Spontaneous coronary artery dissection: A review of diagnostic methods and management strategies
2022-11-02NikolaosLionakisAlexandrosBriasoulisVirginiaZouganeliStavrosDimopoulosDionisiosKalpakosChristosKourek
Nikolaos Lionakis, Alexandros Briasoulis, Virginia Zouganeli, Stavros Dimopoulos, Dionisios Kalpakos, ChristosKourek
Abstract Spontaneous coronary artery dissection (SCAD) is a rare non-atherosclerotic cause of acute coronary syndromes defined as non-iatrogenic, non-traumatic separation of the coronary artery wall. The most common profile is a middle-aged woman between 44 and 53 years with few cardiovascular risk factors. SCAD is frequently linked with predisposing factors, such as postpartum, fibromuscular dysplasia or other vasculopathies, connective tissue disease and hormonal therapy, and it is often triggered by intense physical or emotional stress, sympathomimetic drugs, childbirth and activities increasing shear stress of the coronary artery walls. Patients with SCAD usually present at the emergency department with chest discomfort, chest pain, and rapid heartbeat or fluttery. During the last decades, the most common problem of SCAD was the lack of awareness about this condition which has led to significant underdiagnosis and misdiagnosis. However, modern imaging techniques such as optical coherence tomography, intravascular ultrasound, coronary angiography or magnetic resonance imaging have contributed to the early diagnosis of the disease. Treatment of SCAD remains controversial, especially during the last years, where invasive techniques are being used more often and in more emergent cardiac syndromes. Although conservative treatment combining aspirin and betablocker remains the recommended strategy in most cases, revascularization could also be suggested as a method of treatment in specific indications, but with a higher risk of complications. The prognosis of SCAD is usually good and long-term mortality seems to be low in these patients. Follow-up should be performed on a regular basis.
Key Words: Spontaneous coronary artery dissection; Non-atherosclerotic coronary artery disease; Angiographic classification; Percutaneous coronary intervention
lNTRODUCTlON
Spontaneous coronary artery dissection (SCAD) is a rare non-atherosclerotic cause of acute coronary syndromes (ACS) defined as non-iatrogenic, non-traumatic separation of the coronary artery wall[1]. It usually occurs in young and middle-aged women with few cardiovascular risk factors. Recent studies have shown SCAD to be the underlying cause of myocardial infarction (MI) in 22%-43% of women < 50 years and approximately 21%-27% of pregnancy-associated MI[2-4]. In Canada, SCAD was responsible for 24% of women < 50 years with MI[5] while in Japan this percentage was 35%[3]. In a French series, SCAD was reported in 36% of women under 60 years with ACS and one or few conventional cardiovascular risk factors[6]. Finally, a smaller Australian registry found a prevalence of SCAD of 23% in women under 60 years presenting with ACS[7].
During the last decades, the most common problem in SCAD is the lack of awareness about this condition among healthcare providers, despite the fact that the first case report of this syndrome was a female patient back in 1931[8]. This awareness led to significant underdiagnosis and misdiagnosis in cardiology departments. However, more and more cases are now being identified due to the increased awareness and earlier use of invasive angiographic methods in patients with acute chest pain presenting to emergency departments. Modern imaging techniques such as optical coherence tomography (OCT), coronary angiography or magnetic resonance imaging (MRI) have also contributed to the early diagnosis of the disease. SCAD is not as rare as previously thought. It was supposed to be the main cause for 0.1% to 0.4% of all ACS but these percentages have increased up to 4% over the last years[2,9]. In sudden cardiac deaths, SCAD was reported in 0.5% of cases after autopsy[10]. It is frequently linked with predisposing factors, such as postpartum, fibromuscular dysplasia (FMD) or other vasculopathies, connective tissue disease and hormonal therapy, and it is often triggered by intense physical or emotional stress, sympathomimetic drugs (cocaine, amphetamines), childbirth and activities increasing the intra-abdominal and intra-thoracic pressure (coughing, retching, vomiting) and, thus, shear stress of the coronary artery walls[11].
Treatment of SCAD remains controversial, especially during the last years, where invasive techniques are being used more often and in more emergent cardiac syndromes. Conservative treatment combining aspirin and beta-blocker remains the recommended strategy in most cases. However, the high rate of technical complications in SCAD treated with primary percutaneous coronary intervention (PCI), has been shown to be associated with adverse clinical outcomes[12,13].
The aim of the present review was to demonstrate the existing knowledge regarding the diagnostic methods and the treatment strategies of the underdiagnosed syndrome of SCAD, and highlight the role of primary PCI.
