Simulation analysis and pharmacokinetic evaluation of atractylide I for the treatment of diabetic nephropathy
2022-11-03ShuQingCaoYuJingZhangMengChenYuanYuanZhangChenJieGuoYaoYao
Shu-Qing Cao,Yu-Jing Zhang,Meng Chen,Yuan-Yuan Zhang,Chen-Jie Guo,Yao Yao
1School of Forensic Medicine,Shanxi Medical University,Jinzhong 030600,China.2School of Public Health,Shanxi Medical University,Taiyuan 030001,China.3School of Clinical Medicine,Shanxi Medical University,Taiyuan 030000,China.
Abstract Objective:The study aims to investigate the molecular mechanism and druggability of Atractylide I (AT-I) for the treatment of diabetic nephropathy (DN) using network pharmacology,molecular docking,and pharmacokinetic analysis.Method: Multiple databases and service platforms were used to predict and screen the potential genes of AT-I for the prevention and treatment of DN.Further,gene ontology and kyoto encyclopedia of genes and genomes enrichment analyses were performed on the genes to evaluate the mechanism of AT-I against DN.The protein interaction network was constructed by the STRING database,and the key gene targets of AT-I against DN were screened by Cytoscape software.The binding activity of AT-I with the essential target proteins was analyzed by molecular docking technique.Finally,the pharmacokinetic parameters of AT-I were evaluated to determine its druggability.Result:AT-I has 45 drug action targets intersecting with DN targets,which may be the potential targets of AT-I for the prevention and treatment of DN.The enrichment analysis indicated that gene expression regulation,insulin resistance,hypoxia inducible factor-1 signaling pathway,tumor necrosis factor signaling pathway,and PI3K-Akt signaling pathway determine the biological mechanism of AT-I in the prevention and treatment of DN.Further,network pharmacology and molecular docking results indicated that key genes such as AKT1,MAPK8,EGFR,MAPK1,MMP9,and MTOR bind well with AT-I,while AKT1 is probably the primary target gene of AT-I against DN.Pharmacokinetic analysis showed that AT-I conforms to Lipinski’s rule of five drug-like properties,has good gastrointestinal absorption,can pass the blood-brain barrier,and has superior biological properties.Conclusion:AT-I has desirable druggability and multi-target,multi-pathway,and multi-mechanism activity against DN.This study provides an important basis for future research on the activity of AT-I against DN.
Keywords: Atractylide I;diabetic nephropathy;inflammatory response;AKT1;pharmacokinetics;network pharmacology
Introduction
DN is one of the most common and serious microvascular complications of diabetes mellitus.In recent years,DN incidence has been on the rise,with a gradual increase in the diabetic population in China and aging [1].Microproteinuria and reduced glomerular filtration rate are the major diagnostic indicators of early-stage DN[2].A rapid decline in renal function can lead to a gradual development of DN into end-stage renal disease.Due to the complex pathogenesis of DN and the lack of specific drugs,the current clinical prevention measures of DN include reduction in cardiovascular risk,controlling blood glucose,controlling blood pressure,and inhibiting the renin-angiotensin-aldosterone system [3].Despite current therapeutic approaches,DN onset and progression risk remains high.Therefore,the search for more effective anti-DN drugs is imperative.
Chinese medicine has a long history of treating DN.Based on its clinical manifestations,Chinese medicine classifies DN under“edema”,“kidney dissipation”,“asthenia”,“urinary turbidity”,etc.,and has significant advantages over common Western medicine treatment [4].Atractylide,the rhizome of Atractylide,species of genus Atractylodes in the family Asteraceae,is bitter,sweet,and spleen and stomach-friendly.It can tonify the qi to strengthen the spleen,dry dampness,and promote water retention [5].Wenshun et al.reviewed the modern DN literature and based on the statistical analysis of the Ancient and Modern Medical Case Cloud platform,concluded that Atractylide ranked ninth in terms of frequency of use (with 42 times)for the treatment of early DN,and was one of the major herbal medicines used in TCM for DN treatment [6].A study pointed out that metformin combined with Atractylide powder from Scutellaria baicalensis could significantly reduce the levels of Interleukin (IL)-6 and tumor necrosis factor (TNF)-α in DN patients and improve the insulin sensitivity of the body through anti-inflammatory effects,thus improving DN[7].It was observed that Atractylide Dihuang decoction could significantly reduce urinary microalbumin in DN patients,and there was no toxic clinical side.The efficacy was shown to be better combined with Western medical foundations than conventional Western medical treatment alone [8].Atractylide is rich in volatile oil and polysaccharides,and volatile oils of Atractylide have been attributed to multiple pharmacological activities such as antioxidant,anti-inflammatory,immune modulation,and glucose metabolism [9].A research study by Qinhua et al.highlighted that AT-I could promote significant changes in the cytokine expression levels of inflammatory macrophages,and its anti-inflammatory activity was stronger than that of Atractylide-III [10].CQ et al.screened the anti-inflammatory component of Atractylide by leukocyte membrane chromatography and concluded that AT-I had significant anti-inflammatory effects in both acute and chronic inflammation models in mice [11].The anti-inflammatory activity of AT-I was similar to that of dexamethasone.However,it is noteworthy that there are only a few studies on the anti-DN effects of single drug Atractylide and its active ingredient AT-I.The mechanism of action of AT-I against DN is not well understood.
