox regression test were used in the analysis. A value of P<0.05 was considered statistically significant.
3. Results
The mean age of 123 participants in the post-COVID period was(54.8±13.8) years (range: 21-84). The mean time of admission to the outpatient clinic was (75.4±46.1) days (range: 24-330). A total of 85 (69.1%) patients admitted to outpatient clinic visit in the first three months after acute infection. There was at least one additional disease in 54 (63.5%) of our patients admitted within the first 3 months after acute COVID-19 (group 1) and in 30 (78.9%) of the patients admitted after the 3rd month (group 2). The most common comorbidities observed in group 1 patients were hypertension(29.4%), chronic lung diseases (21.2%), diabetes mellitus (DM)(15.3%) and cardiovascular diseases (15.3%). The most common comorbidities in group 2 patients were chronic lung diseases(34.2%), hypertension (23.7), DM (18.4%) and neuropsychiatric diseases (18.4%), respectively. At least one symptom was present in 91.9% (n=110) of the patients during the acute disease and 61.8%(n=76) in the post-COVID period. The most common symptoms were weakness-fatigue (n=63, 51.2%), loss of taste/smell (n=25,20.3%), myalgia (n=24, 19.5%), others (n=22, 17.9%) whereas the most common respiratory symptoms were cough (n=52, 42.3%),dyspnea (n=45, 36.6%) and chest pain (n=5, 4.1%) consequently.At least one symptom was present in 78 (91.8%) of our group 1 patients and 35 (92.1%) of our group 2 patients. The most common symptoms observed in both group 1 and group 2 patients were fatigue-fatigue, cough and dyspnea, respectively. Comorbidities and symptoms by groups are listed in Table 2. DM (n=2), HT (n=1),respiratory failure (n=2), pulmonary embolism (n=1), organized pneumonia (n=2), pulmonary nodule (n=1), and neuropsychiatric disease (n=1) were among the new diagnosed diseases after COVID-19. Thirty-five (28.5%) of the participants used systemic corticosteroid (1 mg/kg/day methyl prednisolone) during the acute illness. The detailed data of sociodemographic characteristics and symptom distribution of the participants are given in Table 1.
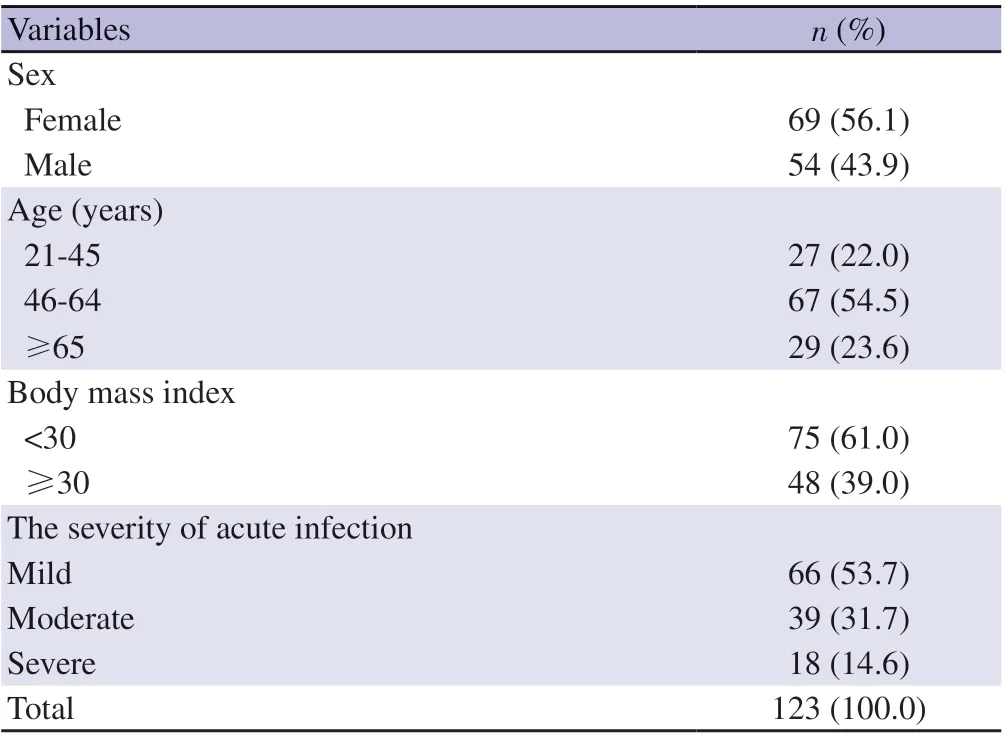
Table 1. Sociodemographic and clinical characteristics.