EPlDEMlOLOGY AND PATHOGENESlS OF SCAD
Although SCAD was primarily considered a disease of young adults, nowadays, the most usual patient is a middle-aged woman between 44 and 53 years with few cardiovascular risk factors[14,15]. There is evidence from a Canadian series that female patients with SCAD are older, with less isometric exercise and higher emotional stress compared to male patients[16]. Clinical presentation and outcomes of SCAD may be similar, not only in white patients, but also in other populations such as Hispanic Americans and Blacks[17]. The fact that most case reports include white patients could be explained by the fact that it may be underestimated in other populations or patients belonging in these populations may not present at the emergency department on time. Patients with SCAD usually have lower levels of hypertension, hyperlipidemia or tobacco use compared with patients who have atherosclerotic acute MI[18]. However, the prevalence of these factors is similar between different ages and sex[18]. Hypertension ranges from 32% to 37% while hyperlipidemia from 20% to 35%[14,15]. The prevalence of SCAD in patients presenting with ACS has risen up to 4% and is the underlying cause of up to 35% of all ACS cases in women ≤ 50 years[2,9].
Pregnancy has a strong association with SCAD. In pregnant women, where the percentage of SCADassociated MI is up to 27%[4,19], dissection may be a consequence of increased physiological hemodynamic stress or high progesterone levels, leading to weakening of the coronary arterial wall[20]. SCAD episodes are presented either antepartum or post-partum, and specifically, early post-partum (within the first 6 wk of childbirth), late post-partum (from 6 wk to 1 year), and very late post-partum (1 to 2 years)[20]. The most common period of an episode is the first week after childbirth[21]. Women with post-partum SCAD tend to be older at first childbirth and multigravidas compared to non-postpartum SCAD women[21]. Hemodynamic changes affecting cardiac parameters could lead to higher levels of shear stress and, as a result, vessels including the aorta and coronary arteries present structural alterations[22].
Secondary iatrogenic dissection is also a major cause of additional risk in patients with already established SCAD undergoing invasive treatment. Increased risk of secondary iatrogenic dissections during coronary angiography (2%), non-SCAD angiography (0.2%) and PCI (14.3%) in SCAD patients has been previously observed in case series[23].
There is still a gap in the literature regarding the understanding of the potential underlying mechanism of non-atherosclerotic SCAD. Two potential theories regarding the initiation of the coronary artery wall have been proposed. The first theory indicates sudden disruption of the coronary artery wall caused by an intimal tear[24,25]. Specifically, the intimal tear results in separation of the inner intimal lining from the outer vessel wall, allowing blood to enter into the false lumen and create an intramural hematoma within or between one of the three layers (intima, media, or adventitia)[24,26]. Pressuredriven expansion of the hematoma causes propagation of the dissection plane with formation of a true lumen, and a thrombus containing a false lumen. The second theory suggests spontaneous rupture and bleeding of the vasa vasorum within the vessel wall, followed by intramedial hemorrhage[24,25,27]. Intramural hematoma (IMH), which compresses the true lumen, causes ischemia of the myocardium, being thus, the primary cause of ACS with SCAD[1]. Occlusion of the lumen may also be worsened by thrombi in the true or false lumen having, however, a small pathophysiologic role compared to IMH[28].
SCAD may affect either normal vessels or vessels with weakened arterial wall architecture due to predisposing arteriopathies. As a result, dissection of the coronary artery walls could be extensive both anterograde and retrograde[1,29,30].
RlSK FACTORS
This syndrome is mainly caused by a combination of factors including sex, hormonal fluctuations, underlying arteriopathies, genetics, and environmental, physical and emotional precipitants. As already mentioned above, pregnancy is a strong predisposing factor of SCAD in women[21]. However, a number of additional factors have been associated with the onset of SCAD in more than half of patients. Extreme physical or emotional stress is probably the most important predisposing factor. Triggering factors leading to a Valsalva-like increase in thoracoabdominal pressure or raising catecholamines, including cocaine exposure or sympathomimetic drugs, intense physical activity, coughing, retching, vomiting and bowel movement, in combination with underlying predisposing arteriopathies, can increase cardiocirculatory shear stress resulting in SCAD[11,15,31,32]. Specifically, emotional stressors seem to appear more often in women, whereas physical stressors have been reported in men among precipitants[16,29].
A significant risk factor of SCAD is FMD. The first case series of concomitant SCAD and extracoronary FMD was reported back in 2011[33]. Since then, high prevalence of FMD has been observed in 72% to 86% of SCAD patients who were routinely screened[11,34]. FMD is an idiopathic, nonatherosclerotic and noninflammatory systemic vasculopathy characterized by unique angiographic findings reflective of perturbations in the structure of the arterial wall leading to luminal stenosis, especially in small and medium-sized arteries[35]. Although the pathophysiology of FMD causing SCAD remains unknown, it is suggested that fibrosis of the vasa vasorum leads to coronary vessel wall ischemia and proliferation of myofibroblasts resulting in dissection[36]. Apart for stenoses, FMD has variable arterial manifestations including aneurysms and dissections. Multifocal FMD, characterized by multiple stenoses in an artery, is the most common type in adults, mainly affecting women. The difference between multifocal and focal FMD is that the first corresponds with medial fibroplasia, perimedial fibroplasia, and medial hyperplasia on histopathology while the second with adventitial and intimal disease[35,37]. A previous study[38] showed that the predominant arteries involved in FMD are renal (66.67%), iliac (44.44%), carotid (37.04%), and vertebral (33.33%). As a result, the prevalence of FMD may be even higher when screening is performed in patients with hypertension[38,39]. FMD and SCAD are directly associated, as prevalence of FMD among patients with SCAD is > 50%[40,41].
