Non-albicans candidemia in cancer patients as an increasing health problem: A comprehensive review and meta-analysis
2022-11-09AynazGhojoghiMaryamErfaninejadEhsanAhmadpourEisaNazarAleksandraBaracMahnazFatahinia
Aynaz Ghojoghi, Maryam Erfaninejad, Ehsan Ahmadpour, Eisa Nazar, Aleksandra Barac, Mahnaz Fatahinia✉
1Department of Medical Mycology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
2Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Biostatistics, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran
4Clinic for Infectious and Tropical Diseases, Clinical Centre of Serbia, Belgrade, Serbia
ABSTRACT
KEYWORDS: Candidemia; Non-albicans Candida; Cancer;Epidemiology; Systematic review; Meta-analysis
1. Introduction
Bloodstream infections (BSIs) are defined as the presence of live bacterial or fungal pathogens in the bloodstream that cause clinical diseases[1]. In patients with serious underlying illnesses, such as hemato-oncological malignancies, these infections are a major source of life-threatening complications[2]. Bloodstream infections in these individuals result in a high rate of morbidity and mortality,as well as longer hospital stays and higher healthcare expenses[3,4].Metabolic dysfunction, ulcerating lesions on mucosal surfaces,growing catabolic state, and secondary immunological deficits to immune-modulating treatments chemotherapy, radiation therapy,and the tumor itself may all contribute to an elevated risk of BSIs in cancer patients[5]. Candida spp. is among the most important medical fungi because of the high frequency with which they infect human hosts, particularly cancer patients. The proportion of BSI caused by Candida spp. has risen globally to date[6,7]. Candida spp.is the 4thmost common cause of bloodstream nosocomial infections in the United States, according to reports[8]. Candidaemia is a leading cause of death, especially when caused by Candida spp.that are resistant to antibiotics[9]. Recent studies have shown that the attributable mortality of nosocomial candidaemia among cancer patients ranges between 10% and 50%, making it a public health concern worldwide[10-12]. The prevalence of Candida spp. blood infection in cancer patients varies by geographic region, hospital characteristics, health-care help, treatment intensity, mucosal barrier disruption, and comorbidities[6,13]. Despite decades of research,candidemia remains a major problem that requires prompt attention to the prevalence, local epidemiologic trends, potential risk factors,and outcome of this infection in cancer patients[14].
In recent years, non-albicans Candida (NACs) have been observed to be on the rise. Given to this change in the etiology, it is necessary to identify Candida species to deduce the optimal antifungal therapy in different high-risk groups[15]. Remarkably, this changing trend in the epidemiology may be due to the varying susceptibility to antifungal agents to some of these emerging NACs[16]. There has been insufficient data provided on the prevalence of NACs among cancer patients in low-income countries to date, which could be attributed to a lack of accurate diagnostic tools[10,17]. The studies showed that azoles, echinocandins, and liposomal amphotericin B(AmB) are the most common antifungal medications used to treat candidemia[2,6,9]. Recently, some researchers observed changes in the susceptibility pattern of Candida species causing bloodstream infection to antifungal agents in cancer patients. There is strong evidence that prophylactic use of azoles such as fluconazole and itraconazole during anticancer therapy has influenced alterations in the distribution of etiologic agents[2,18]. On the other hand, longterm use of other antifungal drugs has resulted in the establishment of resistant Candida spp.[19,20]. Despite our knowledge about candidemia in cancer patients, many aspects of this infection remain unknown. Indeed, clinicians face challenges due to the epidemiological differences related to Candida species and their resistance to antifungal agents. In light of the aforementioned problem, the goal of this systematic review and meta-analysis of published findings was to describe epidemiological aspects of candidemia in hemato-oncological patients around the world, as well as to provide an overview of NACs trends in these patients.Furthermore, this study reveals the potential risk factors for candidemia in cancer patients, as well as Candida spp. antifungal susceptibilities, in order to provide relevant views on these patients.
2. Materials and methods
2.1. Research design
The protocol for the systematic review was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines[21]. Between January 1st, 2000, and April 21st,2021, literature searches were conducted using the databases of MEDLINE (including PubMed), Web of Science, Scopus, Science Direct, and Google Scholar. Several combinations of the following English-language search phrases were employed in each electronic database: blood stream infection, candidemia, fungemia, Candida species, Candida spp., non-albicans Candida, neoplasm, cancer,malignancy, tumor, oncology, epidemiology, and prevalence.