Of the 123 participants in the study, 25 (20.3%) had restrictive,8 (6.5%) had obstructive type of respiratory dysfunction, and 47(38.2%) had a decrease in the diffusing capacity of the lung for carbon monoxide (DLCO). In the first three months, PFTs were normal in 60 (70.6%), restrictive in 19 (22.4%) and obstructive in 6(7.1%) of the participants. In the 91 days to one-year period, PFTs were normal in 30 (78.9%), restrictive in 6 (15.8%) and obstructive in 2 (5.3%) of the participants. The FEV1(L) and DLCO (%) in the first three months after acute infection were found to be statistically significantly lower (P=0.027 and P=0.025, respectively) than later periods. When the PFTs in post-COVID era were examined according to the severity of the acute illness, it was discovered that there was a statistically significant difference between the post-COVID period FVC (% pred), FVC (L), and FEV1/FVC values; in the post-COVID period, severe patients had lower FVC (% pred)and FVC (L) compared to mild and moderate patients, while FEV1/FVC was higher (Table 3).
The radiologic features were normal in 42.4% and 55.3% of Chest X-Ray and computerized tomography respectively in the first three months. After three months, 22.4% of chest X-rays and 7.9% of computerized tomography revealed progression. Ofthe patients in group 1 with progression observed in thoracic CT,ground glass opacity was detected in 8 (9.4%), consolidation in 6(7.1%), fibrosis in 5 (5.9%) and organized pneumonia in 1 (1.2%).Mosaic attenuation was observed in 2 patients (2.3%) in whom non-COVID-19 findings were detected, and cavitation was observed in 1 (1.2%). Fibrosis was detected in 3 (7.9%) patients in group 2 with progression on thorax CT. The detailed data about radiologic features is presented in Figure 2.

Table 2. Comorbidities and symptoms by time of admission to the outpatient clinic.
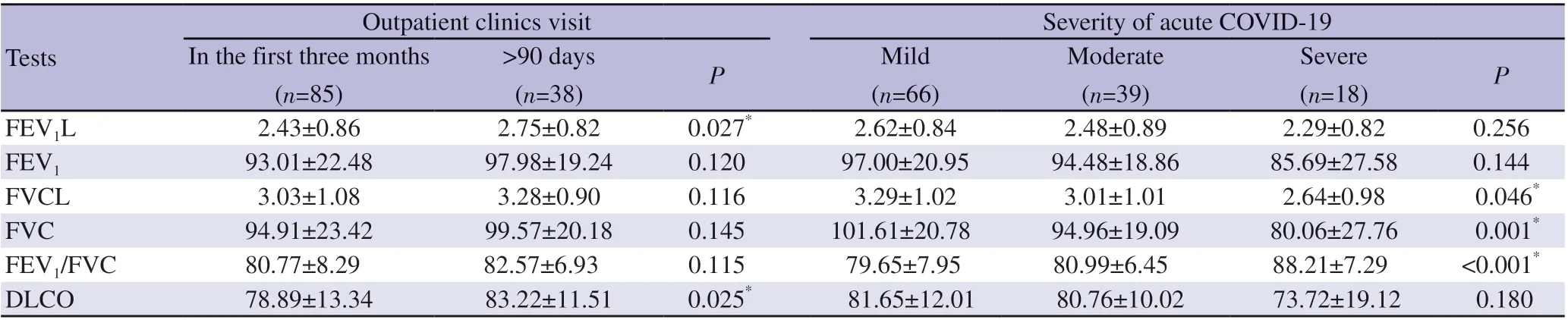
Table 3. Pulmonary function tests according to admission time and disease severity (%).

Figure 2. Radiologic features in post-COVID period.

Table 4. Logistic regression analysis for post-COVID symptoms.