Long-term exposure to exogenous estrogen or progesterone has been shown to cause long-term changes in coronary arterial architecture, being thus, a significant risk factor for SCAD[11].
A relation between systemic inflammation and SCAD seems to exist[42]. Frequent chronic inflammatory systemic diseases including systemic lupus erythematosus, inflammatory bowel disease and sarcoidosis are suggested to have a potential relationship with SCAD[43-45]. A potential vasculitic inflammatory mechanism could be the activation of eosinophils from the adventitial and periadventitial layers a mechanism which leads to the establishment of SCAD[46]. There are data supporting this hypothesis which shows the presence of peri-arterial eosinophils in patients with SCAD compared to patients with iatrogenic or traumatic dissections where eosinophils are absent[47].
Specific connective tissue disorders such as Marfan and Ehler-Danlos type 4 syndromes also seem to have a relation with SCAD, with a reported frequency between 1% and 2%[11]. Some connective tissue disorders such as Marfan syndrome are associated with mutations in a single gene (FBN1), whereas others such as hypermobility type Ehlers-Danlos syndrome are thought to be multifactorial[48]. Defective fibrillin protein caused by mutations in the FBN1 gene causes structural and functional perturbation of connective tissues may predispose individuals to SCAD[49]. However, no specific connective tissue disorders or extracoronary vascular phenotypes in patients with SCAD have been observed[48]. A recent study[50] highlighted the role of extracellular matrix dysfunction in SCAD. Specifically, in this large cohort, across all patients with SCAD, rare disruptive variants were found within 10 collagen genes among individuals with SCAD compared with 2506 constrained genes expressed in the coronary artery[50]. Furthermore, patients with SCAD were 1.75-fold more likely to carry disruptive rare variants within fibrillar collagen genes[50]. Other collagen vascular disorders that have been proposed to be associated with SCAD are Alport syndrome and Nail-patella syndrome[51,52].
Thyroid dysfunction could be another possible risk factor for SCAD. In hypothyroidism, the lack and impairment of thyroid hormones might lead to impairments in the structure of the artery wall, making coronary arteries more prone to SCAD[46]. In a previous study, investigators showed that the prevalence of hypothyroidism was significantly higher (26%) in patients with SCAD compared to patients with ACS[53]. In the same study, patients with both SCAD and hypothyroidism had more distal lesions and more tortuous coronary arteries. However, more data is required to support this finding.
Finally, the role of genetics in SCAD is a complex issue. Most recent studies have involved genetics as a predisposing factor in SCAD. SCAD has not been shown to be strongly familial, but it is more likely that cases within families are scattered and independent of specific genes. However, a recent study[54] highlighted the PHACTR1/EDN1 gene as directly associated with SCAD and other cardiovascular conditions such as coronary artery disease. The authors showed that patients who carried the rs9349379-A allele had an increased risk of FMD and SCAD[54]. This association requires further investigation.
CLlNlCAL SYMPTOMS
There is a wide spectrum of clinical presentation and severity of SCAD. Patients with SCAD usually present at the emergency department with chest discomfort as the most common symptom[55], chest pain, and rapid heartbeat or fluttery. Less frequent symptoms include pain to the arms or neck, nausea or vomiting, unusual or extreme tiredness, shortness of breath and back pain[16]. A percentage between 24% and 87% of patients with SCAD demonstrate STEMI alterations in the ECG and elevation of cardiac enzymes[7,13,56,57]. A smaller percentage of patients, present complications such as ventricular arrhythmias (3% to 10%), cardiogenic shock (< 3%) and sudden cardiac death (< 1%)[11,13,30]. Another characteristic of SCAD is reduced left ventricular ejection fraction (EF) (< 50%), observed in almost 44%-49% of patients. However, there are usually significant improvements in the EF after treatment of the arterial dissection[13,58]. In Canada, patients with SCAD expressed their chest pain either as radiation to the arm (approximately 50%) and/or neck (approximately 22%), dyspnea (approximately 19%) and pain in the back (approximately 12%) or as nausea and vomiting (approximately 23%) and hyperhidrosis (approximately 21%)[55]. However, in a minority of patients, chest pain could also be atypical[55].
DlAGNOSTlC METHODS AND ANGlOGRAPHlC CLASSlFlCATlON
Accuracy and early diagnosis are the most important factors for the management of SCAD. Coronary angiography is the gold standard and the first-line imaging technique for patients presenting with ACS. Administration of intracoronary nitrates, intracoronary imaging when safe and available, and/or follow-up noninvasive or invasive coronary imaging may be helpful in order to distinguish SCAD from other etiologies of ACS[18].