2.2. Inclusion and exclusion criteria
The following criteria were used to determine eligibility: (a)original papers, (b) investigations on the prevalence of Candida species in cancer patients’ blood, (c) full-text availability, (d)literature published in English, and (e) the research received a moderate or high-quality score. On the other hand, studies that met the following criteria were excluded from the review process: (a)the articles irrelevant to the topic under study, including identifying other candidiasis such as oral or cutaneous candidiasis, articles without identifying Candida species, and studies on other highrisk groups except cancers, (b) the research did not have enough information, (c) full-text papers that are unavailable, (d) conference abstracts, case reports, comments, and review articles. Only papers that met the inclusion criteria and provided enough information for the qualitative analysis were considered.
2.3. Quality assessment
The Newcastle Ottawa Scale was used to assess the quality of the research included in this review[22]. Each study was scored independently by two authors (AG and EA), and disagreements were resolved by consensus, or with a third author (MF) when necessary. This checklist was planned to a case-control study based on 9 points in 3 different categories, including selection (0-4 points), comparability (0-2 points), and exposure (0-3 points). For cross-sectional and cohort studies, 7 parameters were considered to score the articles in the 3 categories including selection (0-3 points),comparability (0-2 points), and outcome (0-2 points). In this regard,case-control studies were rated as high, medium, or low quality based on total scores of 7-9, 4-6, and 3 points, respectively. The 6-7,3-5, and 1-2 scales were rated high-, moderate-, and low-quality in cross-sectional and cohort studies, respectively. As a result, the meta-analysis included studies of high and intermediate quality.
2.4. Studies selection and data extraction
Two members of the research team (AG and ME) scrutinize potentially relevant articles by title and abstract and then reviewed the eligibility and quality of potential studies by full-text review.We scanned the reference lists in relevant original and review publications to find additional published articles. All of the publications were downloaded into EndNote V.X8.0.1 software, and duplicate citations of the same data were removed. Each study’s data was extracted independently by two authors (EA and AG).The first author’s last name, year of publication, study design,geographical location, applied diagnostic techniques, type of cancer, frequency of Candida species, participants’ demographic information, and antifungal treatment were retrieved from each article that met the inclusion criteria. Chemotherapy, neutropenia,total parenteral nutrition, intensive care unit, central vascular catheters (CVC), diabetes, dialysis, Candida colonizations,mucositis, ventilation, organ-transplantation, surgery, mortality,and use of immunosuppressive drugs, antibiotics, and antifungal susceptibility were all extracted from each article. All extracted data from each trial was eventually recorded in an Excel file.
2.5. Meta-analysis
For each study included in the meta-analysis, the crude proportion of patients with NACs was calculated. Due to significant heterogeneity in some studies, the total pooled estimates with matching 95% confidence intervals (CI) were derived using random-effects models for the continents and type of cancer. We also supplied Forest plots to show the thorough representation of the included studies based on proportions and 95% CI. In addition,we conducted a meta-analysis utilizing random-effects model to estimate antifungal susceptibility to Candida spp. isolated from cancer patients’ bloodstreams, as well as to look into the most common risk factors for candidemia in cancer patients. Statistical heterogeneity was examined using X2and I2statistics to assess the inconsistency of study data[23]. We also used the funnel plot, Egger,and Begg tests to measure publication bias. At a significance level of 0.05, all percentage meta-analyses were performed using metaprop in Stata version 14.10 and meta for packages in R version 4.1.1. at the significant level of 0.05.
3. Results
3.1. Literature search results
A total of 4 546 documents were discovered after a thorough search of five databases. Because of redundancy, 1 343 records were removed from the analysis. Following that, 3 040 papers were eliminated based on a preliminary review of the titles and/or abstracts. After reviewing the full-text version with the inclusion and exclusion criteria, 94 articles were eliminated from the remaining 163 records. In the end, 69 studies satisfied our criteria and were evaluated for meta-analysis. The search process in this systematic review and meta-analysis is depicted in detail in Figure 1.

Figure 1. The flowchart of the study selection process.

Table 1. Characteristics of included studies.