Several factors are involved in the pathogenesis of DN,including genetic factors,environmental factors,and pathophysiological factors such as inflammatory response,oxidative stress,and renal hemodynamic alterations [12].The traditional one-drug,one-target model is no longer suitable for the treatment of complex diseases such as DN.In this context,the multi-component,multi-pathway,and multi-target characteristics of traditional Chinese medicine bring a ray of hope for the prevention and treatment of DN.Therefore,network pharmacology and computer-aided drug design methods are used in this study to understand the mechanism of action of AT-I against DN.The pharmacokinetic characteristics of AT-I can provide a scientific basis for the clinical development of Atractylide for the prevention and treatment of DN (Figure 1).
Materials and methods
AT-I target acquisition
In this study,the drug targets of AT-I were evaluated using two strategies,namely,database search and molecular conformational relationship search.
The targets and SMILE codes of AT-I were retrieved from TCMSP(https://tcmspw.com/tcmsp.php) and Herb (http://herb.ac.cn/) with“Atractylide” as the keyword.The 3D chemical structure of AT-I was obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/).The energy minimized structure of AT-I was obtained using the minimum energy module of the MM2 procedure in ChemBio3D Ultra 14.0 software.The structure was saved in mol2 format and then imported into the Pharm Mapper server(http://www.lilab-ecust.cn/pharmmapper). The pharmacophore matching method was used to predict the potential targets of AT-I.The top 100 human target proteins were selected according to the “Norm Fit” score in descending order.Further,the SMILE code of AT-I was entered into the Swiss Target Prediction web server(http://www.swisstargetprediction.ch/) to predict the potential targets based on the molecular structure of AT-I.The UniProt database(https://www.uniprot.org/) was used to identify the genes for the above target proteins and integrate the above four sources to obtain AT-I targets.
AT-I target gene acquisition for DN treatment
For identification of genes,the keyword “diabetic nephropathy” was used,and the species was limited to “Homo sapiens”.The target genes associated with DN were obtained from DisGeNET (https://www.disgenet.org/),GeneCards (https://www.genecards.org/),and CTD (https://ctdbase.com) databases.The top 300 genes with high scores were selected and combined after screening out duplicates to develop a DN gene collection.The DN genes thus obtained and AT-I drug genes were provided as inputs into Venny 2.1.0(https://bioinfogpcnb.csic.es/),and the intersections of the two sets of genes were the targets of Atractylide against DN.
Bioenrichment analysis
The gene ontology enrichment analysis and kyoto encyclopedia of genes and genomes pathway enrichment analysis were performed using DAVID (https://david.ncifcrf.gov/) to identify Atractylide targets against DN.The species was limited to “Homo sapiens”,and the biological functions and signaling pathways withP<0.001 were screened out.Thus,the potential biological mechanism of AT-I against DN was evaluated.
Screening of key targets of AT-I against DN and construction of protein-protein interaction network
AT-I targets against DN were imported into the STRING database(https://string-db.org/),and protein interaction analysis was conducted by setting the species as “Homo sapiens”.The protein interaction network was constructed by fixing the confidence score as“medium confidence=0.4” and other default settings.The tab-separated values file was saved and imported into Cytoscape 3.9.1 software.The top 10 genes were selected as the key target genes of AT-I for DN control using the maximal clique centrality algorithm in the CytoHubba plug-in.
Molecular docking validation
For further verification of AT-I affinity for crucial target genes,the 3D structures of key target genes were identified and downloaded from PDB (http://www.rcsb.org/) using the original mol2 format of AT-I.Then,the key proteins were pre-processed using AutoDockTools for dehydration,hydrogenation,detection of molecular center nodes,etc.After the identification of the key protein as a high molecular weight receptor with AT-I as a low molecular weight ligand for molecular docking,the results were visualized using Pymol software.
Pharmacokinetic evaluation

Figure 1 Flowchart of research design.AT-I,Atractylide I;DN,diabetic nephropathy;PPI,protein-protein interaction;GO,gene ontology;KEGG,kyoto encyclopedia of genes and genomes.
The SMILES code of AT-I was imported into the SwissADME server(http://www.swissadme.ch/) for analyzing and evaluating the pharmacokinetic parameters of AT-I such as druggability,blood-brain barrier permeability,gastrointestinal absorption,and hepatic pharmacase inhibition.