The main limitation of coronary angiography is that the 2-dimensional luminogram does not allow us to display the arterial wall[24,26]. As a result, further imaging techniques are required in order to set a definitive diagnosis. Intracoronary imaging, including OCT and intravascular ultrasound (IVUS) (Figure 1), is the supplementary method that improves SCAD diagnosis and has the possibility to display the arterial wall layers compared to coronary angiography[24,26]. Important disadvantages are that it is not widely available, it is often associated with additional risks and higher costs and, it requires instrumentation of the coronary artery, a situation which in SCAD could pose a challenge. Coronary computed tomography angiography has lower spatial resolution compared with conventional angiography and presents challenges in the evaluation of lumens and walls of small coronary arteries[9].
Optical coherence tomography, with an axial resolution of 15 μm, is a supplementary intracoronary imaging method for the diagnosis of SCAD that provides higher spatial resolution[40]. Despite this important advantage, OCT requires blood clearance using a high-pressure contrast injection, a situation that could lead to a further extension of the dissection, and particularly of the false lumen[59]. Nevertheless, the reduction of depth penetration, which is by definition limited in OCT, could lead to safe and accurate diagnosis[6,60]. OCT could also provide specialists with very significant information regarding the true lumen, characteristics related to the false lumen such as the type, size, and the point of extension, as well as its relation with side-branches[60]. In order to locate the characteristic crescentic shape of the false lumen, meticulous image analysis and evaluation are necessary. The practical usefulness of OCT in SCAD patients requiring coronary revascularization is to assure us, before any intervention, the location of the guidewire in the right lumen; thus, avoiding stenting of the false lumen that could cause hazardous complications[61,62].
IVUS, with an axial resolution of 150 μm, is also a supplementary method in SCAD diagnosis, and is able to differentiate atherosclerotic plaques from SCAD[27,59,63,64]. This method clearly illustrates and differentiates the two lumens and can show the severity of false lumen thrombosis. This technique readily depicts the true and the false coronary lumens and is also able to demonstrate the extent of false lumen thrombosis. IVUS presents great advantages over OCT. The major advantages are that pressurized contrast injection to clear blood from the lumen is not required and depth penetration is superior, enabling full imaging through the thrombus of the vessel wall up to the exterior elastic lamina[59]. The primary drawback of IVUS is its poor spatial resolution, making it difficult to identify small structures related to the disease, such as the intimal-medial membrane and localized fenestrations linking the two lumens[65,66].
MRI is another new diagnostic method for patients with SCAD. Specifically, MRI can diagnose SCAD when its underlying diagnosis cannot be initially recognized on angiography[67]. The characteristic findings of MRI in patients with SCAD are (1) late gadolinium enhancement which could be transmural, affected by myocardium, subendocardial, and with patchy enhancement; (2) microvascular obstruction; and (3) IMH[67,68]. Cardiac magnetic resonance (CMR) imaging demonstrating delayed gadolinium enhancement in an area with suspected dissection could help in the confirmation of SCAD. However, normal CMR imaging may not exclude SCAD. A substantial minority of patients with angiographicallyconfirmed SCAD do not have CMR evidence of infarction[18,68].
An important key-point in the differential diagnosis of SCAD would be the discrimination between non-atherosclerotic SCAD and coronary artery dissection caused by atheromatic plaque rupture in patients with atherosclerosis or catheter-induced iatrogenic dissections. The absence of atheroma or calcification in patients with non-atherosclerotic SCAD causes more fragile arterial walls and it is difficult to limit the expansion of the dissection[24]. Therefore, patients in this category often present more extensive dissections, while non-affected coronary artery segments appear smooth on angiography[24]. A proposed diagnostic algorithm from our Institution is demonstrated in Figure 2.

Figure 1 lntravascular imaging techniques in spontaneous coronary artery dissection. A: Optical coherence tomography; B: Intravascular ultrasound.

Figure 2 Flowchart diagram for the diagnosis of spontaneous coronary artery dissection. OCT: Optical coherence tomography; IVUS: Intravascular ultrasound; SCAD: Spontaneous coronary artery dissection.
The classification of SCAD is based on angiographic imaging techniques. There are 3 widely approved types of SCAD[7] (Figure 3): Type 1 describes the pathognomonic appearance of arterial wall contrast staining with multiple radiolucent lumens with or without dye hang-up or slow contrast clearing[30]. Type 2 describes diffuse smooth stenoses of varying severity and length (typically > 20 mm). It usually has abrupt changes in the arterial caliber from the normal diameter to diffuse smooth narrowing. The diffuse narrowing is bordered by normal artery segments proximally and distally to the IMH in type-2A variant, while it extends to the apical tip of the artery in type-2B variant[24]. Type 3 describes a focal or tubular (typically < 20 mm) stenosis mimicking atherosclerosis requiring supplementary techniques to diagnose IMH or double-lumen, such as OCT and IVUS[24].
The most common angiographic type of SCAD is type 2, observed in approximately 67% of dissected arteries[7,11,40,69]. The left anterior descending artery is the most frequently affected (32%-46%)[7,11,40,69].