3.2. Study characteristics
There were 42 retrospective studies, 19 prospective studies, 7 cross-sectional studies, and 1 case control study included in this analysis, with a total of 9 706 patients. The NOS scale scores in the studies ranged from moderate to high. Table 1 lists the characteristics of the 69 studies[36-83] as well as their quality ratings. According to the data, 54 studies relied solely on traditional methods to identify Candida species, while 12 investigations employed combined traditional and molecular methods. The metaanalysis included only three research that employed traditionalindependent methodologies. In most of the researches, standard methods such as Sabouraud Dextrose Agar and/or CHROMagarTMCandida as culture media and/or biochemical assays were used.The included studies were from six continents including Europe(Slovakia, Italy, Spain, UK, France, Portugal, Germany, Netherland,Russia, Greece, Turkey, Poland), Asia (South Korea, Iran, Taiwan,China, Qatar, India, Pakistan), Africa (Egypt, Ethiopia), North America (the United States), South America (Brazil), and Australia.The United States and Brazil were the most often mentioned countries in this meta-analysis.
3.3. NACs prevalence in cancer patients with candidemia
According to the current meta-analysis, the total pooled prevalence of NACs infection in cancer patients was 0.62 (95% CI 0.58-0.67)using the random-effects model (Figure 2). There was significant intra-group heterogeneity (P<0.05) in the results. In addition, there was significant inter-group heterogeneity (P<0.05), corroborating the report of pooled prevalence for each continent individually.Furthermore, for each of the 69 studies included in the metaanalysis, we assessed publication bias. The funnel plot, shown in Figure 3, is a graphical representation of the standard errors plotted against the proportion reported. The funnel plot shows that there will be no significant publication bias because the research will be scattered almost evenly around the mean proportion. Furthermore,neither Egger’s nor Begg’s tests found significant evidence of publication bias in meta-analysis (Egger’s test: P=0.13; Begg’s test:P=0.71).
3.4. NACs prevalence in cancer patients with candidemia based on type of cancer
There was statistical significance intra-group and inter-group heterogeneity, as demonstrated in Table 2. P value less than 0.05 indicating that achieving the pooled prevalence with 95% CI for each type of cancer subgroup is feasible. As a result, random-effects meta-analyses were performed in two subgroups: hematologic(n=29) and solid (n=21) cancer. In patients with hematologic and solid cancers, the combined prevalence of NACs infection was 68%(95% CI 65%-70%) and 52% (95% CI 49%-54%), respectively.

Figure 2. Forest plot for non-albicans Candida proportions in cancer patients with candidemia, stratified by continent.
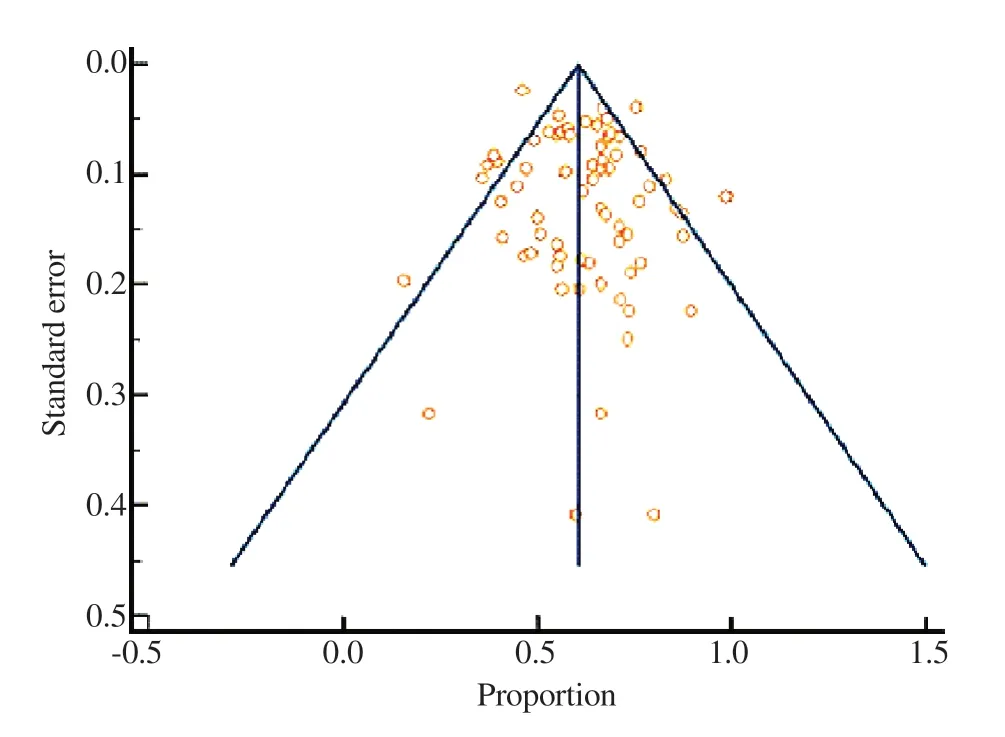
Figure 3. Funnel plot to assess the publication bias.