Result
Anti-DN gene screening for AT-I
The AT-I drug targets obtained from TCMSP,Herb,Swiss Target Prediction,and Pharm Mapper databases were combined and de-weighted to obtain 158 AT-I targets.Then based on the relevant score,the top 300 human DN genes were obtained from GeneCards,DisGeNET,and CTD databases.After combining and de-weighting,621 human DN major target genes were obtained.The above two sets of genes were then intersected to obtain 45 anti-DN genes for AT-I(Figure 2).
Bioenrichment analysis

Figure 2 Potentially useful AT-I genes for DN prevention and treatment.AT-I,Atractylide I;DN,diabetic nephropathy.

Figure 3 Bar chart showing gene ontology bioenrichment analysis
For elucidation of the biological processes and enrichment pathways involved in the action of AT-I genes against DN,a bioenrichment analysis was conducted on 45 potential genes.The top ten smallest biological processes,cellular components,and molecular function withP< 0.001 were visualized for gene ontology functional enrichment analysis (Figure 3).The enrichment results indicated that the major DN genes were involved in the negative regulation of the apoptotic process,positive regulation of transcription from RNA polymerase II promoter,and negative regulation of cellular response to insulin stimulation.The negative regulation of the apoptotic process,positive regulation of transcription from RNA polymerase II promoter,negative regulation of cellular response to insulin stimulus,and positive regulation of gene expression are biological processes.The cellular components and molecular function are primarily related to the cell surface receptor complex,steroid hormone receptor activity,insulin binding,and phosphatidylinositol 3-kinase binding.Thus,it is implied that AT-I can interfere with DN progression by affecting gene expression and hormone binding.
The top 20 smallest kyoto encyclopedia of genes and genomes enrichment entries withP<0.001 were visualized (Figure 4).The enrichment results indicated that AT-I mainly inhibited DN development through the hypoxia inducible factor-1 (HIF-1) signaling pathway,pathways involved in cancer development,TNF signaling pathway,insulin resistance,PI3K-Akt signaling pathway,vascular endothelial growth factor signaling pathway,etc.(Table 1).
Anti-DN key target screening and protein-protein interaction construction for AT-I
For elucidation of the interactions between the major target genes and for extracting the key genes of AT-I for DN prevention and treatment,45 major target genes were imported into the String database for protein-protein interaction analysis.The genes with disconnected nodes were hidden,and 45 nodes with 378 edges were obtained.This indicated that the 45 commonly associated genes were interlinked by 378 edges.After saving the file in the tab-separated values format and mapping it in Cytoscape software,the top 10 key genes(Table 2) were filtered using the maximal clique centrality algorithm in the CytoHubba plugin (Figure 5).
Molecular docking validation
The molecular docking validation of the obtained 10 key gene targets of AT-I was performed using AutoDockTools software,and the binding activities of their high molecular weight receptors and low molecular weight ligands are shown in Table 3.It can be observed that the binding energy of all AT-I and 10 key anti-DN genes were less than or equal to -5.0 kcal-mol-1,i.e.,AT-I has a strong binding with the key genes(Figure 6).

Figure 4 Scatter plot of kyoto encyclopedia of genes and genomes enrichment results.VEGF,vascular endothelial growth factor;HIF-1,hypoxia inducible factor-1;TNF,tumor necrosis factor;AMPK,adenosine 5’-monophosphate-activated protein kinase.

Figure 5 PPI diagram of key genes.PPI,protein-protein interaction.
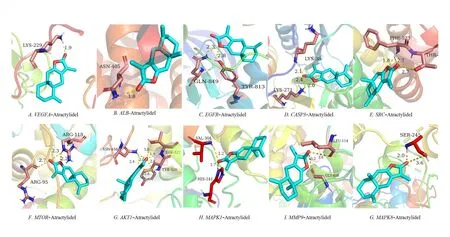
Figure 6 Molecular docking map of AT-I and key genes.AT-I,Atractylide I.