THERAPEUTlC STRATEGlES

Figure 3 Types of spontaneous coronary artery dissection based on angiographic imaging techniques.
One of the most controversary issues of modern cardiology is the management strategy of SCAD. There are no randomized controlled trials to compare the effect of medical therapies and revascularization strategies in these patients in order to contribute to the determination of the optimal management of SCAD. It also remains unclear whether guideline-indicated medical therapies administered for ACS would be beneficial in SCAD[70]. Therefore, management strategies are usually based on expert opinions. There is a special category of patients with SCAD, often presenting with cardiogenic shock. Pregnancy-associated SCAD is associated with dissection of more prominent epicardial vessels, leading to extensive myocardial injury and life-threatening arrhythmias[21]. As a result, these patients typically present with ACS complicated by acute heart failure or cardiogenic shock[21]. The therapeutic approach in these patients may include immediate hemodynamic support with mechanical circulatory support, extracorporeal membrane oxygenation, intra-aortic balloon pump (IABP), and left ventricular assist device (LVAD), in combination with revascularization or even cardiac transplantation[71,72]. Case reports have demonstrated beneficial effects of these therapeutic approaches in pregnant women with SCAD and cardiogenic shock[73].
Conservative management
The aim of conservative management in the acute phase of SCAD is to restore or preserve myocardial perfusion and cardiac function. Medical therapy includes beta-blockers and aspirin. The beneficial effect of beta-blockers in reducing shear stress of the coronary artery walls is associated with lower risk of recurrence, especially in patients with significant impairment of left ventricular systolic function[1,40]. Other medical therapies related to the reduction of arterial pressure and protection of the myocardium, such as angiotensin converting enzyme, angiotensin receptor blocker or mineralocorticoid receptor antagonist, are also indicated in SCAD[74,75].
The use of antiplatelets and anticoagulants, as well as the duration of treatment, remain controversial in SCAD. Patients who undergo stenting have an indication to receive dual antiplatelet therapy for 12 mo and lifelong monotherapy (usually aspirin) while acute dual antiplatelet therapy (usually with aspirin and clopidogrel) is suggested for patients managed conservatively[34,40,74,75]. The indication for acute administration of anticoagulants is only during revascularization while the indication for chronic use is in the case of left ventricular thrombus or thromboembolism[65]. However, all the above approaches remain questionable and further evidence is required.
The role of lipid-lower therapies is unclear and controversial. Statins could be recommended for routinely use after MI and in patients with pre-existing dyslipidemia[40,76]. SCAD is characterized by a general lack of atherosclerosis, and, therefore, statins may not be effective in patients with a normal lipidemic profile.
Thrombolysis is contraindicated in the acute management of SCAD as it might extend dissection and cause coronary rupture, leading to cardiac tamponade[77,78]. Adverse outcomes related to thrombolytics, including extension of dissection or hematoma, have been reported in SCAD[79].
Revascularization
Percutaneous coronary intervention is associated with poor success rates (50%-70%) due to the increased risk of potential coronary complications including iatrogenic catheter-induced dissections, extension of dissections, and failure to enter into the true lumen[61,80]. Furthermore, there is an increased risk of proximal and/or distal false lumen propagation during stent deployment[44,81] and major side branch restriction or occlusion by propagation of the hematoma[59]. Secondary iatrogenic dissection is a major risk for patients undergoing invasive treatment. Increased risk of secondary iatrogenic dissections during coronary angiography (2%), non-SCAD angiography (0.2%) and PCI (14.3%) has been reported in SCAD patients[23]. Another study also demonstrated an increased percentage of secondary dissections related to catheterization maneuvers in patients with SCAD (5%)[12]. Taking all these factors into consideration, meticulous co-axial catheter technique and avoidance of aggressive catheterization maneuvers are suggested for patients with an indication for revascularization, while a conservative approach would be the preferred strategy in clinically stable SCAD patients with no evidence of ongoing ischemia[11,13]. Moreover, in SCAD patients, catheterization through femoral access would be preferable than radial access. Data have shown a 3-fold higher risk for catheter-induced iatrogenic dissection in patients with SCAD undergoing coronary angiography by radial access compared to femoral access[11,23].
There are specific indications for revascularization. Patients with hemodynamic instability, ongoing or recurrent ischemia, ventricular arrhythmias, or left main dissection should be considered for PCI[1,9]. A necessary condition for PCI is the suitable anatomy of the coronary arteries. Meticulous catheter wire and guide-wire manipulation, avoidance of deep catheter engagement, noncoaxial positioning of the catheter tip, catheter dampening, and/or strong contrast injections are measures that should be implemented in order to minimize complications[13,82]. All medical procedures should be undertaken with extreme attention in order to prevent catheter-induced dissection. Less conventional interventional approaches include minimal plain old balloon angioplasty to restore flow[83], longer stents to lower the possibility of further extension of the hematoma[59], and other similar techniques[84-87].