The model developed to predict post-COVID syndrome is significant (Omnibus test P<0.001), with an accuracy rate of 80.5 percent and a Nagelkerke R square value of 45%. Patients who developed acute complications (severe pneumonia, acute respiratory distress syndrome, irregularity of blood sugar level due to the use of corticosteroids, etc.) (OR 9.91, 95% CI 1.93-50.87), had 2 or more symptoms at the time of admission (OR 7.73, 95% CI 2.56-23.33),had 1% to 14% CT involvement (OR 3.05, 95% CI 1.06-8.79), or had 50% or more CT involvement (OR 14.68, 95% CI 1.24-172.55)had a higher risk of developing post-COVID syndrome, among the variables included in the model (Table 4).
4. Discussion
The study revealed that nearly 90% of patients during the acute illness and 60% during post-COVID period had at least one symptom according to their own notifications. The most common symptoms were fatigue/weakness, cough and dyspnea. Pulmonary function tests were significantly deteriorated in patients with chronic airway diseases, systemic steroid need in acute illness, and moderate-severe illness in acute infection phase. Those who had two or more symptoms at the time of their first hospital admission,as well as those who experienced complications such as severe pneumonia, acute respiratory distress syndrome, or blood sugar dysregulation during the acute illness phase, were shown to have a higher risk of PCS. Furthermore, patients with 1%-49% CT involvement at the time of admission had a 3 times higher chance of PCS, while those with 50% or more had a 14.6 times higher risk.
In a meta-analysis with 14-118 days of follow-up, the most common symptoms were fatigue (58%), headache (44%),attention deficit (27%), hair loss (25%), and shortness of breath(24%)[20]. A cohort study conducted from January to May 2020 with a mean follow-up of 186 days found that fatigue, muscle weakness (63%), sleep difficulties (26%) and anxiety or depression(23%) were the most common disorders[21]. In a multicenter study of patients in the first wave of the pandemic in Spain, participants were evaluated an average of seven months after hospital discharge,and only 18.6% found that they were completely free of any post-COVID symptoms, and the most common symptoms were fatigue(60.8%), hair loss (26.3%) and shortness of breath (23.5%)[22]. At the end of a one-year follow-up of the same group, the prevalence of cough, chest pain, dyspnea, and fatigue were 2.5%, 6.5%, 23.3%,and 61.2%, respectively[23]. The reported disparity in long-term COVID symptom prevalence rates can be explained by the different methodologies used, as greater prevalence with active inquiry than with passive inquiry. As is typically noted in the literature,the frequency of symptoms continues to decrease as the duration of post-COVID follow-up is prolonged. The overall symptom frequency in our findings was consistent with the literature, but unlike many other studies, the frequency of cough and dyspnea were higher than selected studies. This was thought to be due to the inclusion of both inpatients and outpatients in the study, and thus patients of different severity.
A few limited studies evaluating respiratory function in MERSCoV and SARS-CoV-1 pandemics reported deterioration in exercise capacity and DLCO in SARS-CoV-1 survivors with a followup period of 6 months to 15 years, suggesting that there may be damage to the intra-alveolar diffusion pathway[24-26]. According to a two-year follow-up research, 52% of SARS-CoV-1 survivors exhibited chronic impaired diffusion function and reduced exercise capacity[27]. In another multicenter study, which included 145 patients who recovered from acute COVID-19 infection, and systematically evaluated cardiopulmonary injury by following the 60th and 100th day; decreased static and/or dynamic lung volumes or impaired lung functions reflected by impaired DLCO were found in 42% and 36% of cases, respectively[28]. One hundred days after being diagnosed with COVID-19, 22% of patients had decreased FVC and/or FEV1, 11% had decreased TLC, and 21% of all patients had impaired DLCO[28]. In our study, compatible with literature,as predicted, FEV1and DLCO improved after three months compared to the first three months. In a review including seven studies evaluating the respiratory functions of post-COVID patients,diffusion capacity deterioration, restrictive pattern and obstructive pattern were 39%, 15% and 7%, respectively[29] and our results were coherent.
The decrease in diffusion capacity, which is directly related to the severity of the acute disease, is the most frequently reported physiological disorder after acute COVID-19[5,21,30]. It has been observed that other respiratory function test parameters may also be impaired in the post-COVID period in those who have had a severe or severe acute disease process[21,31,32]. In another retrospective study examining hospitalized COVID-19 cases, a very high prevalence of diffusion capacity deterioration (66%) was detected especially in those with severe disease who were more likely to develop pulmonary fibrosis, and those with high levels of inflammatory markers such as C-reactive protein and interleukin-6 [33]. In our study, we found a significant decrease in FVC and an increase in FEV1/FVC in the post-COVID-19 period, in line with the literature,especially in severe COVID-19 patients. This was thought to be due to the preservation of FEV1values despite the significant decrease in FVC values as a result of widespread lung involvement and sequelae in severe COVID-19 patients.