Figure 4. The combined proportion of non-albicans Candida in cancer patients with candidemia in different continents.

Table 2. Pooled prevalence of non-albicans infection isolated from bloodstream in patient with hematologic and solid tumors.
3.5. Prevalence of NACs in cancer patients with candidemia based on continent and year
Candidaemia caused by NACs in cancer patients was studied in 23 countries across six continents. Figure 4 shows the cumulative fraction of NACs in cancer patients with candidemia from various continents around the world. Accordingly, most NACs cases in cancer patients were in the following order: Africa>North America>South America>Europe>Asia. Herein we unequivocally demonstrate that the proportion of NACs to C. albicans has been significantly higher in all continents. In recent years, NACs have been linked to more incidences of candidemia in cancer patients than C. albicans, according to our findings (Supplementary Figure 1). Moreover, a linear regression model provided new data on the trend of NACs in recent years in these patients (Figure 5). According to the findings, among cancer patients with candidemia, there was a weak connection between year and NACs proportion (slope=0.004,P=0.25).

Figure 5. Non-albicans Candida prevalence in cancer patients with candidemia plotted against publication year with fitted regression line and 95% CI.
3.6. NACs distribution in cancer patients with candidemia
Candida albicans and NACs species were responsible for 3 965(39.2%) and 5 980 (59.2%) of the 10 112 cases identified in the studies, respectively. In addition, C. albicans and NACs co-infected 167 species (1.6%) of cancer patients’ blood samples. As shown in Figure 6, the Candida spp. isolated from the patients were C. albicans,C. tropicalis, C. parapsilosis, C. glabrata, C. krusei, C. guilliermondii, C.kefyr, C. lusitaniae, C. famata, C. rugosa, C. pelliculosa, C. norvegensis,and other species. Of 5 980 non-albicans Candida species detected,C. parapsilosis was the most frequent isolate (15.9%, 1 609/5 980),followed by C. tropicalis (14.9%, 1 508/5 980) and C. glabrata(14%, 1 417/5 980). Supplementary Table 1 summarizes the data extracted from the studies related to the Candida species. According to the findings, C. parapsilosis and C. glabrata were responsible for the majority of NACs cases in Europe and North America,respectively. In Asia, Africa, and South America, C. tropicalis was the most common species. It’s also worth noting that only one study from Australia was included, indicating a high frequency of C.parapsilosis.
3.7. Risk factors associated with candidemia in cancer patients
We used a meta-analysis with a random effects model to evaluate the prevalence of different risk variables and the death rate in cancer patients with candidemia in the current study. Supplementary Table 2 shows the combined or pooled effect sizes (ES) for the prevalence of each risk factor, the significance of the ES=0 test(s), and the results of the heterogeneity test between studies included in the meta-analysis. The test of heterogeneity between studies included in the meta-analysis found that all risk factors have substantial interstudy heterogeneity (P<0.05). In addition, the combined prevalence rates of all risk variables in these patients were statistically significant and had a significant difference with zero (P<0.05),according to the results of the significance test. The combined ES of death rate in cancer patients with candidemia obtained 36 % based on our findings (95% CI 0.30-0.42). Furthermore, therapeutic use of antibiotics with 85% (95% CI 0.81-0.89), central vascular catheters with 69% (95% CI 0.62-0.77), and chemotherapy with 56% (95%CI 0.46-0.67) had the highest combined prevalence rate and were most frequently occurring risk factors in these patients.
3.8. Comparisons of antifungals
A meta-analysis was conducted using a random effect model on the in vitro antifungal activity of the most important drugs against Candida species. In our systematic review, 14 articles[19,20,72-83]had the necessary criteria to evaluate antifungal susceptibility(Supplementary Table 3). C. krusei displayed the highest resistance(pooled ES=0.85, P<0.05) and sensitive-dose dependent(pooled ES=0.008, P<0.05) to fluconazole, as well as the lowest susceptibility (pooled ES=0.008, P<0.05). As a result, fluconazole had the highest resistance to the isolated NACs (P<0.05).Furthermore, when compared to other antifungals, caspofungin and AmB showed the lowest resistance and the maximum sensitivity to Candida spp. Surprisingly, NACs had a much greater rate of drug resistance than C. albicans (P<0.05).