Coronary artery by-pass grafting (CABG) could be considered for patients with left main dissections with ongoing ischemia, extensive dissections, in cases where PCI failed and in patients who are not anatomically suitable for PCI[1,59]. CABG as a treatment strategy for SCAD is usually associated with promising in-hospital outcomes[12]. However, there are no clinical trials to prove the beneficial effect of CABG compared to PCI. In contrast, retrospective studies have shown high rates of graft failure at follow-up[12]. There are only case reports suggesting CABG over PCI in peripartum SCAD in order to avoid complications or sudden cardiac death due to extension of the dissection and aneurysm formation[88]. Although CABG has not been shown to prevent recurrent SCAD[12], it still remains an important therapeutic strategy, providing coronary blood flow and myocardial perfusion in critically ill patients. Despite concern for inadequacy of distal targets in diffusely dissected vessels, successful graft anastomoses are usually achieved in every primary vessel and almost all secondary vessels in all patients treated with CABG during index hospitalization, including patients initially managed conservatively or with PCI[12]. A potential risk of CABG is the fact that dissected coronary artery tissues are profoundly fragile, unlikely to hold suture, and prone to anastomotic complications[18]. A medical history of connective tissue disorders may increase the risk. Another possible complication of CABG in patients with SCAD is the high rates of bypass conduit failure due to the fact that healing of the native coronary arteries results in increasing competitive flow[12,18]. There is still not enough evidence on whether arterial or venous grafting demonstrates better outcomes in patients with SCAD. Thus, the use of reliable and immediately high-flow venous conduits could be suggested, particularly in hemodynamically unstable patients with large territories of myocardium on ongoing ischemia[18].
A proposed management algorithm for SCAD is shown in Figure 4.
PROGNOSlS AND FOLLOW-UP
The prognosis of SCAD is usually good and patients surviving SCAD demonstrate low long-term mortality[11,12,13]. Specifically, 49.7% to 89.7% of patients with SCAD received medical therapy as initial treatment, and 2.6% to 8.5% of conservatively treated patients eventually required revascularization during the index hospitalization[11,12,13]. Revascularization with PCI is the initial treatment for SCAD in 16.7% to 47.1% of patients[11,12,13]. Reported PCI success rates vary from 36.4% to 72.5%, which is significantly lower than the success rates in control subjects with atherosclerotic ACS[11,12,13]. Emergency CABG is required in 2.2% to 7.4% of patients who initially received medical therapy or PCI. An initial CABG revascularization approach was used in 0.6% to 3.7%, and initial success was high in this small group of patients, ranging from 87.5% to 100%[11,12,13]. During a follow-up of 2 to 3 years, major adverse cardiac events related to recurrent SCAD were reported in 10% to 30% of cases[3,11,29,40]. At longer-term follow-up, major adverse cardiac events predominantly related to recurrent SCAD were reported in 15% to 37% at 5 to 7 years, while the estimated rate of major adverse cardiac events was approximately 50% at 10 years[3,11-13,29]. Conservative therapeutic strategies including medical therapy have shown excellent long-term prognosis at 6 years with event-free survival rates between 88% and 94%[89]. Another case series in United States reported a 10-year survival of 92%[29]. Similarly, a case series in Italy reported a 94.4% 6-year survival[13] while, in Canada, mortality was estimated at 1.2% in 3.1 years[40]. Studies with a small number of SCAD patients, such as the Swiss series and the Japanese series, either did not demonstrate deaths in 63 patients at a median 4.5-year follow-up[56] or demonstrated a single death among 63 patients who were followed up for 34 mo[3], respectively. However, recurrent dissections and high rates of target vessel failure are significant factors that caused major adverse cardiac events ranging from 14.6% to 47.4% in SCAD patients who underwent PCI in the above case series[3,13,29,40]. SCAD recurrence was 17% in United States patients within a 4-year followup, approximately 29% within 10 years, and the period between the two events was at 2.8 years[29]. The corresponding percentage in the Canadian case series was 10.4% within 3.1 years, while the percentage of a new MI event was approximately 17%[40]. The Japanese series study showed recurrent SCAD of 11% after 1 mo from the first episode, during their 34-mo follow-up[3]. In the Swiss series study, 3 patients had recurrent SCAD out of 63 patients followed-up for a median of 4.5 years[56]. Finally, the Italian series study reported a 4.7% recurrence rate over a median 22-mo follow-up[13].