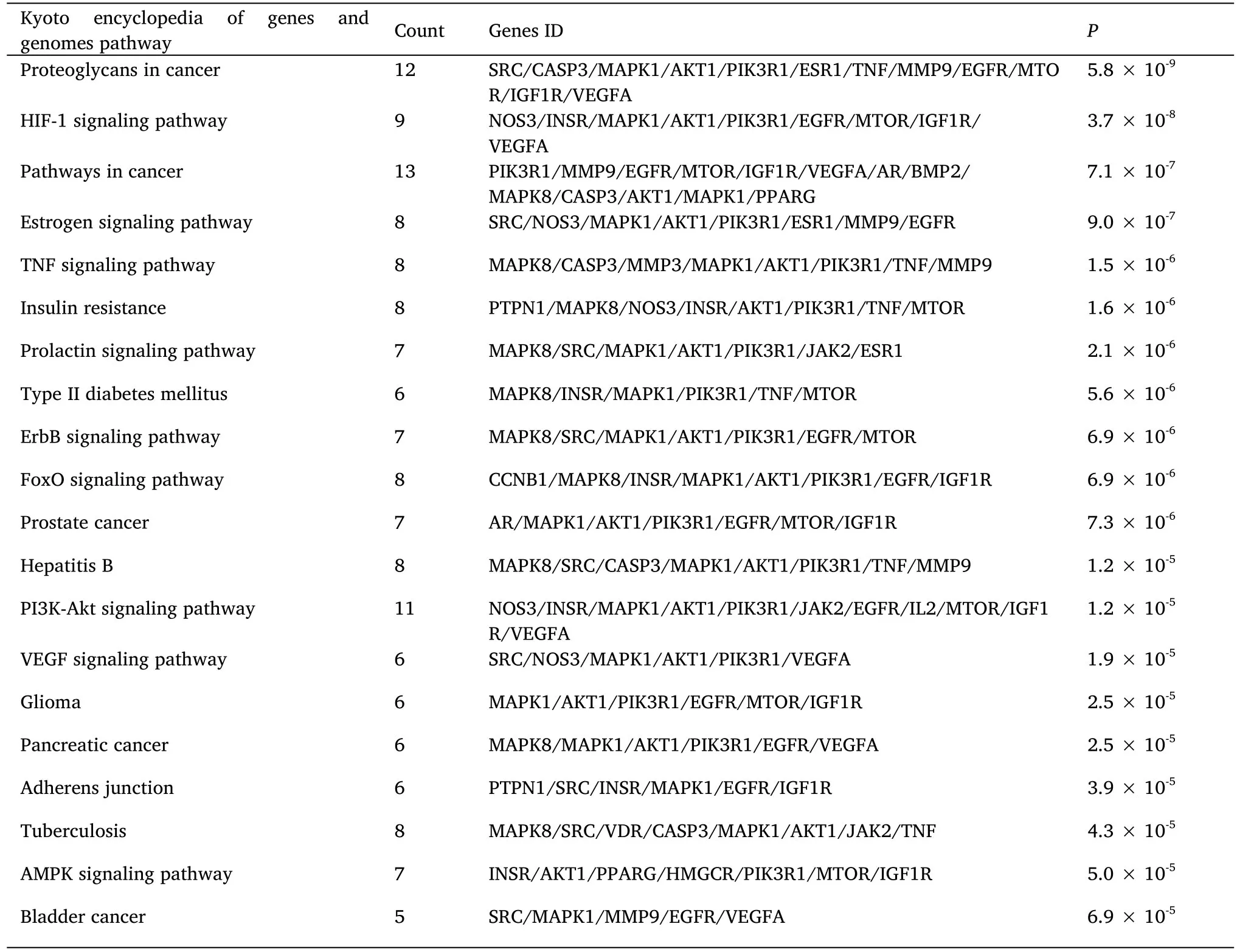
Table 1 Kyoto encyclopedia of genes and genomes pathway enrichment analysis
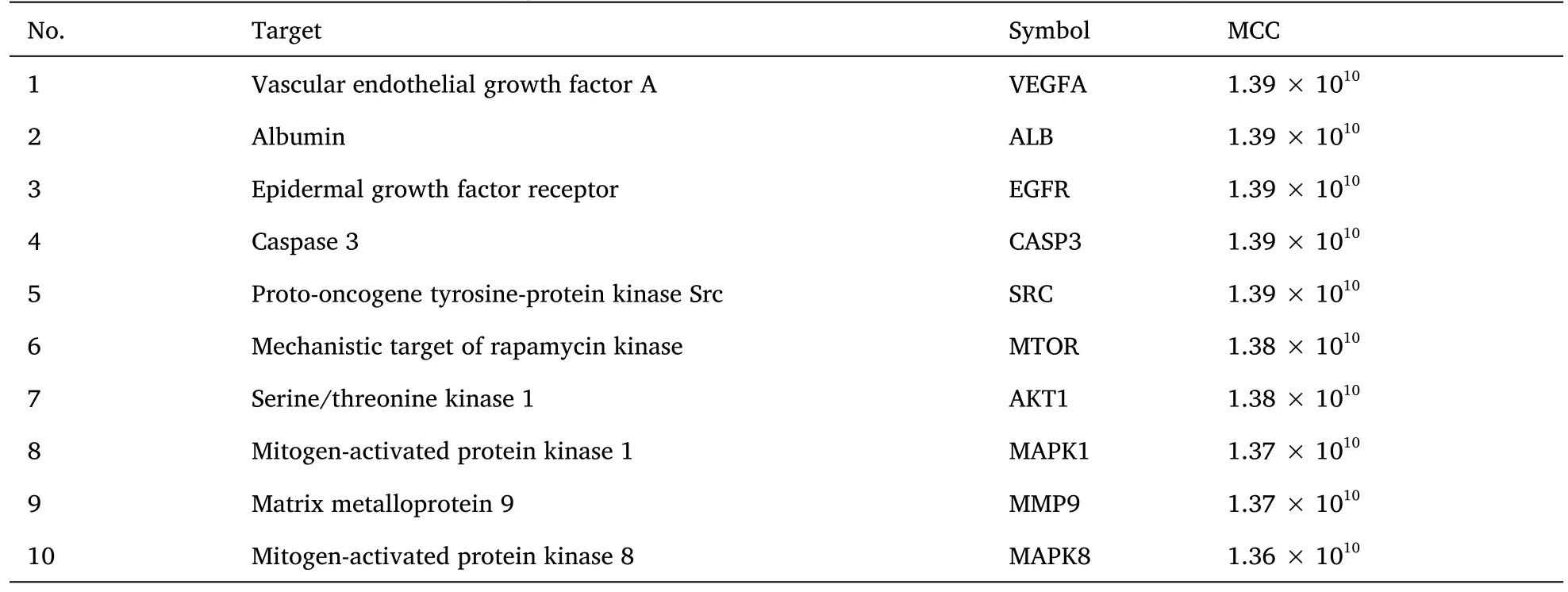
Table 2 Key genes involved in the prevention and treatment of DN by AT-I
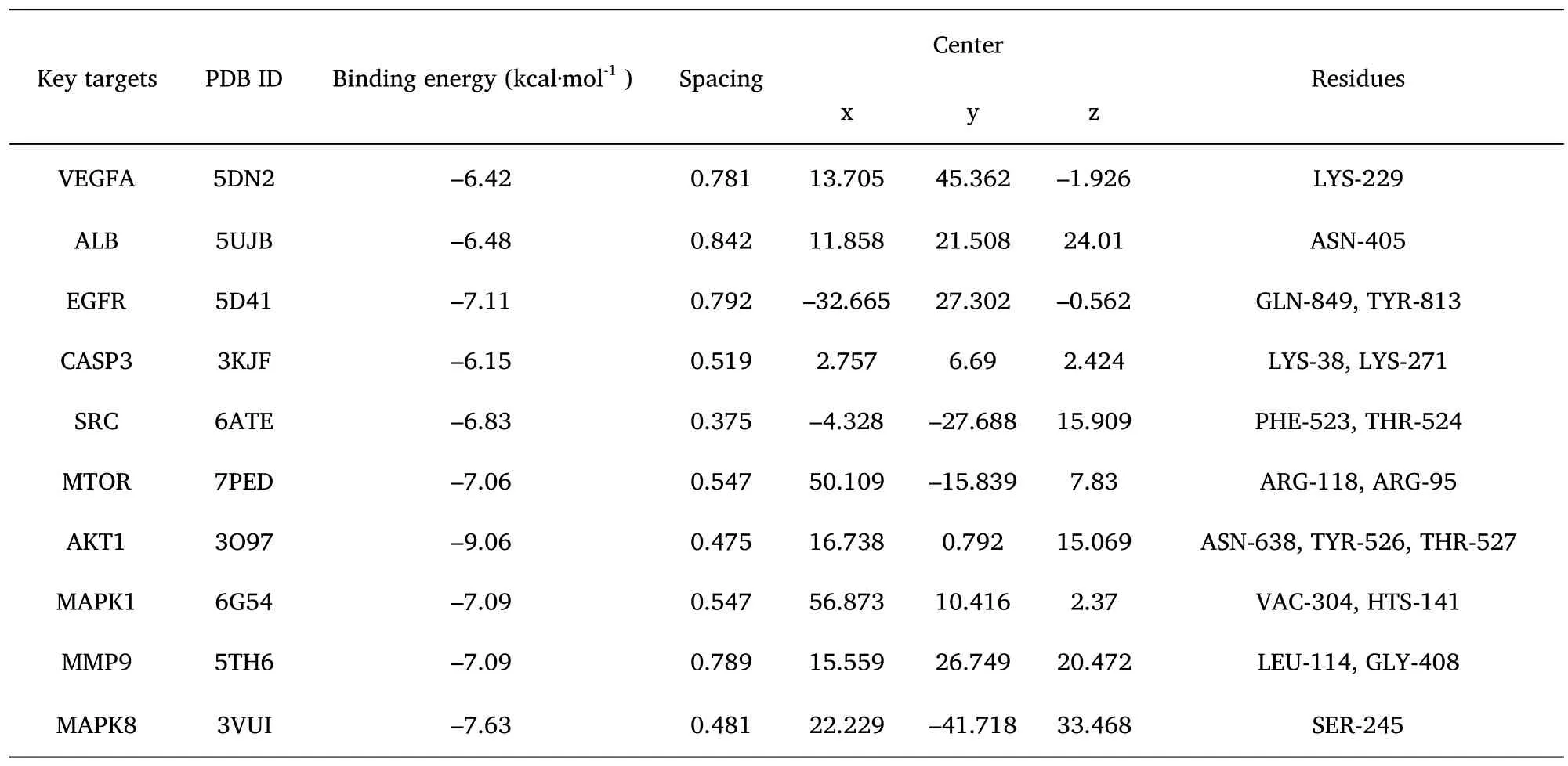
Table 3 Results of AT-I molecular docking
Pharmacokinetic evaluation
The SMILE code CC1=C2CC3C(=C)CCCC3(C=C2OC1=O)C of AT-I was imported into the SwissADME platform,and its prototype pharmacokinetic parameters were analyzed.As shown in the bioavailability radar plot (Figure 7B),it can be observed that the six physicochemical properties of Atractylide,namely,lipophilicity,size,polarity,solubility,saturation,and flexibility are ideal.AT-I conforms to the five rules of Lipinski-like drugs and has good druggability(Table 4).