Figure 6. Distribution of Candida species in cancer patients with candidemia.
4. Discussion
Candidemia is an increasing health problem in cancer patients due to high morbidity, mortality and increased costs worldwide[71].Although C. albicans is the leading cause of candidemia, the proportion of other Candida species also shows an increasing trend as important pathogens. In our meta-analysis, the overall pooled prevalence of NACs infection as the cause of current disease in cancer patients was 62%. Several publications from throughout the world have demonstrated that NACs cause a progressive increase in candidemia in cancer patients, with a significant mortality rate[8,32,56,72]. Confirming this, our data showed that in recent years,NACs were responsible for most candidemia cases compared to C. albicans, except in 2007 and 2018. It is important to emphasize that in the two years mentioned; only 2 studies were eligible for analysis in the present review. Hence, we do not have more data for a detailed evaluation of the distribution of species and other important variables affecting candidemia in cancer patients. There is persuasive evidence that the shift from C. albicans to NACs as the dominant species may be correlated with age, antifungal drug resistance, types of health care facilities, the use of medical devices such as catheters, parenteral nutrition, broad-spectrum antibacterial agents, the techniques employed by different researchers for the species identification, and geographic variation[84]. In this respect,the recent studies revealed that the species distributions among different age groups were very similar, with some minor exceptions.For example, C. glabrata may be more common among older persons[31,85]. Furthermore, the increased use of azoles (fluconazole,itraconazole) for prophylactic and empiric antifungal therapy among high-risk cancer patients may be contributing to this shift in etiology.On the other hand, several investigations have linked the use of implanted or semi-implantable synthetic materials, such as central venous catheters, to an increase in the prevalence of C. parapsilosis as a causal agent of candidemia[19,86,87]. As shown in our results,there were various diagnostic methods for identifying Candida species in blood samples of oncology patients. Although blood culture is considered the “gold standard” for detecting BSI caused by Candida spp., combining culture with additional approaches such as molecular amplification techniques can help with accurate diagnosis and/or screening of patients with candidemia caused by NACs[51,88]. The data suggest that C. parapsilosis was the most common NACs in oncologic patients, followed by C. tropicalis and C. glabrata fungemia. The increased isolation of C. parapsilosis may be attributed to the widespread use of automated blood culture systems or foreign medical devices (intravascular catheters, cardiac valves, endotracheal tubes, and so on) in cancer patients, where their adherence to the surface of these devices and tendency to form a biofilm can facilitate candidemia[89]. The study conducted by Kremery et al. demonstrated the increased frequency of C.parapsilosis candidemia associated with intravascular catheters from 7.1% in 1996 up to 15% in 1997[90]. On the other hand,geographic differences may play a role in raising the percentage of candidemia caused by NACs. Keeping this in mind, C. parapsilosis candidemia was found to be the most prevalent NACs species isolated from blood in cancer patients in Europe in the current investigation. Meanwhile, Asia, Africa, and Latin America have seen a considerable increase in C. tropicalis infections. Furthermore,the majority of isolated C. glabrata cases, a species that develops azole resistance quickly, occurred in North America[31]. More importantly, the rise in the incidence of rare NACs (such as C.rugosa, C. zeylanoides, C. stellatoidea, and others) in cancer patients’blood, particularly in Europe and North America, may be attributed to the use of effective and precise techniques like polymerase chain reaction or matrix-assisted laser desorption/ionization time-of-flight(MALDI/TOF) in infection detection[31,60,72].