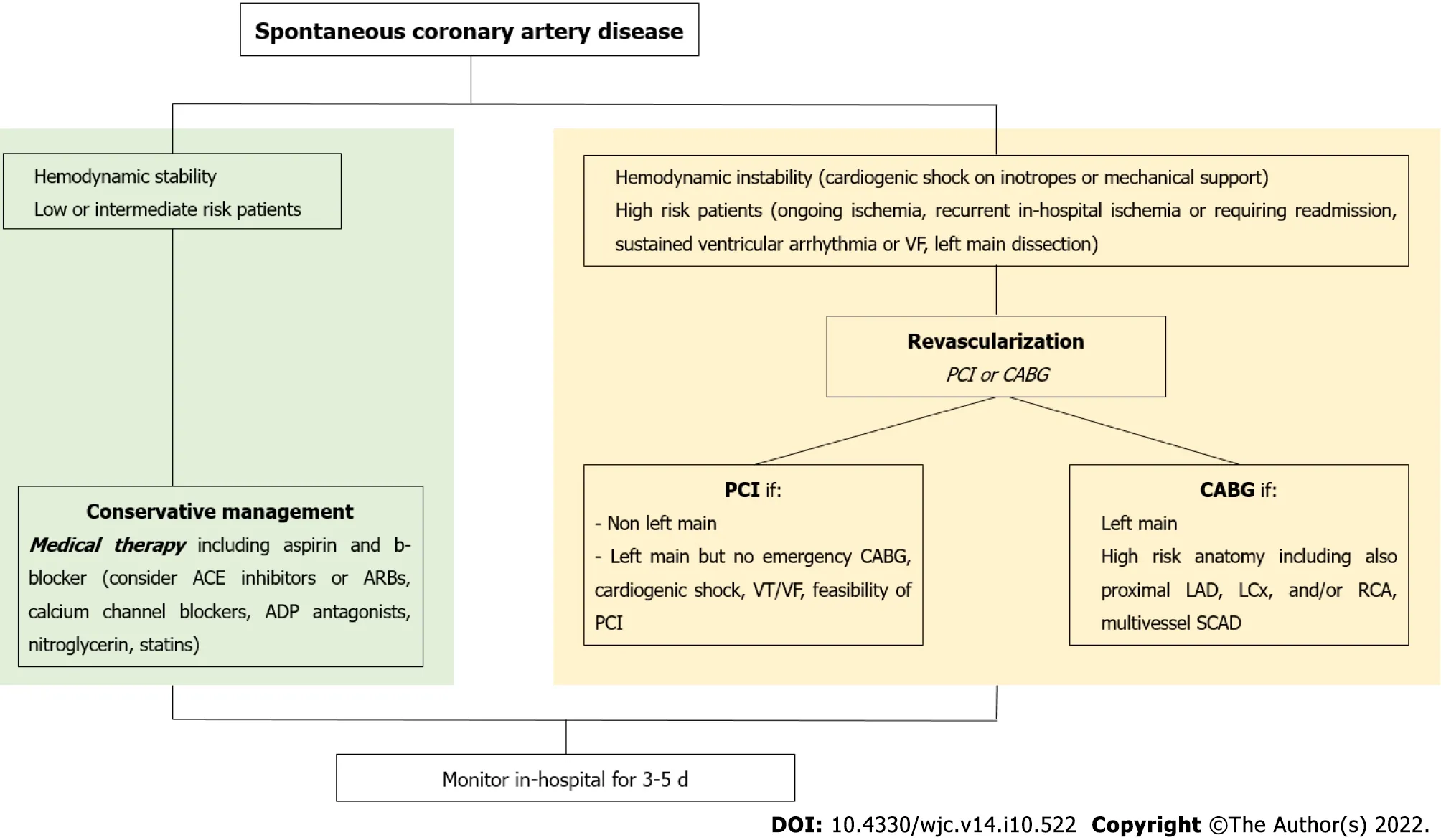
Figure 4 Proposed management algorithm for spontaneous coronary artery dissection. VF: Ventricular fibrillation; ACE: Angiotensin converting enzyme; ADP: Adenosine diphosphate; CABG: Coronary artery by-pass grafting; VT: Ventricular tachycardia; PCI: Percutaneous coronary intervention; LAD: Left anterior descending artery; LCx: Left circumflex coronary; RCA: Right coronary artery; SCAD: Spontaneous coronary artery dissection.
Follow-up of SCAD patients should be performed every year with imaging methods including simple techniques such as echocardiography and cardiac magnetic resonance imaging, more complex techniques such as computed tomography-peripheral angiography or magnetic resonance-angiography, and invasive techniques such as coronary angiography[59]. Coronary computed tomography angiography was shown to be a valuable and useful method, without complications, for noninvasive follow-up of patients with SCAD, especially in those with large-caliber coronary arteries[57,90,91]. A significant limitation of coronary computed tomography angiography is the poor visualization of SCAD lesions in distal coronary arteries or side branches, or of vessel caliber < 2.5 mm[11,92]. For all patients, either treated conservatively or those who underwent PCI, a follow-up angiography in the catheter laboratory is suggested 6 mo after the event, in cases where coronary computed tomography angiography is not feasible or misdiagnosing. Repeat angiograms can be performed by the femoral or radial approach during a short hospital stay or on an ambulatory basis[93]. Patients should also have outpatient appointments at the referring hospital or with cardiologists in private practice twice within the first year of the event; typically, 1 to 3 mo and 12 mo after the event. Thereafter the appointments depend on the clinical status of each patient[93]. Other non-invasive follow-up techniques including cardiopulmonary exercise stress test and echocardiography should be conducted every year. In pregnancy, pregnant women with a medical history of SCAD should be referred to specialized medical centers for assessment, counseling for the potential increased risk of dissection and complications during pregnancy, and follow-up by a multidisciplinary team that includes maternal-fetal medicine specialists, cardiologists with experience managing SCAD, and obstetric anesthesiologists[83]. In patients with systemic arteriopathy, an extracoronary dissection or an aneurysm, subsequent use of alternative imaging methods that do not expose the patient to ionizing radiation such as magnetic resonance angiography and duplex ultrasonography should be considered[94]. Major adverse cardiac events should be registered in all patients with SCAD at follow-up, especially in the first 5 years.