Discussion
DN has become the leading cause of chronic kidney disease in China,and its growth has adversely affected the quality of life of patients and imposed a great burden on their families and society [13].As a core drug in the treatment of DN in TCM,the lack of research to understand the mechanism of action and druggability of a single drug and its primary active ingredient against DN has restricted the clinical use of Atractylide and the discovery of effective novel anti-DN drugs to a certain extent.
Using the data from multiple platforms,a total of 45 major targets of AT-I against DN were obtained in this study.The bioenrichment analysis indicated that AT-I can exert anti-DN activity through multi-target and multi-pathway characteristics.HIF is a major regulator of the body’s adaptation to hypoxia and can interact with signaling pathways such as Notch and NF-kB for directly stimulating the expression of hundreds of target genes to maintain cellular oxygen homeostasis [14].In a study including 1,165 US type 1 diabetic patients with and without DN,researchers determined a protective association between the hypoxia inducible factor-1 alpha (HIF1A)Pro582Ser polymorphism and DN in male subjects by identifying polymorphism of the HIF1A gene through in vivo and in vitro experiments [15].Moreover,the animal experiments indicated that conditional knockout of HIF-1α exacerbates renal tubular injury in DN mice [16].This phenomenon did not occur when there was an overexpression of HIF-1α and in the HO-1 agonist groups,thus implying the alleviation of renal tubular injury in DN mice by HIF-1α via HO-1-mediated mitochondrial dynamics.
On the other hand,AT-I attenuated tissue injury by inhibiting hypoxia-induced HIF-1α and downregulating the Akt signaling pathway [17].Diabetes significantly increases the risk of cancer in diabetic patients when compared to non-diabetic patients and leads to more than one-fold higher cancer mortality than non-diabetic patients[18,19].A study conducted by Luo et al.showed that the disturbance of calcium metabolism in DN rats was associated with their susceptibility to kidney cancer [20].Dong et al.analyzed the co-expressed differential genes between DN and kidney cancer using bioinformatics and observed a significant association between DN and kidney cancer [21].In our previous study,the differentially expressed genes,namely,VEGFA and MMP9,in DN were also significantly upregulated in renal clear cell carcinoma.Moreover,the study also indicated that high MMP9 expression levels were associated with a poorer prognosis for patients.Interestingly,AT-I has been shown to inhibit cell proliferation and induce cancer cell death.It has also been clinically tested for the treatment of cancer-induced cachexia [9].Insulin resistance (IR) refers to a decrease in the sensitivity of insulin-dependent receptor cells or disruption of insulin-mediated signaling pathways.IR plays an essential role in the development of diabetes and its complications [22].In addition,the PI3K-AKT pathway,an important signaling pathway for IR production,inhibits hepatic gluconeogenesis and promotes glucose transporters 4 to transfer glucose into the cell,thereby causing IR when AKT is inactivated,inhibited,or continuously activated [23].A study by Han et al.indicated that Atractylide extracts promoted glucose transport by increasing glucose transporters 4,PI3K,and IRS-1 levels in addition to organismal lipid differentiation [24].However,the role of AT-I was not determined in that study.A meta-analysis suggested that TNF-α-308G/A polymorphism may enhance DN susceptibility in Asian patients with type 2 diabetes [25].A research study by Xu et al.showed that the expression levels of TNF-α and IL-6 were significantly higher in DN patients when compared to those of normal human subjects [26].When DN-affected rats were treated with TNF-α inhibitors for 12 weeks,it was found that TNF-α inhibition significantly reduced the expression of NLRP3 inflammatory vesicles and IL-6 and L-17A mRNA in the renal tubules,thereby resulting in reduced albuminuria levels and glomerular and tubular damage in DN rats [27].Moreover,it has been observed that AT-I can significantly inhibit the production of TNF-α,IL-6,and IL-1β through the TLR4/NF-kB signaling pathway,thus acting as a protective barrier[28,29].Lastly,due to the lack of relevant literature,it is difficult to directly understand the mechanism of action and effect of AT-I against DN.However,based on the available literature,it can be predicted that multiple pathways are involved in the action of AT-I against DN.

Figure 7 Structural formula of AT-I (A) and radar diagram of bioavailability (B).AT-I,Atractylide I.