Examining the impact of numerous risk factors on the development of candidemia in cancer patients is one of the potentially beneficial approaches in our research. In the present study, major risk factors for candidaemia based on the estimated combined prevalence were including therapeutic use of antibiotics (85%), CVC (69%),chemotherapy (56%), and neutropenia (45%). It is well recognized that rapid laboratory diagnosis of causal organisms in cancer patients will improve clinical outcomes, save costs, and reduce the usage of broad-spectrum antibiotics. Importantly, catheterassociated candidemia can lead to significant complications such endocarditis, meningitis, and septic thrombosis[32]. The results of the present study showed that NACs were mostly isolated in males than females[34,91]. Importantly, we discovered that 36% of cancer patients with candidemia died. Because the retrospective studies considered in this analysis lacked a thorough patient history and integrated laboratory data, it was unable to draw clear conclusions concerning mortality and other risk factors. Surprisingly, based on the kind of cancer, our meta-analysis revealed a substantial difference between NACs and C. albicans infection. In relation to this, despite the fact that individuals with solid cancer are considered to be at higher risk for candidemia, more incidences of NACs in the bloodstream have been observed in patients with hematologic malignancy[24,92]. In contrast to individuals with solid tumors, most patients with hematologic malignancy receive prophylactic antifungal medicines such as fluconazole or AmB. This could be one cause for this occurrence. This may have influenced the incidence of candidemia caused by NACs in leukemia and lymphoma patients[33]. One of the main goals of this study was to compare the findings of susceptibility tests done on Candida spp. isolated from cancer patients in diverse investigations. In vitro activity of anti-antifungal drugs revealed that caspofungin was the most active agent, followed by amphotericin B and voriconazole.As a result, caspofungin have promising activity against most Candida species and may represent a promising agent against isolates with primary and secondary resistance to fluconazole or itraconazole. Several studies have reported highly promising findings relevant to the potential use of caspofungin in cancer patients with invasive Candida infections[93,94]. In the other hand,the finding unequivocally demonstrated that voriconazole was the most active azole assayed against NACs. Voriconazole may be a suitable alternative for treating candidemia in cancer patients whose infections are due to NACs which have developed resistance to fluconazole. The majority of cancer patients infected with antifungal-resistant Candida strains were infected with C. krusei or C. glabrata. Our findings support a prior study that found that prophylactic use of azole antifungal drugs, such as C. krusei and C.glabrata, can result in the formation of NACs, which indicate lower resistance to these agents[95]. However, due to their design, many of the studies are unable to properly address the subject of Candida spp. shifts toward species that are less susceptible or resistant to azoles over time.
The following are some of the study’s limitations: (Ⅰ) many studies were retrospective, which could result in missing data due to a lack of data in medical records; (Ⅱ) a number of studies were performed in a single center with relatively small size; consequently, it may have compromised the statistical power of the study; (Ⅲ) the results can be greatly influenced by the application of several approaches with differing degrees of sensitivity and specificity; MALDI-TOF or DNA sequencing, for example, can be utilized as precise and sensitive diagnostic procedures to confirm Candida spp.; (Ⅳ) there is a lack of data in some of studies to assess the influence of in vitro susceptibility on BT candidemia; as a result, we are unable to assess whether or not a given therapy is appropriate for the entire research group, and (Ⅴ) another significant constraint is the scarcity of published studies in many parts of the world, as a result, future studies in these areas should be conducted with bigger sample sizes.In the current systematic review, we found that NACs were responsible for the majority of candidemia cases in cancer patients over the last two decades. It’s worth noting that patients with hematologic cancer have had higher cases of NACs BSI than those with solid malignancy. Candida parapsilosis and C. tropicalis were the most common isolates among the NACs detected in the patients. In this review, due to the lack of a comprehensive history of patients and integrated laboratory data in the retrospective studies used, it is not possible to draw firm conclusions about mortality and some risk factors. The authors have suggested that more prospective multi-center studies with valuable diagnostic methods should be conducted in diverse regions of the world, especially in low- and middle-income nations, to address the epidemiological characterization of NACs candidemia in this population.
Conflict of interest statement
Authors have no conflict of interest to disclose.
Ethics approval and consent to participate
The authors confirm that the ethical policies of the Journal, as noted on the Journal’s author guidelines page, have been adhered to.No ethical approval was required as this is a review article with no original research data.
Acknowledgements
The authors sincerely thank all the people who helped in conducting the current study.
Funding
No funding was used for this study.
Authors’contributions
MF and AG conceived and designed the study. AG, ME and MF searched the literature; AG, and ME extracted the data. AG, MF, and AB wrote the manuscript and EN performed the statistical analysis.EA designed figures. All authors have read, critically revised and approved the final manuscript.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- The predictors of long-COVID in the cohort of Turkish Thoracic Society-TURCOVID multicenter registry: One year follow-up results
- A cross-sectional study of post-COVID syndrome at a tertiary care center in Turkey
- Genetic diversity of Leishmania donovani isolates from cutaneous lesions of military personnel in the Mullaitivu and Kilinochchi districts of the Northern Province, Sri Lanka
- Will COVID-19 vaccination rates for children be still low in the future?
- A looming twindemic of COVID-19 and dengue on post-flood scenario in the developing countries
- Recurrent Marburg virus disease outbreaks from 1967 to 2022: A perspective on challenges imposed and future implications