EXERClSE AND LlFESTYLE AFTER SCAD
In most SCAD patients, especially those with recurrence or noncoronary aneurysms or dissections, it is suggested thay they avoid extreme endurance training, exercise until exhaustion, elite competitive sports, or vigorous exertion in extremes of ambient temperature. Additionally, patients should avoid lifting or carrying heavy objects that require straining or prolonged Valsalva maneuvers[18]. The onset of SCAD symptoms has been correlated with physical activity in up to 32% of patients after SCAD[95]. After the onset of chest pain, patients should be immediately evaluated according to proposed algorithms in previous articles[18].
High levels of psychological distress including depression, anxiety, and post-traumatic stress disorder are associated with post-SCAD patients, especially women. Psychological support is always recommended in patients with cardiovascular diseases[96].
CURRENT DEVELOPMENTS AND FUTURE PERSPECTlVES
The current progress in early recognition and appropriate therapeutic management of SCAD has improved the understanding of the pathophysiology background of the disease, its association with potential risk factors and the role of genetics. Diagnostic management includes high-resolution diagnostic methods such as OCT, IVUS, cardiac MRI and duplex ultrasonography. Progress has also been noted in the therapeutic strategies of the disease. Life-saving technologies, especially in patients with hemodynamic instability, including mechanical circulatory support systems, extracorporeal membrane oxygenation, IABP, and LVADs, as well as new revascularization techniques and progress in cardiac transplantation have increased survival rates and reduced complications in these patients.
However, data remain retrospective, observational, and often based on case reports. Collaborative evaluations across medical centers are further required. National registries and continuous follow-up programs at 1, 3, 6 and 12 mo, with or without intravascular imaging, would provide more evidence for the disease, leading thus, to more comprehensive points of view regarding accurate diagnosis of SCAD among AMI and chest pain, appropriate indications and optimal techniques for revascularization, optimization of medical therapy, individualization of risk factors for recurrent SCAD and better development of genetic model systems for better understanding of genetic variations of SCAD. Invasive approaches with the use of bioresorbable vascular scaffolds should be encouraged in proximal/middle vessel lesions, occurring in larger vessels (≥ 3.0 mm vessel diameter) and in cases where patients are still symptomatic or hemodynamically unstable[97,98]. Finally, the establishment of new accurate scores for the diagnosis and therapeutic management of SCAD is mandatory.
CONCLUSlON
SCAD is a rare cardiovascular disease which mostly affect women (approximately 90%), but there are limited data regarding diagnosis and treatment. The increased awareness of SCAD during the last decade has improved our knowledge regarding the potential mechanisms of the disease. Alertness and education are the most important alliances of the medical community in the early and accurate diagnosis of SCAD, especially among women, and its treatment strategies. Coronary angiography is the gold standard treatment method in unstable patients with symptoms of acute ischemia. OCT and IVUS are supplementary intracoronary imaging techniques in the diagnosis of SCAD, and they should be performed before each intervention in order to increase safety and reduce secondary iatrogenic dissections related to stenting. Further randomized controlled trials in medical centers, investigating different diagnostic methods and different therapeutic approaches, are required.
FOOTNOTES
Author contributions:Kourek C performed the majority of the writing and prepared the figures; Lionakis N, Briasoulis A, Zouganeli V and Dimopoulos S provided the input in writing the paper; Lionakis N, Kourek C and Kalpakos D designed the outline and coordinated the writing of the paper; all authors revised and approved the final draft. Lionakis N and Kourek C contributed equally to this work.
Conflict-of-interest statement:All authors declare no conflict of interests for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Greece
ORClD number:Christos Kourek 0000-0003-4348-2153; Stavros Dimopoulos 0000-0003-2199-3788.
S-Editor:Liu JH
L-Editor:Webster JR
P-Editor:Liu JH
杂志排行
World Journal of Cardiology的其它文章
- Euglycemic diabetic ketoacidosis: A rare but serious side effect of sodium-glucose co-transporter 2 inhibitors
- Is Takotsubo cardiomyopathy still looking for its own nosological identity?
- Role of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cryoballoon ablation outcomes for paroxysmal atrial fibrillation
- Virulent endocarditis due to Haemophilus parainfluenzae: A systematic review of the literature