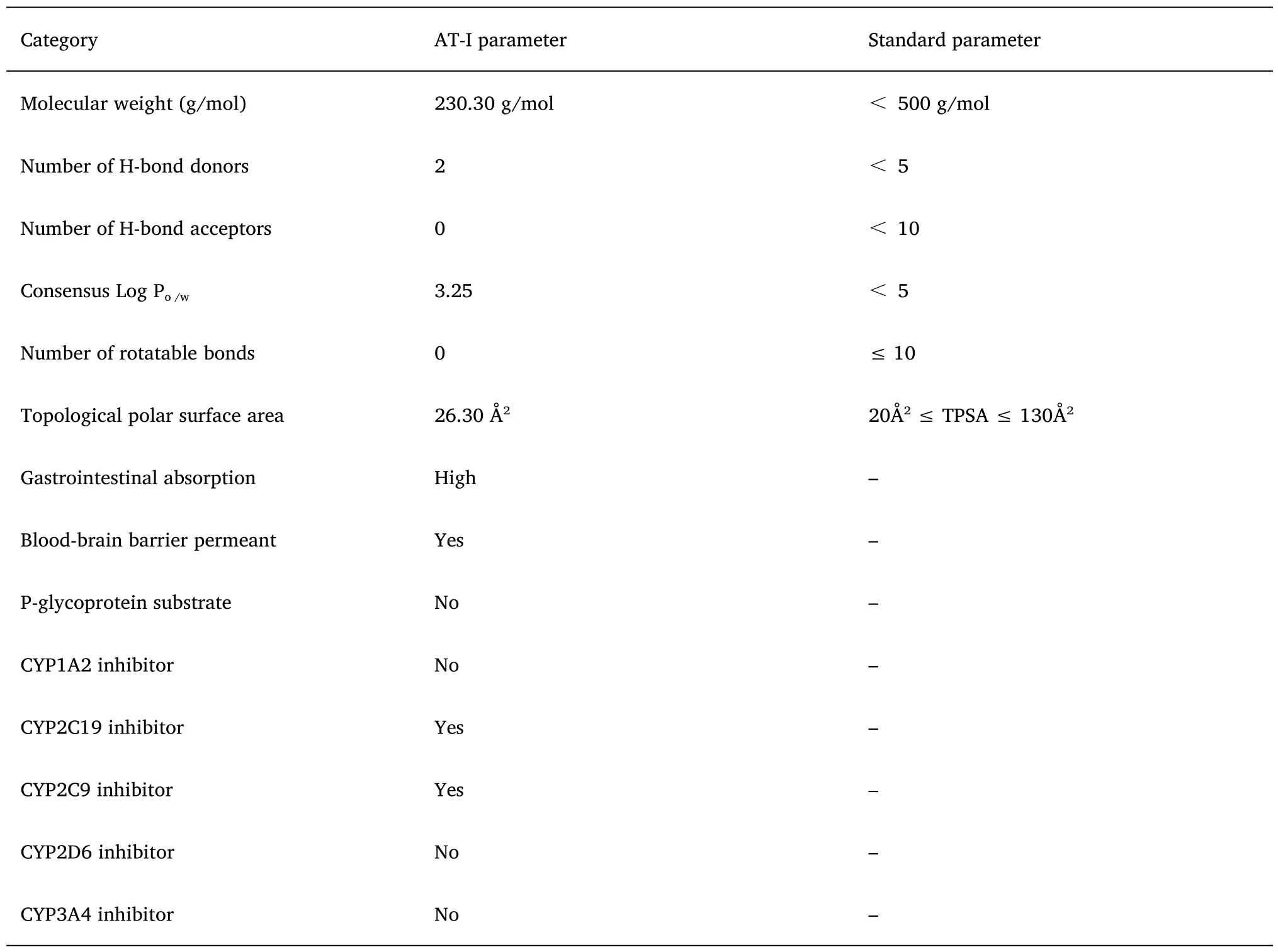
Table 4 Pharmacokinetic parameters of AT-I
Next,the algorithm was used to screen the ten key genes of AT-I against DN,and their AT-I binding activity was verified by molecular docking.The results indicated that all the key genes have a strong binding activity with AT-I,and there is some similarity between the active sites of AT-I and the inhibitors of key genes.This further illustrates the reliability and authenticity of the results from this study,with AKT1 found to have the strongest binding activity with AT-I,and is presumed to be the possible major target of AT-I against DN.AKT is a downstream effector of the PI3K pathway that regulates many cellular functions,including glucose metabolism,glycogen synthesis,cell proliferation,death,and hypertrophy.It is also involved in several signaling pathways [30].AKT is an important mediator of insulin action,which regulates glucose uptake in muscles,adipocytes,liver,and other tissues by promoting the translocation of glucose transporter proteins from intracellular stores to the plasma membrane in response to insulin stimulation [31].A study conducted by Xing et al.indicated that repeated low-dose radiation activated AKT phosphorylation in kidney tissues of DN mice,which in turn,significantly ameliorated diabetes-induced renal inflammatory damage [32].Further,a study done by Rane et al.also showed that a prolonged hyperglycemic environment in the body downregulated the level of AKT activation and accelerated the renal tubular cell apoptosis via enhanced p38 MAPK activation,which in turn,contributed to the DN development [33].AKT1,one of the isoforms of AKT,regulates neuronal insulin signaling,glucose uptake,and insulin resistance.Numerous experimental studies have indicated that AKT1 inhibition leads to increased levels of uncoupled mitochondrial respiration and oxidative stress found in the renal tubules,while activation of AKT1 signaling prevents the development of acute kidney damage and subsequent chronic kidney disease [34,35].However,it should be noted that the role of AKT1 in the development of DN was confirmed in our previous study and other network pharmacology studies [36,37].Although previous studies have shown that mixed herbs in combination with Atractylide lead to good DN control,there is still a lack of studies on the activity of Atractylide and AT-I on DN and AKT1.It is important to experimentally verify whether AKT1 is the primary target of AT-I against DN.However,the present study undoubtedly highlights the importance of Atractylide as an active ingredient.In addition,other key genes also have a major impact on DN.In a study that included 650 Chinese Han patients with type 2 DN and 580 patients with only diabetes mellitus,genetic variants at the rs2010963 and rs69947 loci of the VEGFA gene were found to increase the risk of DN possibly [38].Moderate amounts of VEGFA are beneficial for maintaining the glomerular structure;however,excess VEGFA leads to abnormal angiogenesis and thereby promotes the development of DN [39].AT-I downregulates vascular endothelial growth factor expression,and VEGFA,one of the isoforms of vascular endothelial growth factor,may improve DN by inhibiting renal tissue angiogenesis[40].A study by Zhu et al.highlighted that the ALB gene encoding serum albumin