In a review of the post-acute COVID-19 syndrome, those who were severely ill during acute COVID-19 and/or required intensive unit care, the elderly, those with multiple organ comorbidities and/or active cancer, those after transplantation, and those with a high symptom burden during acute infection phase were more susceptible to the development of PCS[32]. In a prospective and longitudinal study, it was reported that male sex, advanced age,presence of multiple symptoms, diarrhea, anosmia, and baseline IgG titers between 1.2 and 4 may be risk factors for a PCS after 7 months[11]. In another study, it was reported that the risk of developing PCS is high in people with advanced age, obesity and a history of pulmonary disease[34]. Patients with more than two symptoms at the time of admission, those with acute complications,and those with CT involvement all had a higher risk of PCS,according to our research. To the best of our knowledge, this is the first data indicating the increased risk according to the involvement in radiological examination at the time of acute infection.
In a thorough 6-month follow-up analysis, around 50% of the 353 patients who had lung computed tomography had at least one abnormality; the most frequent were ground-glass opacities and irregular lines, and 1% had thickening of the interlobular septum[35].Risk factors for the development of interstitial lung disease, such as ICU follow-up, invasive MV support, and bacterial superinfections,have been observed in a study looking at post-COVID pulmonary sequelae[36]. Regardless of the underlying cause, 70% of acute respiratory distress syndrome patients who recover have abnormal imaging results at 6 months, and in some situations, they may also develop sequelae such fibrosis and pulmonary hypertension[37].Some authors asserted that after a three-month follow-up, a higher incidence of fibrosis was connected to prior coronavirus-related epidemic illnesses, such as the Middle East respiratory syndrome and the severe acute respiratory syndrome, in 33% and 38% of patients, respectively[38]. Our results were also consistent with the literature. Approximately 45% of patients in the early post-COVID period had at least 1 abnormality on thoracic CT. While the most common abnormality was ground glass opacity, we found the rate of post-COVID fibrosis to be 5.9% in group 1 patients and 7.9% in group 2 patients.
This was a single-center, cross-sectional study with lack of active surveillance so the conclusions do not represent the entire population. There is a requirement for data verification in trials with a high number of participants, and prospective periodical follow-up.
SARS-CoV-2 infects a wide variety of tissues and has multi-organ and multi-system effects. Long-COVID, on the other hand, has no recognized genesis or biological foundation. Meanwhile, the prevalence, type, and related factors of such post-acute symptoms remain unclear. Long-COVID’s burden must be quantified at the population level in order to assess its impact on the healthcare system and allocate resources properly. In addition, it would not be correct to completely associate the symptoms and clinical findings after acute COVID-19 with PCS. These cases should definitely be evaluated in multidisciplinary councils established on this subject and all other causes should be excluded. Long-term symptoms must be contextualized, and risk factors must be definitively identified in order to enhance the management of such cases.
Conflict of interest statement
The authors declare that they have no potential conflict of interest including any financial, personal or other relationships with other people or organizations that could inappropriately influence or be perceived to influence the presented work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’contributions
EG, OBT, BM, SK have made significant contributions to the working concept and design. EG, OBT, YS, BC, SK contributed to the data’s collection, analysis, and interpretation. EG, OBT,BM, YS, BC, SK have written the piece or significantly edited it,accepted the submitted version, and agreed to be held personally liable for their contributions. They have also promised to ensure that any concerns regarding the truthfulness or integrity of any portion of the work are resolved. All authors read and approved the final version of the manuscript.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Non-albicans candidemia in cancer patients as an increasing health problem: A comprehensive review and meta-analysis
- The predictors of long-COVID in the cohort of Turkish Thoracic Society-TURCOVID multicenter registry: One year follow-up results
- Genetic diversity of Leishmania donovani isolates from cutaneous lesions of military personnel in the Mullaitivu and Kilinochchi districts of the Northern Province, Sri Lanka
- Will COVID-19 vaccination rates for children be still low in the future?
- A looming twindemic of COVID-19 and dengue on post-flood scenario in the developing countries
- Recurrent Marburg virus disease outbreaks from 1967 to 2022: A perspective on challenges imposed and future implications