levels were associated with chronic vascular complications in Chinese patients with type 2 diabetes [41].A network pharmacology study indicated that AT-I can act on the ALB gene and then participate in the regulation of metabolic syndrome by the Atractylide-Porin drug pair and improving glucolipid metabolism and insulin sensitivity [42].AT-I was shown to inhibit the expression of EGFR,and the activation of EGFR has a protective effect on the kidney [43].However,sustained activation exacerbates DN progression,and the pathophysiology of DN involves several factors such as altered hemodynamics,metabolic disturbances,and inflammatory responses [44].CASP3-dependent scorch death pathway has attracted attention as a newly discovered regulatory mechanism involved in DN progression,and studies have highlighted that AT-I can inhibit CASP3 activity while retaining mitochondrial function[45].The analogs to CASP3,MTOR,and AKT1 are together involved in the PI3K/AKT/mTOR signaling pathway,and it has been observed that the activation of MTOR reduces SOD activity and GSH levels and activates CASP3,which in turn,promotes oxidative stress-induced DN thylakoid cell apoptosis [46-48].MAPK1 and MAPK8 are MAPK family proteins that play a key role in regulating gene expression and cytoplasmic functional activities.AT-I can inhibit apoptosis and oxidative stress by inhibiting the MAPK pathway and inhibiting the activities of JAK2/STAT3,PI3K/Akt,p38 MAPK,and Wnt/β-linked protein to reduce renal fibrosis[49,50].Although there are only a few studies on the mechanism of AT-I against DN,the major key targets of AT-I against DN screened by the data platform and algorithm in this study directly or indirectly corroborated with the previous studies,thereby implying that the action of AT-I against DN involves multiple targets.
Finally,the pharmacokinetics of AT-I was evaluated by simulation.It was found that AT-I conforms to Lipinski’s rule of five drug-like properties,has good druggability and gastrointestinal absorption,and can cross the blood-brain barrier.Therefore,it is expected to be an excellent drug against DN.However,due to its inhibitory effect on some hepatic enzymes,utmost care should be taken when used in combination with other drugs.
Lastly,this study utilizes network pharmacology and computer-aided drug design methods to overcome the limitations of traditional experiments,such as time-consuming processes,high cost,and fewer data.This study investigates the pharmacological mechanism of AT-I against DN,which provides a theoretical basis for further research on the active ingredients of Atractylide and the development of novel anti-DN drugs in the future.
杂志排行
Medical Data Mining的其它文章
- Highly expression of MYBL2 is correlated with a poor prognosis and immune infiltration in clear cell renal carcinoma
- Bibliometric analysis of literature review of osteoarthritis in the Cochrane database
- Advancing biological fluorescence microscopy with deep learning: a bibliometric perspective
- Feature selection and machine learning approach for carotid atherosclerosis in asymptomatic adults
- Investigating public perceptions regarding the Long COVID on Twitter using sentiment analysis and topic modeling
- A novel prognosis signature based on two cuprotosis-related genes for kidney renal clear cell carcinoma
